Wireless Flexible System for Highly Sensitive Ammonia Detection Based on Polyaniline/Carbon Nanotubes
Abstract
:1. Introduction
2. Experiment
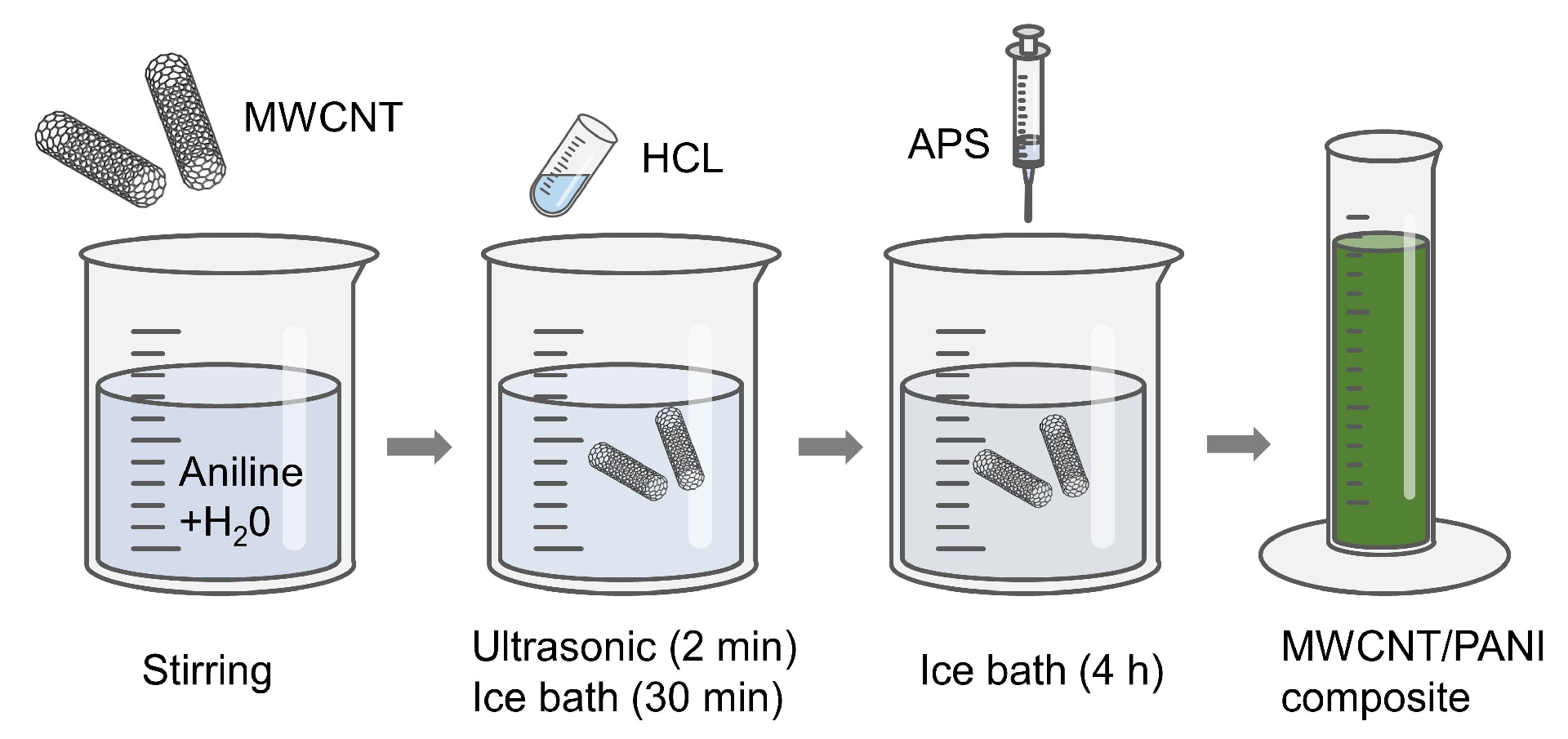
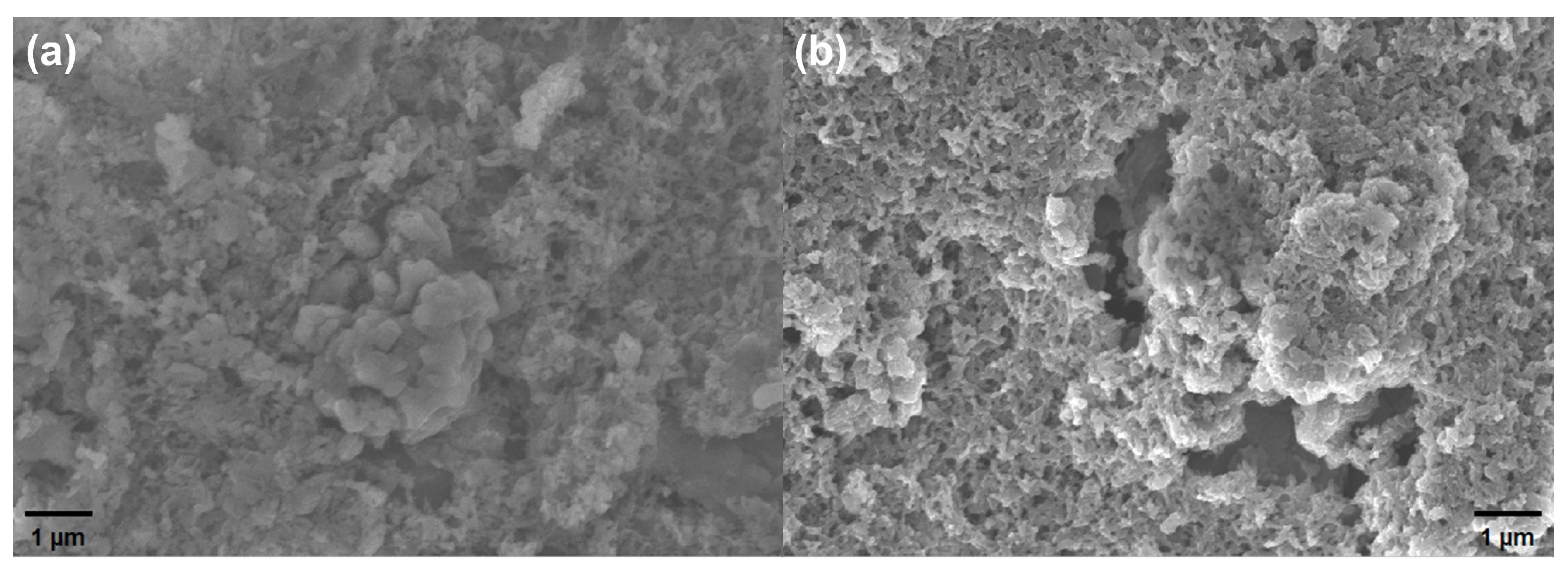
2.1. Material Synthesis
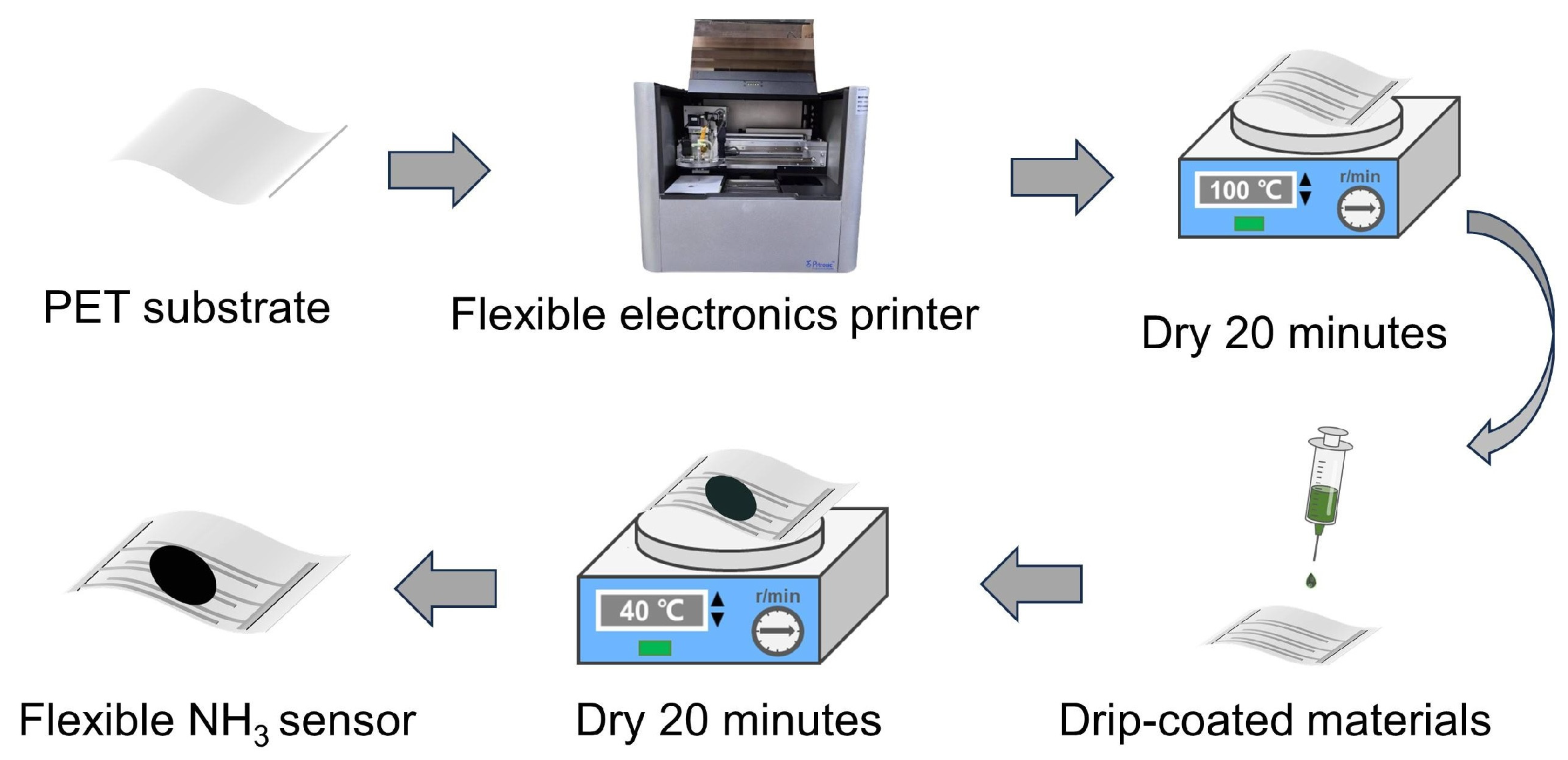
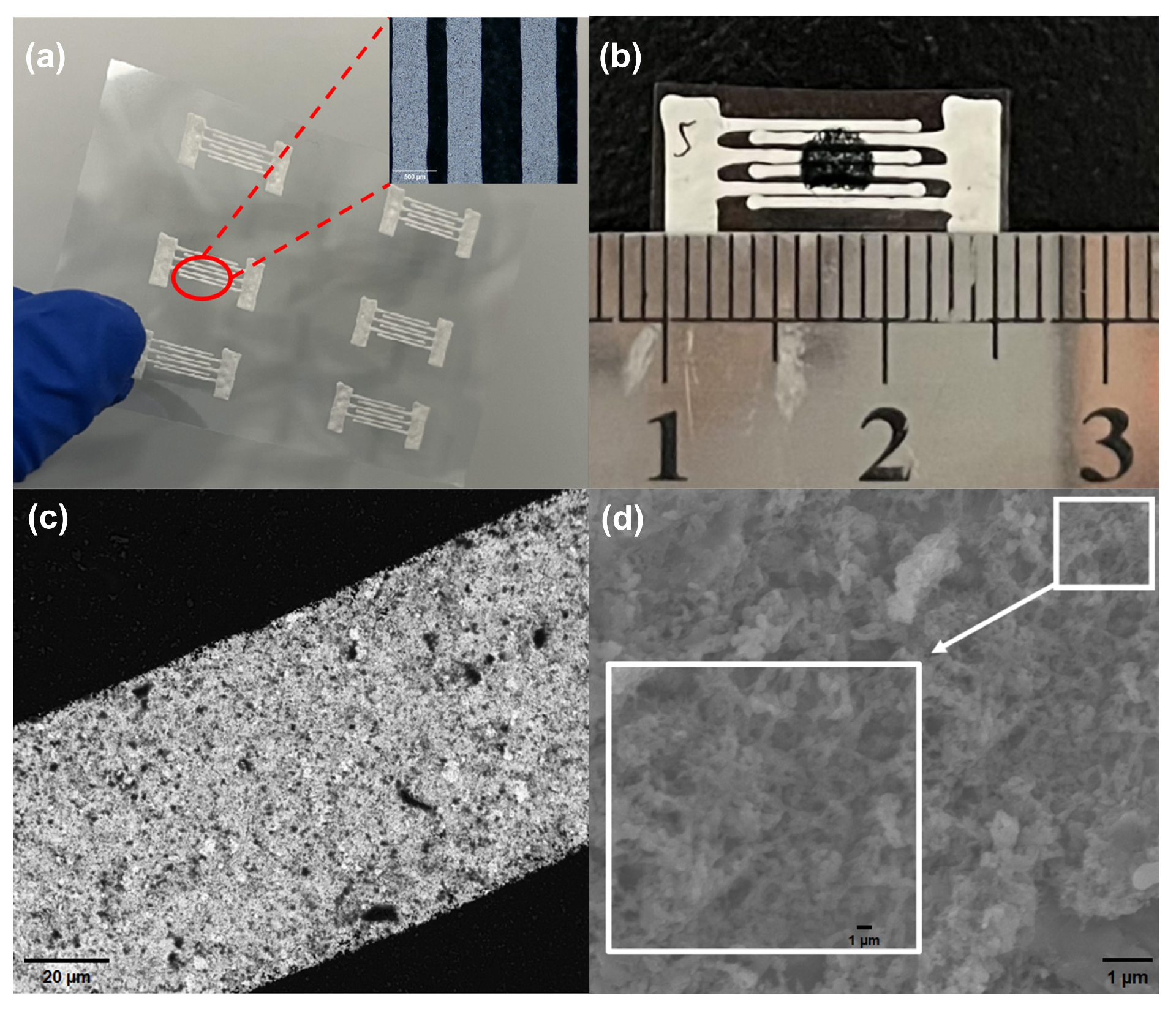
2.2. Sensor Preparation
3. Results and Discussion
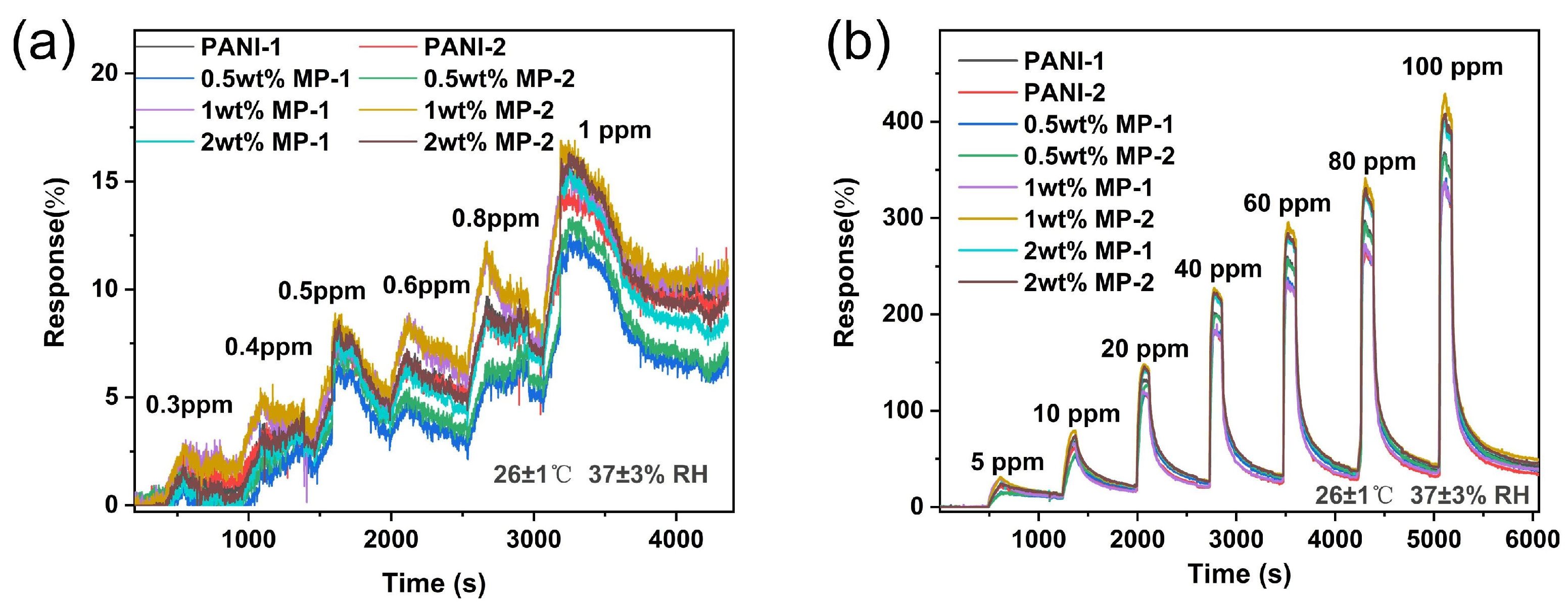
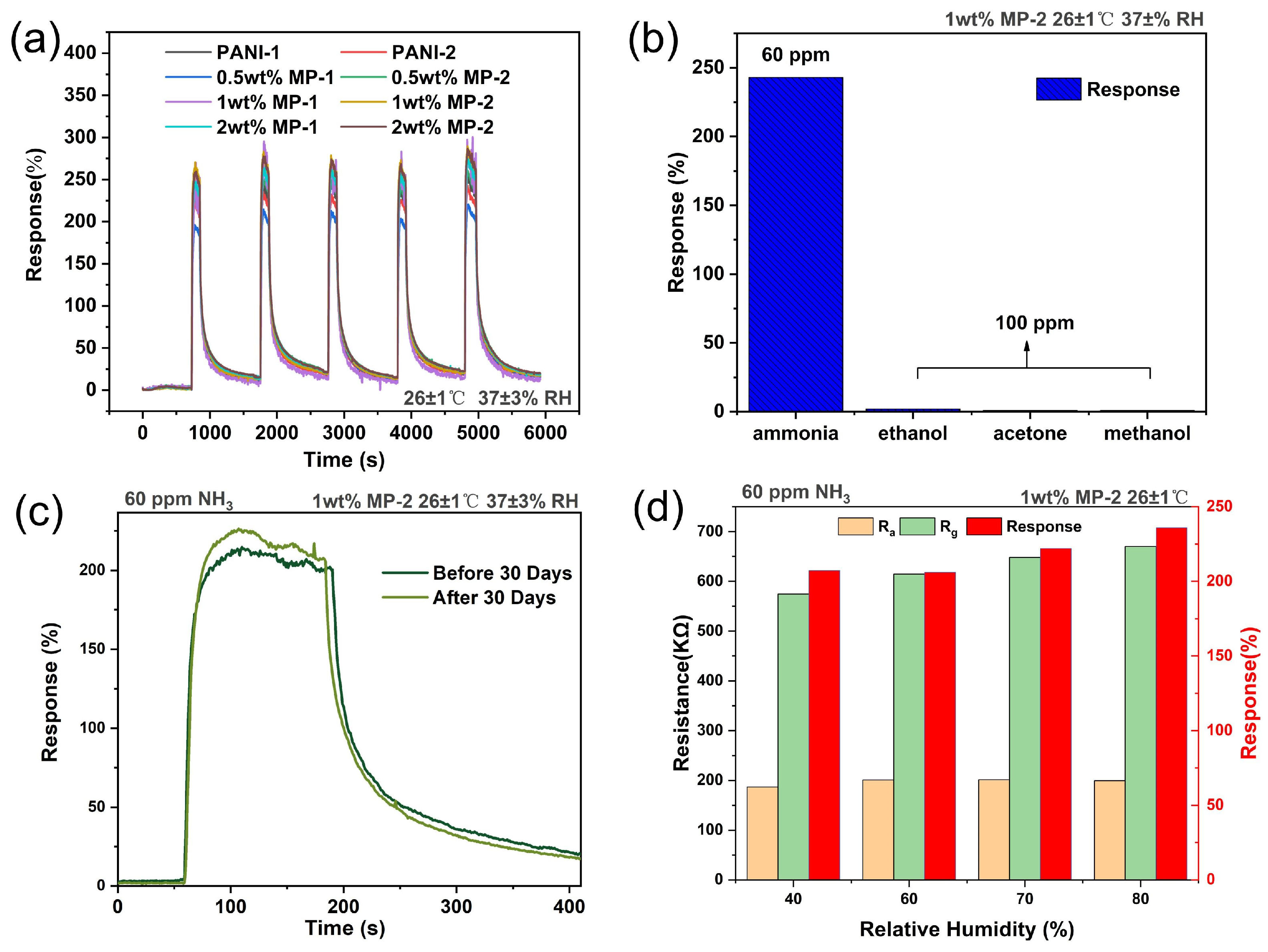
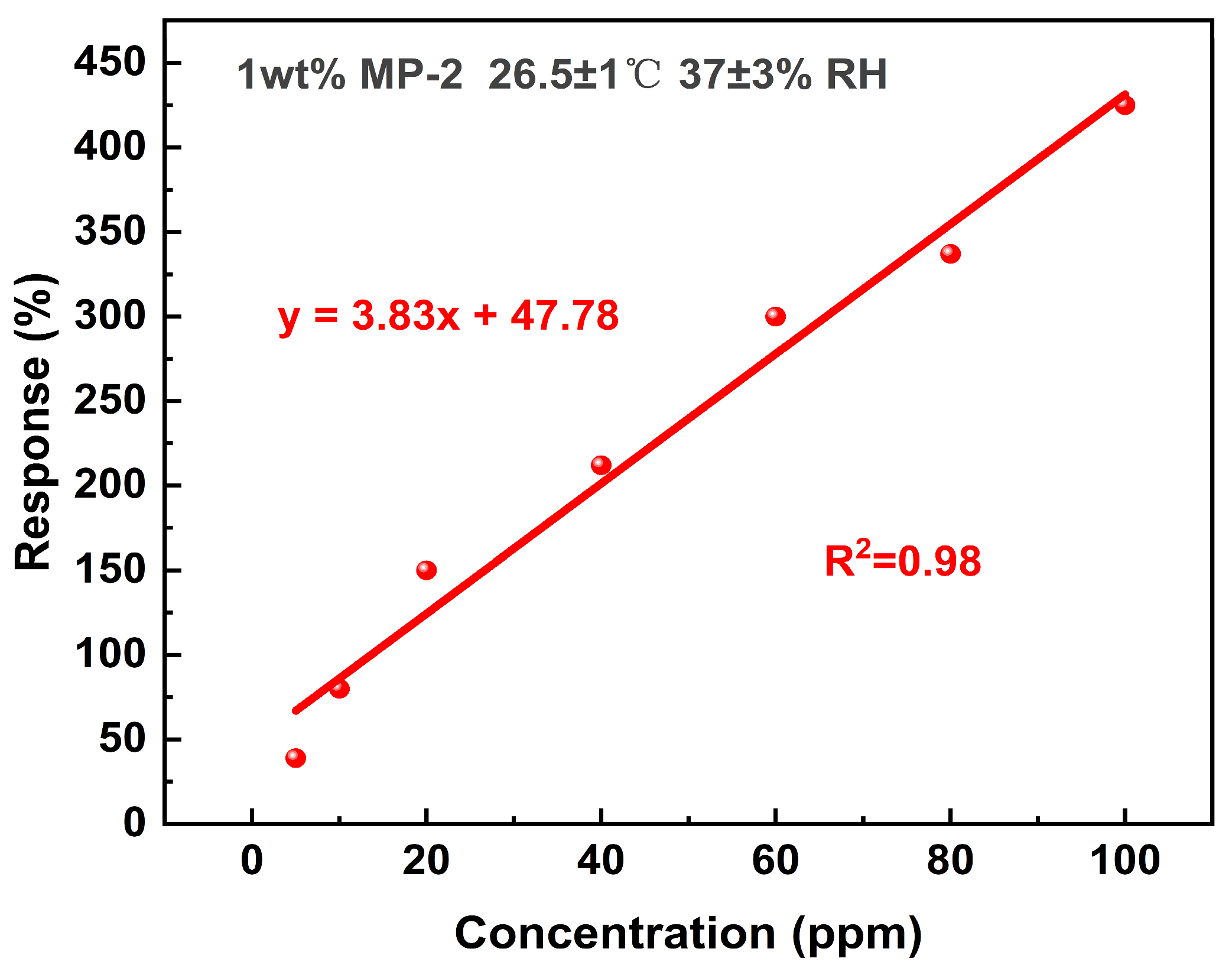
3.1. Gas Sensing Performance
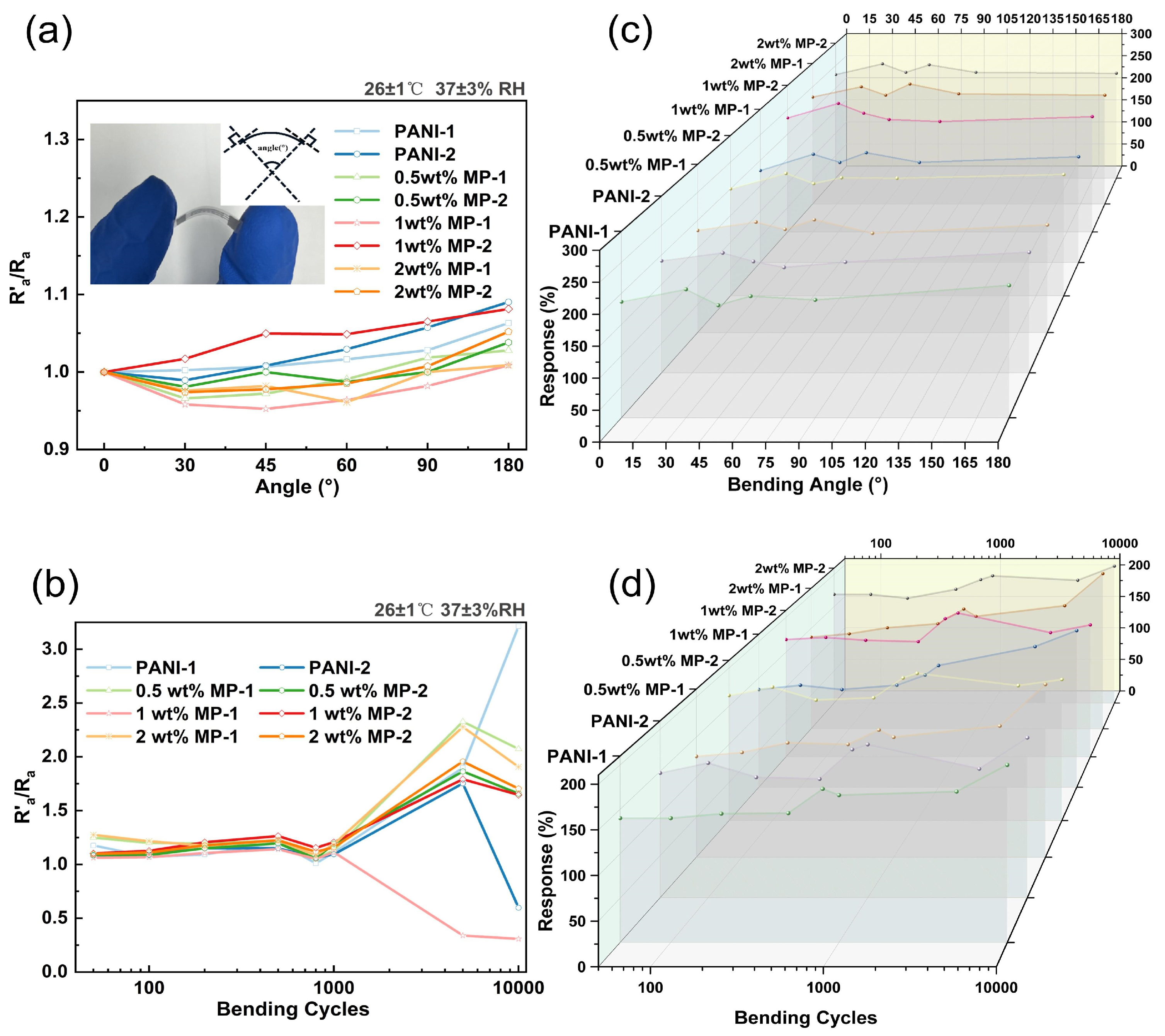
3.2. Anti-Bending Performance
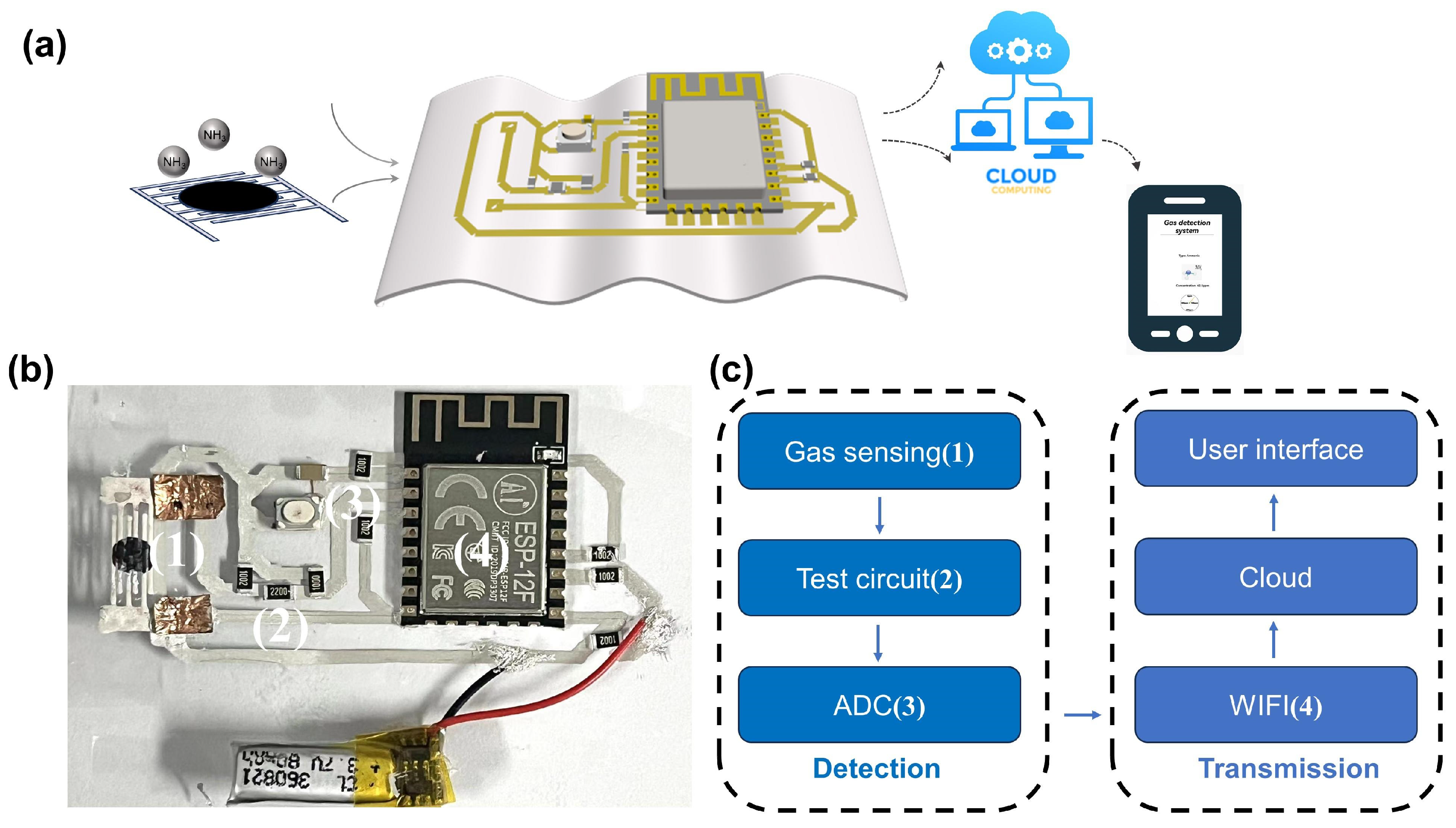
3.3. Wireless Sensing Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, H.; Shi, J.; Liu, C.; Chen, X.; Lv, W.; Zhou, Y.; Zeng, M.; Yang, J.; Wei, H.; Zhou, Z.; et al. Design of p–p Heterojunctions Based on CuO Decorated WS2 Nanosheets for Sensitive NH3 Gas Sensing at Room Temperature. Nanotechnology 2021, 32, 445502. [Google Scholar] [CrossRef] [PubMed]
- Achary, L.S.K.; Kumar, A.; Barik, B.; Nayak, P.S.; Tripathy, N.; Kar, J.P.; Dash, P. Reduced Graphene Oxide-CuFe2O4 Nanocomposite: A Highly Sensitive Room Temperature NH3 Gas Sensor. Sens. Actuators B Chem. 2018, 272, 100–109. [Google Scholar] [CrossRef]
- Wang, H.; Nie, S.; Li, H.; Ali, R.; Fu, J.; Xiong, H.; Li, J.; Wu, Z.; Lau, W.-M.; Mahmood, N.; et al. 3D Hollow Quasi-Graphite Capsules/Polyaniline Hybrid with a High Performance for Room-Temperature Ammonia Gas Sensors. ACS Sens. 2019, 4, 2343–2350. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xin, X.; Zhang, S.; Sui, J.; Yu, J.; Wang, X.; Long, Y.-Z. Silver-Loaded Carbon Nanofibers for Ammonia Sensing. E-Polymers 2020, 20, 606–612. [Google Scholar] [CrossRef]
- Timmer, B.; Olthuis, W.; Berg, A.V.D. Ammonia Sensors and Their Applications—A Review. Sens. Actuators B Chem. 2005, 107, 666–677. [Google Scholar] [CrossRef]
- Wu, M.; He, M.; Hu, Q.; Wu, Q.; Sun, G.; Xie, L.; Zhang, Z.; Zhu, Z.; Zhou, A. Ti3C2 MXene-Based Sensors with High Selectivity for NH3 Detection at Room Temperature. ACS Sens. 2019, 4, 2763–2770. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Lv, S.; Jiang, L.; Liu, F.; Zhao, L.; Wang, J.; Hu, X.; Yang, Z.; He, J.; Wang, C.; et al. The Gas Sensor Utilizing Polyaniline/MoS2 Nanosheets/SnO2 Nanotubes for the Room Temperature Detection of Ammonia. Sens. Actuators B Chem. 2021, 332, 129444. [Google Scholar] [CrossRef]
- Gavgani, J.N.; Hasani, A.; Nouri, M.; Mahyari, M.; Salehi, A. Highly Sensitive and Flexible Ammonia Sensor Based on S and N Co-Doped Graphene Quantum Dots/Polyaniline Hybrid at Room Temperature. Sens. Actuators B Chem. 2016, 229, 239–248. [Google Scholar] [CrossRef]
- Balamurugan, C.; Lee, D.-W. A Selective NH3 Gas Sensor Based on Mesoporous P-Type NiV2O6 Semiconducting Nanorods Synthesized Using Solution Method. Sens. Actuators B Chem. 2014, 192, 414–422. [Google Scholar] [CrossRef]
- Fedoruk, M.J.; Bronstein, R.; Kerger, B.D. Ammonia Exposure and Hazard Assessment for Selected Household Cleaning Product Uses. J. Expo. Sci. Environ. Epidemiol. 2005, 15, 534–544. [Google Scholar] [CrossRef]
- Obermeier, J.; Trefz, P.; Happ, J.; Schubert, J.K.; Staude, H.; Fischer, D.-C.; Miekisch, W. Exhaled Volatile Substances Mirror Clinical Conditions in Pediatric Chronic Kidney Disease. PLoS ONE 2017, 12, e0178745. [Google Scholar] [CrossRef] [PubMed]
- Fenske, J.D.; Paulson, S.E. Human Breath Emissions of VOCs. J. Air Waste Manag. Assoc. 1999, 49, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhu, Z.; Lv, W.; Wu, J.; Yang, J.; Zeng, M.; Hu, N.; Su, Y.; Liu, R.; Yang, Z. E-Nose System Based on Fourier Series for Gases Identification and Concentration Estimation From Food Spoilage. IEEE Sens. J. 2023, 23, 3342–3351. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, Y.; Kang, S.; Cui, M.; Xu, H. Development of pH-Responsive Antioxidant Soy Protein Isolate Films Incorporated with Cellulose Nanocrystals and Curcumin Nanocapsules to Monitor Shrimp Freshness. Food Hydrocoll. 2021, 120, 106893. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, P.; Niu, G.; Luo, D.; Wang, Q.; Wang, F. Rapid and Efficient Detection of NH3 at Room Temperature Using CuO/WS2 Nanohybrids. IEEE Sens. J. 2022, 22, 12539–12546. [Google Scholar] [CrossRef]
- Ke, F.; Zhang, Q.; Ji, L.; Zhang, Y.; Zhang, C.; Xu, J.; Wang, H.; Chen, Y. Electrostatic Adhesion of Polyaniline on Carboxylated Polyacrylonitrile Fabric for High-Performance Wearable Ammonia Sensor. Compos. Commun. 2021, 27, 100817. [Google Scholar] [CrossRef]
- Ren, W.; Zhao, C.; Niu, G.; Zhuang, Y.; Wang, F. Gas Sensor Array with Pattern Recognition Algorithms for Highly Sensitive and Selective Discrimination of Trimethylamine. Adv. Intell. Syst. 2022, 4, 2200169. [Google Scholar] [CrossRef]
- Zhuang, Y.; Liu, X.; Wang, X.; Niu, G.; Cheng, R.; Wang, F. Pulse Heating Combined with Machine Learning for Enhanced Gas Identification and Concentration Detection With MOS Gas Sensors. IEEE Sens. Lett. 2023, 7, 6006304. [Google Scholar] [CrossRef]
- Niu, G.; Wang, F. A Review of MEMS-Based Metal Oxide Semiconductors Gas Sensor in Mainland China. J. Micromech. Microeng. 2022, 32, 054003. [Google Scholar] [CrossRef]
- Zhao, C.; Gong, H.; Niu, G.; Wang, F. Ultrasensitive SO2 Sensor for Sub-Ppm Detection Using Cu-Doped SnO2 Nanosheet Arrays Directly Grown on Chip. Sens. Actuators B Chem. 2020, 324, 128745. [Google Scholar] [CrossRef]
- Kroes, J.M.H.; Pietrucci, F.; Chikkadi, K.; Roman, C.; Hierold, C.; Andreoni, W. The Response of Single-Walled Carbon Nanotubes to NO2 and the Search for a Long-Living Adsorbed Species. Appl. Phys. Lett. 2016, 108, 033111. [Google Scholar] [CrossRef]
- Peng, L.-M.; Zhang, Z.; Wang, S. Carbon Nanotube Electronics: Recent Advances. Mater. Today 2014, 17, 433–442. [Google Scholar] [CrossRef]
- Schroeder, V.; Savagatrup, S.; He, M.; Lin, S.; Swager, T.M. Carbon Nanotube Chemical Sensors. Chem. Rev. 2019, 119, 599–663. [Google Scholar] [CrossRef]
- Vasquez, S.; Costa Angeli, M.A.; Petrelli, M.; Ahmad, M.; Shkodra, B.; Salonikidou, B.; Sporea, R.A.; Rivadeneyra, A.; Lugli, P.; Petti, L. Comparison of Printing Techniques for the Fabrication of Flexible Carbon Nanotube-Based Ammonia Chemiresistive Gas Sensors. Flex. Print. Electron. 2023, 8, 035012. [Google Scholar] [CrossRef]
- Park, J.; Ryu, C.; Jang, I.R.; Jung, S.I.; Kim, H.J. A Study of Strain Effect on Stretchable Carbon Nanotube Gas Sensors. Mater. Today Commun. 2022, 33, 105007. [Google Scholar] [CrossRef]
- Abdulla, S.; Pullithadathil, B. Unidirectional Langmuir–Blodgett-Mediated Alignment of Polyaniline-Functionalized Multiwalled Carbon Nanotubes for NH3 Gas Sensor Applications. Langmuir 2020, 36, 11618–11628. [Google Scholar] [CrossRef] [PubMed]
- Kar, P.; Choudhury, A. Carboxylic Acid Functionalized Multi-Walled Carbon Nanotube Doped Polyaniline for Chloroform Sensors. Sens. Actuators B Chem. 2013, 183, 25–33. [Google Scholar] [CrossRef]
- Kumar, L.; Rawal, I.; Kaur, A.; Annapoorni, S. Flexible Room Temperature Ammonia Sensor Based on Polyaniline. Sens. Actuators B Chem. 2017, 240, 408–416. [Google Scholar] [CrossRef]
- Ma, J.; Fan, H.; Li, Z.; Jia, Y.; Yadav, A.K.; Dong, G.; Wang, W.; Dong, W.; Wang, S. Multi-Walled Carbon Nanotubes/Polyaniline on the Ethylenediamine Modified Polyethylene Terephthalate Fibers for a Flexible Room Temperature Ammonia Gas Sensor with High Responses. Sens. Actuators B Chem. 2021, 334, 129677. [Google Scholar] [CrossRef]
- Abdulla, S.; Mathew, T.L.; Pullithadathil, B. Highly Sensitive, Room Temperature Gas Sensor Based on Polyaniline-Multiwalled Carbon Nanotubes (PANI/MWCNTs) Nanocomposite for Trace-Level Ammonia Detection. Sens. Actuators B Chem. 2015, 221, 1523–1534. [Google Scholar] [CrossRef]
- Wu, T.; Lv, D.; Shen, W.; Song, W.; Tan, R. Trace-Level Ammonia Detection at Room Temperature Based on Porous Flexible Polyaniline/Polyvinylidene Fluoride Sensing Film with Carbon Nanotube Additives. Sens. Actuators B Chem. 2020, 316, 128198. [Google Scholar] [CrossRef]
- Zhu, C.; Zhou, T.; Xia, H.; Zhang, T. Flexible Room-Temperature Ammonia Gas Sensors Based on PANI-MWCNTs/PDMS Film for Breathing Analysis and Food Safety. Nanomaterials 2023, 13, 1158. [Google Scholar] [CrossRef] [PubMed]
- Kan, C.-W.; Lam, Y.-L. Future Trend in Wearable Electronics in the Textile Industry. Appl. Sci. 2021, 11, 3914. [Google Scholar] [CrossRef]
- Jin, C.; Bai, Z. MXene-Based Textile Sensors for Wearable Applications. ACS Sens. 2022, 7, 929–950. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Kandasubramanian, B. Flexible Polymeric Substrates for Electronic Applications. Polym. Rev. 2018, 58, 630–667. [Google Scholar] [CrossRef]
- Park, S.J.; Ko, T.-J.; Yoon, J.; Moon, M.-W.; Oh, K.H.; Han, J.H. Highly Adhesive and High Fatigue-Resistant Copper/PET Flexible Electronic Substrates. Appl. Surf. Sci. 2018, 427, 1–9. [Google Scholar] [CrossRef]
- Ding, W.-T.; Jiao, X.-Y.; Zhao, Y.-M.; Sun, X.-Y.; Chen, C.; Wu, A.-P.; Ding, Y.-T.; Hou, P.-X.; Liu, C. Enhancing the Electrical Conductivity and Strength of PET by Single-Wall Carbon Nanotube Film Coating. ACS Appl. Mater. Interfaces 2023, 15, 37802–37809. [Google Scholar] [CrossRef]
- Niu, G.; Zhang, M.; Wu, B.; Zhuang, Y.; Ramachandran, R.; Zhao, C.; Wang, F. Nanocomposites of Pre-Oxidized Ti3C2Tx MXene and SnO2 Nanosheets for Highly Sensitive and Stable Formaldehyde Gas Sensor. Ceram. Int. 2023, 49, 2583–2590. [Google Scholar] [CrossRef]
- Santos, M.C.; Hamdan, O.H.C.; Valverde, S.A.; Guerra, E.M.; Bianchi, R.F. Synthesis and Characterization of V2O5/PANI Thin Films for Application in Amperometric Ammonia Gas Sensors. Org. Electron. 2019, 65, 116–120. [Google Scholar] [CrossRef]
- Tai, H.; Duan, Z.; He, Z.; Li, X.; Xu, J.; Liu, B.; Jiang, Y. Enhanced Ammonia Response of Ti3C2T Nanosheets Supported by TiO2 Nanoparticles at Room Temperature. Sens. Actuators B Chem. 2019, 298, 126874. [Google Scholar] [CrossRef]
- Kulkarni, S.B.; Navale, Y.H.; Navale, S.T.; Stadler, F.J.; Ramgir, N.S.; Patil, V.B. Hybrid Polyaniline-WO3 Flexible Sensor: A Room Temperature Competence towards NH3 Gas. Sens. Actuators B Chem. 2019, 288, 279–288. [Google Scholar] [CrossRef]
- Konvalina, G.; Haick, H. Sensors for Breath Testing: From Nanomaterials to Comprehensive Disease Detection. Acc. Chem. Res. 2014, 47, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Navale, Y.H.; Navale, S.T.; Galluzzi, M.; Stadler, F.J.; Debnath, A.K.; Ramgir, N.S.; Gadkari, S.C.; Gupta, S.K.; Aswal, D.K.; Patil, V.B. Rapid Synthesis Strategy of CuO Nanocubes for Sensitive and Selective Detection of NO2. J. Alloys Compd. 2017, 708, 456–463. [Google Scholar] [CrossRef]
- Yang, Z.; Coutinho, D.H.; Sulfstede, R.; Balkus, K.J.; Ferraris, J.P. Proton Conductivity of Acid-Doped Meta-Polyaniline. J. Membr. Sci. 2008, 313, 86–90. [Google Scholar] [CrossRef]
- Zeng, F.-W.; Liu, X.-X.; Diamond, D.; Lau, K.T. Humidity Sensors Based on Polyaniline Nanofibres. Sens. Actuators B Chem. 2010, 143, 530–534. [Google Scholar] [CrossRef]
- Kim, S.J.; Koh, H.-J.; Ren, C.E.; Kwon, O.; Maleski, K.; Cho, S.-Y.; Anasori, B.; Kim, C.-K.; Choi, Y.-K.; Kim, J.; et al. Metallic Ti3C2Tx MXene Gas Sensors with Ultrahigh Signal-to-Noise Ratio. ACS Nano 2018, 12, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, Y.; Cho, S.; Park, K.; Kang, H.; Kim, S.J.; Jung, H. In Situ Formation of Multiple Schottky Barriers in a Ti3C2 MXene Film and Its Application in Highly Sensitive Gas Sensors. Adv. Funct. Mater. 2020, 30, 2003998. [Google Scholar] [CrossRef]
- Wen, X.; Cai, Y.; Nie, X.; Xiong, J.; Wang, Y.; Song, H.; Li, Z.; Shen, Y.; Li, C. PSS-Doped PANI Nanoparticle/Ti3C2Tx Composites for Conductometric Flexible Ammonia Gas Sensors Operated at Room Temperature. Sens. Actuators B Chem. 2023, 374, 132788. [Google Scholar] [CrossRef]
- Wu, Q.; Shen, W.; Lv, D. An Enhanced Flexible Room Temperature Ammonia Gas Sensor Based on GP-PANI/PVDF Multi-Hierarchical Nanocomposite Film. Sens. Actuators B Chem. 2021, 334, 129630. [Google Scholar] [CrossRef]
| Materials | Response | Detection Limit | Test Conditions | Ref. |
|---|---|---|---|---|
| PANI/V2O5 | 20%/2 ppm | 1 ppm | RT/70%RH | [39] |
| TiO2/Ti3C2Tx | 3.1%/10 ppm | 0.5 ppm | RT/61%RH | [40] |
| PANI-WO3 | 14%/10 ppm | 5 ppm | RT/40–80%RH | [41] |
| MWCNTs/PANI | 38%/10 ppm | 0.3 ppm | RT/37%RH | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuang, Y.; Wang, X.; Lai, P.; Li, J.; Chen, L.; Lin, Y.; Wang, F. Wireless Flexible System for Highly Sensitive Ammonia Detection Based on Polyaniline/Carbon Nanotubes. Biosensors 2024, 14, 191. https://doi.org/10.3390/bios14040191
Zhuang Y, Wang X, Lai P, Li J, Chen L, Lin Y, Wang F. Wireless Flexible System for Highly Sensitive Ammonia Detection Based on Polyaniline/Carbon Nanotubes. Biosensors. 2024; 14(4):191. https://doi.org/10.3390/bios14040191
Chicago/Turabian StyleZhuang, Yi, Xue Wang, Pengfei Lai, Jin Li, Le Chen, Yuanjing Lin, and Fei Wang. 2024. "Wireless Flexible System for Highly Sensitive Ammonia Detection Based on Polyaniline/Carbon Nanotubes" Biosensors 14, no. 4: 191. https://doi.org/10.3390/bios14040191





