Recent Progress in Organic Electrochemical Transistor-Structured Biosensors
Abstract
:1. Introduction
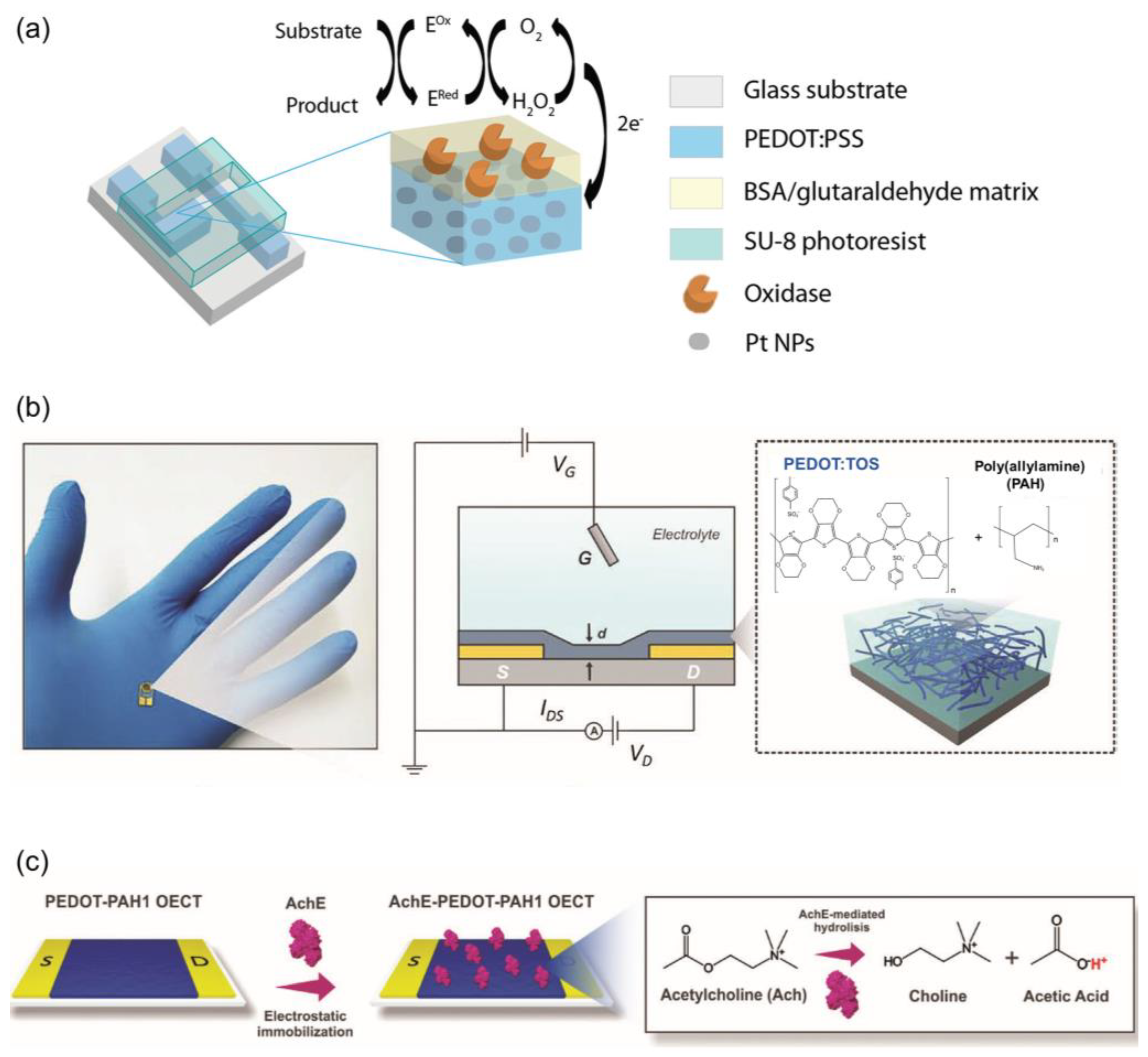
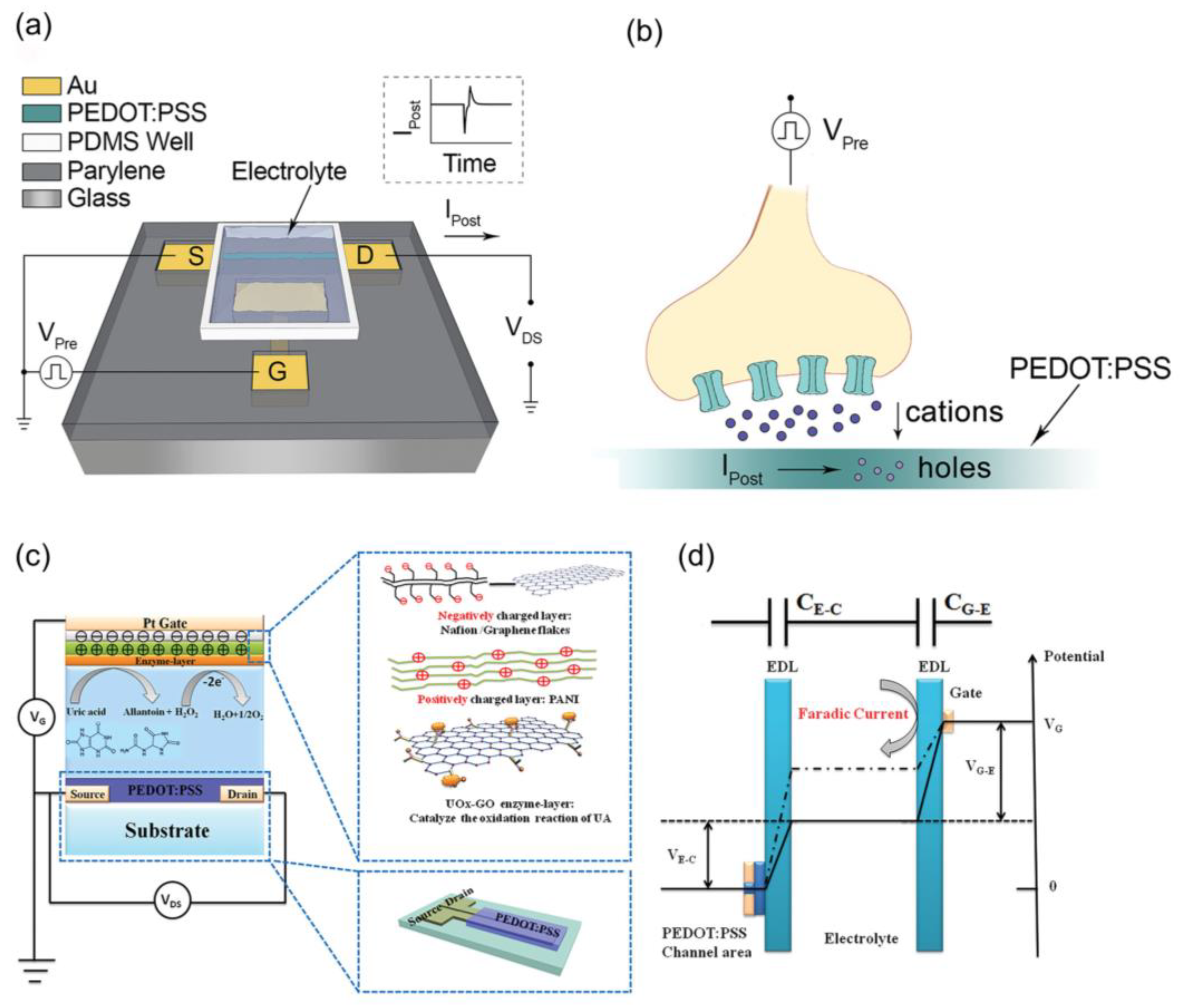
2. Basic Structure and Working Principles of OETBs
3. OETBs for Electroactive Species Detection
3.1. Neurotransmitters Detection

3.2. Vitamins and Antioxidants Category Detection
3.3. Electroactive Metabolites Detection
4. OETBs for Electro-Inactive Species Detection
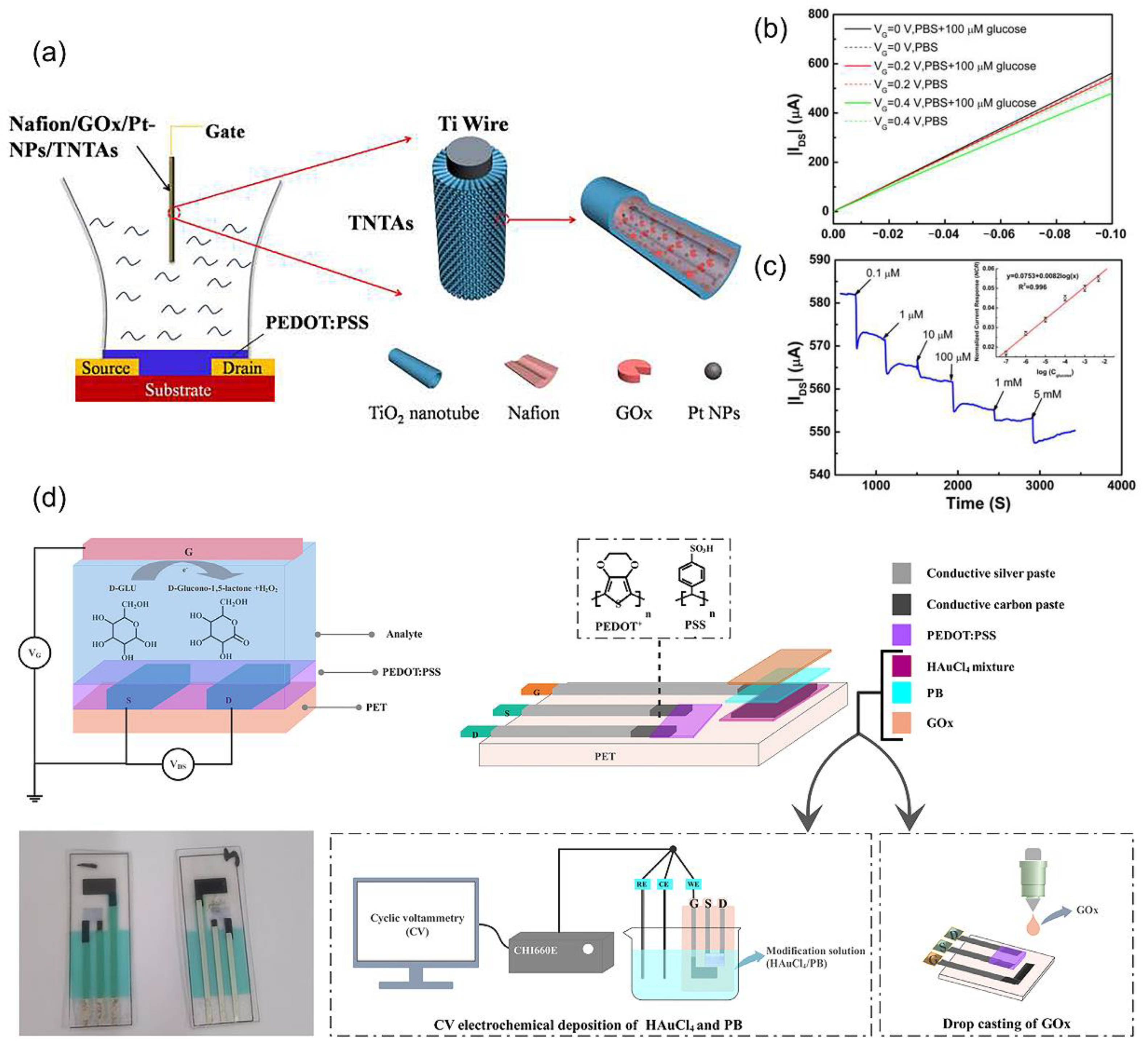
4.1. Electro-Inactive Metabolites Detection
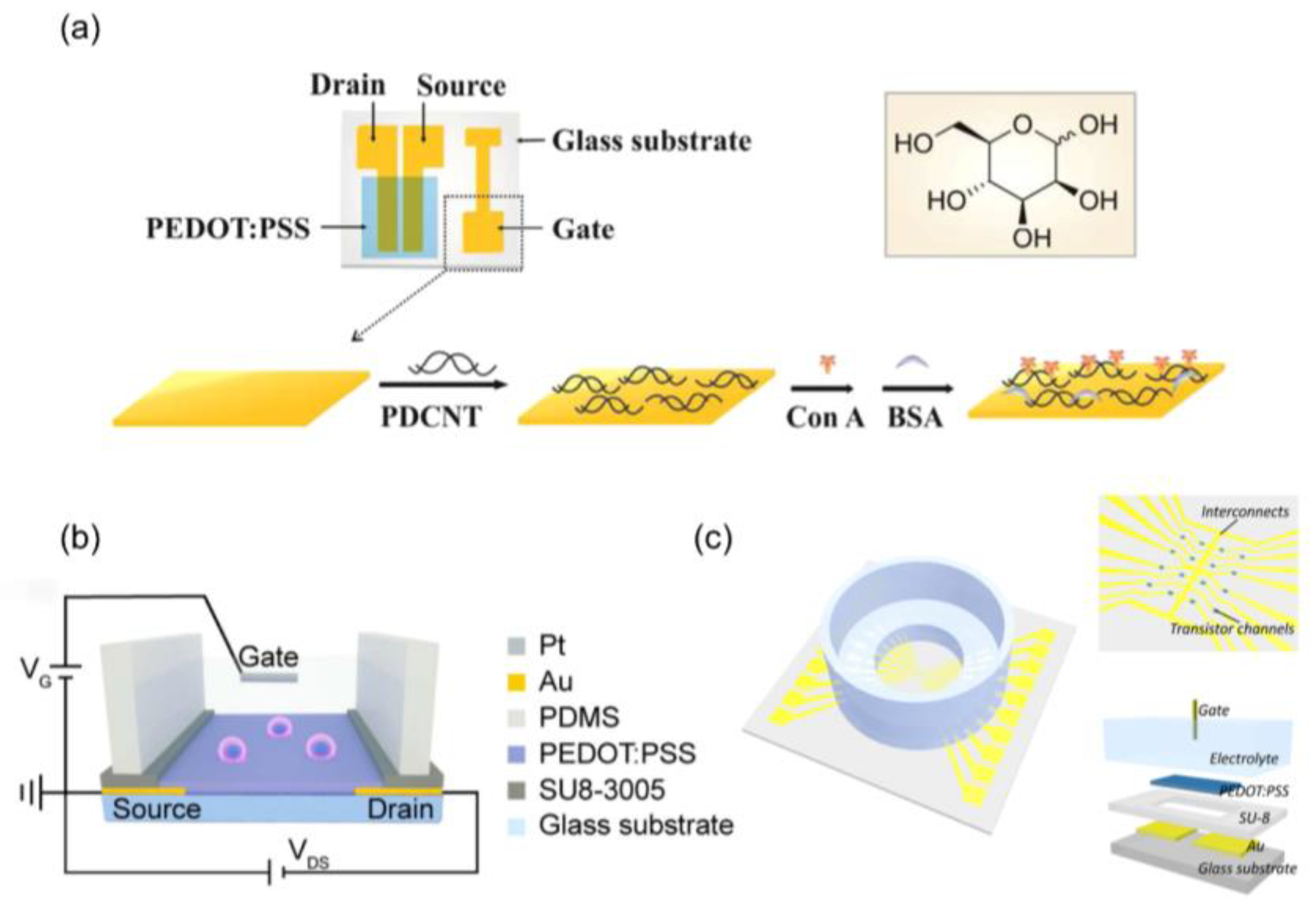
4.2. Biomolecules Detection
5. OETBs for Cancer Cells Detection
5.1. Tumor Markers Detection
5.2. Tissue and Morphological Features Detection
6. OETBs for Wearable and Implantable Human-Machine Interfaces
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Sharma, A.; Ahmed, A.; Sundramoorthy, A.K.; Furukawa, H.; Arya, S.; Khosla, A. Recent Advances in Electrochemical Biosensors: Applications, Challenges, and Future Scope. Biosensors 2021, 11, 336. [Google Scholar] [CrossRef] [PubMed]
- Sardini, E.; Serpelloni, M.; Tonello, S. Printed Electrochemical Biosensors: Opportunities and Metrological Challenges. Biosensors 2020, 10, 166. [Google Scholar] [CrossRef] [PubMed]
- Agius, F.; González-Lamothe, R.; Caballero, J.; Muñoz-Blanco, J.; Botella, M.; Valpuesta, V. Engineering Increased Vitamin C Levels in Plants by Overexpression of a D-galacturonic Acid Reductase. Nat. Biotechnol. 2003, 21, 177–181. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, C.; Sun, Y.; Xu, R.; Li, C.; Wang, C.; Liu, W.; Gu, J.; Shi, Y.; Yang, L.; et al. Synaptic Transistor with Multiple Biological Functions Based on Metal-organic Frameworks Combined with the LIF Model of a Spiking Neural Network to Recognize Temporal Information. Microsyst. Nanoeng. 2023, 9, 96. [Google Scholar] [CrossRef] [PubMed]
- Potje-Kamloth, K. Semiconductor Junction Gas Sensors. Chem. Rev. 2008, 108, 367–399. [Google Scholar] [CrossRef]
- Someya, T.; Dodabalapur, A.; Huang, J.; See, K.C.; Katz, H.E. Chemical and Physical Sensing by Organic Field-Effect Transistors and Related Devices. Adv. Mater. 2010, 22, 3799–3811. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.L.; Miragliotta, J.; Becknell, A.; Katz, H.E. Hydroxy-terminated Organic Semiconductor-based Field-effect Transistors for Phosphonate Vapor Detection. J. Am. Chem. Soc. 2007, 129, 9366–9376. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wang, T.; Li, Y.; Luo, P.; Ni, Y. Organic Synaptic Transistors with an Ultra-Short-Term Weight-Reconstruction for Processing Multiple Types of Signals. Adv. Electron. Mater. 2023, 10, 2300702. [Google Scholar] [CrossRef]
- Ni, Y.; Feng, J.; Liu, J.; Yu, H.; Wei, H.; Du, Y.; Liu, L.; Sun, L.; Zhou, J.; Xu, W. An Artificial Nerve Capable of UV-Perception, NIR–Vis Switchable Plasticity Modulation, and Motion State Monitoring. Adv. Sci. 2022, 9, 2102036. [Google Scholar] [CrossRef]
- Pan, X.; Zhang, Z.; Su, M. Colloidal self-assembly in biosensing strategies for biomarkers diagnosis. Innov. Mater. 2024, 2, 100076. [Google Scholar] [CrossRef]
- Truong, P.L.; Yin, Y.; Lee, D.; Ko, S.H. Advancement in COVID-19 Detection Using Nanomaterial-based Biosensors. Exploration 2023, 3, 20210232. [Google Scholar] [CrossRef]
- Wang, J.; Chen, D.; Huang, W.; Yang, N.; Yuan, Q.; Yang, Y. Aptamer-functionalized Field-effect Tansistor Biosensors for Disease Diagnosis and Environmental Monitoring. Exploration 2023, 3, 20210027. [Google Scholar] [CrossRef]
- Clechet, P.; Jaffrezic-Renault, N. Silica Surface Sensitization and Chemical Sensors. Adv. Mater. 1990, 2, 293–298. [Google Scholar] [CrossRef]
- Harraz, F.A. Porous Silicon Chemical Sensors and Biosensors: A review. Sens. Actuators B Chem. 2014, 202, 897–912. [Google Scholar] [CrossRef]
- Lim, H.J.; Saha, T.; Tey, B.T.; Tan, W.S.; Ooi, C.W. Quartz Crystal Microbalance-based Biosensors as Rapid Diagnostic Devices for Infectious Diseases. Biosens. Bioelectron. 2020, 168, 112513. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Lee, S.S. A Quartz Crystal Microbalance-based Biosensor for Enzymatic Detection of Hemoglobin A1c in Whole Blood. Sens. Actuators B Chem. 2018, 258, 836–840. [Google Scholar] [CrossRef]
- Alanazi, N.; Almutairi, M.; Alodhayb, A. A Review of Quartz Crystal Microbalance for Chemical and Biological Sensing Applications. Sens. Imaging 2023, 24, 10. [Google Scholar] [CrossRef]
- Maas, M.B.; Maybery, G.H.C.; Perold, W.J. Borosilicate Glass Fiber-Optic Biosensor for the Detection of Escherichia coli. Curr. Microbiol. 2018, 75, 150–155. [Google Scholar] [CrossRef]
- Barrio, M.; Cases, R.; Cebolla, V.; Hirsch, T.; Marcos, S.; Wilhelm, S.; Galbán, J. A Reagentless Enzymatic Fluorescent Biosensor for Glucose based on Upconverting Glasses, as Excitation Source, and Chemically Modified Glucose Oxidase. Talanta 2016, 160, 586–591. [Google Scholar] [CrossRef]
- Su, H.; Li, S.; Jin, Y.; Xian, Z.; Yang, D.; Zhou, W.; Kerman, K. Nanomaterial-based Biosensors for Biological Detections. Adv. Health Care Technol. 2017, 3, 19–29. [Google Scholar] [CrossRef]
- Lan, L.; Yao, Y.; Ping, J.; Ying, Y. Recent Advances in Nanomaterial-based Biosensors for Antibiotics Detection. Biosens. Bioelectron. 2017, 91, 504–514. [Google Scholar] [CrossRef]
- Yun, Y.-H.; Eteshola, E.; Bhattacharya, A.; Dong, Z.; Shim, J.-S.; Conforti, L.; Kim, D.; Schulz, M.J.; Ahn, C.H.; Watts, N. Tiny Medicine: Nanomaterial-Based Biosensors. Sensors 2009, 9, 9275–9299. [Google Scholar] [CrossRef]
- Lee, S.H.; Sung, J.H.; Park, T.H. Nanomaterial-Based Biosensor as an Emerging Tool for Biomedical Applications. Ann. Biomed. Eng. 2012, 40, 1384–1397. [Google Scholar] [CrossRef]
- Kurnik, M.; Pang, E.Z.; Plaxco, K.W. An Electrochemical Biosensor Architecture Based on Protein Folding Supports Direct Real-Time Measurements in Whole Blood. Angew. Chem. Int. Ed. 2020, 59, 18442. [Google Scholar] [CrossRef]
- Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Reagentless and Reusable Electrochemical Affinity Biosensors for Near Real-time and/or Continuous Operation. Advances and Prospects. Curr. Opin. Electrochem. 2019, 16, 35–41. [Google Scholar] [CrossRef]
- Bai, L.; Elósegui, C.G.; Li, W.; Yu, P.; Fei, J.; Mao, L. Biological Applications of Organic Electrochemical Transistors: Electrochemical Biosensors and Electrophysiology Recording. Front. Chem. 2019, 7, 2296–2646. [Google Scholar] [CrossRef]
- Wallace, G.G.; Smyth, M.; Zhao, H. Conducting Electroactive Polymer-based Biosensors, Trac-Tend. Anal. Chem. 1999, 18, 245–251. [Google Scholar] [CrossRef]
- Strakosas, X.; Bongo, M.; Owens, R.M. The Organic Electrochemical Transistor for Biological Applications. J. Appl. Polym. Sci. 2015, 132, 41735. [Google Scholar] [CrossRef]
- Rivnay, J.; Inal, S.; Salleo, A.; Owens, R.M.; Berggren, M.; Malliaras, G.G. Organic Electrochemical Transistors. Nat. Rev. Mater. 2018, 3, 17086. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, Y.; Yin, Z. Low-power Soft Transistors Triggering Revolutionary Electronics. Innovation 2024, 5, 100616. [Google Scholar] [CrossRef]
- Nawaz, A.; Liu, Q.; Leong, W.L.; Fairfull-Smith, K.E.; Sonar, P. Organic Electrochemical Transistors for in Vivo Bioelectronics. Adv. Mater. 2021, 33, 2101874. [Google Scholar] [CrossRef]
- Marks, A.; Griggs, S.; Gasparini, N.; Moser, M. Organic Electrochemical Transistors: An Emerging Technology for Biosensing. Adv. Mater. Interfaces 2022, 9, 2102039. [Google Scholar] [CrossRef]
- Friedlein, J.T.; McLeod, R.R.; Rivnay, J. Device physorctics of organic electrochemical transistors. Org. Electron. 2018, 63, 398–414. [Google Scholar] [CrossRef]
- Sophocleous, M.; Contat-Rodrigo, L.; García-Breijo, E.; Georgiou, J. Organic Electrochemical Transistors as an Emerging Platform for Bio-Sensing Applications: A Review. IEEE Sens. J. 2021, 21, 3977–4006. [Google Scholar] [CrossRef]
- Rashid, R.B.; Ji, X.; Rivnay, J. Organic Electrochemical Transistors in Bioelectronic Circuits. Biosens. Bioelectron. 2021, 190, 113461. [Google Scholar] [CrossRef]
- Ravariu, C. From Enzymatic Dopamine Biosensors to OECT Biosensors of Dopamine. Biosensors 2023, 13, 806. [Google Scholar] [CrossRef]
- Shen, H.; Di, C.A.; Zhu, D. Organic transistor for bioelectronic applications. Sci. China-Chem. 2017, 60, 437–449. [Google Scholar] [CrossRef]
- Afreen, S.; Muthoosamy, K.; Manickam, S.; Hashim, U. Functionalized fullerene (C60) as a Potential Nanomediator in the Fabrication of Highly Sensitive Biosensors. Biosens. Bioelectron. 2015, 63, 354–364. [Google Scholar] [CrossRef]
- Moczko, E.; Istamboulie, G.; Calas-Blanchard, C.; Rouillon, R.; Noguer, T. Biosensor Employing Screen-printed PEDOT: PSS for Sensitive Detection of Phenolic Compounds in Water. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 2286–2292. [Google Scholar] [CrossRef]
- Kergoat, L.; Piro, B.; Berggren, M. Advances in Organic Transistor-based Biosensors: From Organic Electrochemical Transistors to Electrolyte-gated Organic Field-effect Transistors. Anal. Bioanal. Chem. 2012, 402, 1813–1826. [Google Scholar] [CrossRef]
- Bernards, D.A.; Malliaras, G.G. Steady-State and Transient Behavior of Organic Electrochemical Transistors. Adv. Funct. Mater. 2007, 17, 3538–3544. [Google Scholar] [CrossRef]
- Romele, P.; Gkoupidenis, P.; Koutsouras, D.A. Multiscale Real Time and High Sensitivity Ion Detection with Complementary Organic Electrochemical Transistors Amplifier. Nat. Commun. 2020, 11, 3743. [Google Scholar] [CrossRef]
- Kukhta, N.A.; Marks, A.; Luscombe, C.K. Molecular Design Strategies toward Improvement of Charge Injection and Ionic Conduction in Organic Mixed Ionic–Electronic Conductors for Organic Electrochemical Transistors. Chem. Soc. Rev. 2022, 122, 4325–4355. [Google Scholar] [CrossRef]
- Tian, X.; Liu, D.; Bai, J.; Chan, K.S.; Ip, L.C.; Chan, P.K.L.; Zhang, S. Pushing OECTs toward Wearable: Development of a Miniaturized Analytical Control Unit for Wireless Device Characterization. Anal. Chem. 2022, 94, 6156–6162. [Google Scholar] [CrossRef]
- Yu, S.; Sun, X.; Liu, J.; Li, S. OECT-Inspired Electrical Detection. Talanta 2024, 275, 126180. [Google Scholar] [CrossRef]
- Zhang, P.; Zhu, B.; Du, P.; Travas-Sejdic, J. Electrochemical and Electrical Biosensors for Wearable and Implantable Electronics Based on Conducting Polymers and Carbon-Based Materials. Chem. Soc. Rev. 2024, 124, 722–767. [Google Scholar] [CrossRef]
- Sun, H.; Gerasimov, J.; Berggren, M.; Fabiano, S. N-Type Organic Electrochemical Transistors: Materials and Challenges. J. Mater. Chem. 2018, 6, 11778–11784. [Google Scholar] [CrossRef]
- Li, X.; Feng, Y.; Shi, L.; Zhou, J.; Ni, Y. Far-gate Synaptic Transistors Utilizing Ion-charge Dual-transfer Mechanism for Neurotransmitter-multiplexing Temporal Coding. Appl. Phys. Lett. 2024, 124, 164101. [Google Scholar] [CrossRef]
- Wu, X.; Chen, S.; Moser, M.; Moudgil, A.; Griggs, S.; Marks, A.; Li, T.; McCulloch, I.; Leong, W.L. High Performing Solid-state Organic Electrochemical Transistors Enabled by Glycolated Polythiophene and Ion-gel electrolyte with a Wide Operation Temperature Range From −50 to 110 °C. Adv. Funct. Mater. 2023, 33, 2209354. [Google Scholar] [CrossRef]
- Bischak, C.G.; Flagg, L.Q.; Ginger, D.S. Ion Exchange Gels Allow Organic Electrochemical Transistor Operation with Hydrophobic Polymers in Aqueous Solution. Adv. Mater. 2020, 32, 2002610. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Hou, K.; Li, T.; Wu, X.; Wang, Z.; Wei, L.; Leong, W.L. Ultra-lightweight, Highly Permeable, and Waterproof Fibrous Organic Electrochemical Transistors for on-skin Bioelectronics. Adv. Mater. Technol. 2023, 8, 2200611. [Google Scholar] [CrossRef]
- Shi, H.; Liu, C.; Jiang, Q.; Xu, J. Effective Approaches to Improve the Electrical Conductivity of PEDOT: PSS: A Review. Adv. Electron. Mater. 2015, 1, 1500017. [Google Scholar] [CrossRef]
- Kayser, L.V.; Lipomi, D.J. Stretchable Conductive Polymers and Composites Based on PEDOT and PEDOT: PSS. Adv. Mater. 2019, 31, 1806133. [Google Scholar] [CrossRef]
- Fan, X.; Nie, W.; Tsai, H.; Wang, N.; Huang, H.; Cheng, Y.; Wen, R.; Ma, L.; YaN, F.; Xia, Y. PEDOT: PSS for Flexible and Stretchable Electronics: Modifications, Strategies, and Applications. Adv. Sci. 2019, 6, 1900813. [Google Scholar] [CrossRef] [PubMed]
- Donahue, M.J.; Sanchez-Sanchez, A.; Inal, S.; Qu, J.; Owens, R.M.; Mecerreyes, D.; Malliaras, G.G.; Martin, D.C. Tailoring PEDOT Properties for Applications in Bioelectronics. Mater. Sci. Eng. R Rep. 2020, 140, 100546. [Google Scholar] [CrossRef]
- Nissa, J.; Janson, P.; Berggren, M.; Simon, D.T. The Role of Relative Capacitances in Impedance Sensing with Organic Electrochemical Transistors. Adv. Electron. Mater. 2021, 7, 2001173. [Google Scholar] [CrossRef]
- Friedlein, J.T.; Rivnay, J.; Dunlap, D.H.; McCulloch, I.; Shaheen, S.E.; McLeod, R.R.; Malliaras, G.G. Influence of Disorder on Transfer Characteristics of Organic Electrochemical Transistors. Appl. Phys. Lett. 2017, 111, 023301. [Google Scholar] [CrossRef]
- Klein, M.O.; Battagello, D.S.; Cardoso, A.R.; Hauser, D.N.; Bittencourt, J.C.; Correa, R.G. Dopamine: Functions, Signaling, and Association with Neurological Diseases. Cell. Mol. Neurobiol. 2019, 39, 31–59. [Google Scholar] [CrossRef]
- Tang, K.; Turner, C.; Case, L.; Mehrehjedy, A.; He, X.; Miao, W.; Guo, S. Organic Electrochemical Transistor with Molecularly Imprinted Polymer-Modified Gate for the Real-Time Selective Detection of Dopamine. ACS Appl. Polym. Mater. 2022, 4, 2337–2345. [Google Scholar] [CrossRef]
- Ji, W.; Wu, D.; Tang, W.; Xi, X.; Su, Y.; Guo, X.; Liu, R. Carbonized Silk Fabric-based Flexible Organic Electrochemical Transistors for Highly Sensitive and Selective Dopamine Detection. Sens. Actuators B Chem. 2020, 304, 127414. [Google Scholar] [CrossRef]
- Xi, X.; Wu, D.; Ji, W.; Zhang, S.; Tang, W.; Su, Y.; Guo, X.; Liu, R. Manipulating the Sensitivity and Selectivity of OECT-Based Biosensors via the Surface Engineering of Carbon Cloth Gate Electrodes. Adv. Funct. Mater. 2020, 30, 1905361. [Google Scholar] [CrossRef]
- Qing, X.; Wang, Y.; Zhang, Y.; Ding, X.; Zhong, W.; Wang, D.; Wang, W.; Liu, Q.; Liu, K.; Li, M.; et al. Wearable Fiber-Based Organic Electrochemical Transistors as a Platform for Highly Sensitive Dopamine Monitoring. ACS Appl. Mater. Interfaces 2019, 11, 13105–13113. [Google Scholar] [CrossRef]
- Wortsman, J. Role of epinephrine in acute stress. Endocrinol. Metab. Clin. North Am. 2002, 1, 79–106. [Google Scholar] [CrossRef]
- Wong, D.L. Epinephrine Biosynthesis: Hormonal and Neural Control During Stress. Cell. Mol. Neurobiol. 2006, 26, 889–898. [Google Scholar] [CrossRef]
- Coppedè, N.; Tarabella, G.; Villani, M.; Calestani, D.; Iannotta, S.; Zappettini, A. Human stress monitoring through an organic cotton-fiber biosensor. J. Mater. Chem. B 2014, 14, 5620–5626. [Google Scholar] [CrossRef]
- Coppedè, N.; Ferrara, L.; Bifulco, P.; Villani, M.; Iannotta, S.; Zappettini, A.; Cesarelli, M.; Fabrizio, E.D.; Gentile, F. Multiscale Modification of the Conductive PEDOT: PSS Polymer for the Analysis of Biological Mixtures in a Super-hydrophobic Drop. Microelectron. Eng. 2016, 158, 80–84. [Google Scholar] [CrossRef]
- Mak, C.H.; Liao, C.; Fu, Y.; Zhang, M.; Tang, C.Y.; Tsang, Y.H.; Chan, H.L.W.; Yan, F. Highly-sensitive epinephrine sensors based on organic electrochemical transistors with carbon nanomaterial modified gate electrodes. J. Mater. Chem. C 2015, 3, 6532–6538. [Google Scholar] [CrossRef]
- Ding, Y.; Tan, K.; Zhang, S.; Wang, S.; Zhang, X.; Hu, P. Wearable and Recyclable Epinephrine Biosensors Based on Molecular Imprinting Polymer Modified Organic Electrochemical Transistors. Chem. Eng. J. 2023, 477, 146844. [Google Scholar] [CrossRef]
- Hasselmo, M.E. The Role of acetylcholine in learning and memory. Curr. Opin. Neurobiol. 2006, 16, 710–715. [Google Scholar] [CrossRef]
- Perry, E. Acetylcholine and Alzheimer’s Disease. Br. J. Psychiatry 1988, 152, 737–740. [Google Scholar] [CrossRef]
- Kergoat, L.; Piro, B.; Simon, D.T.; Pham, M.-C.; Noël, V.; Berggren, M. Detection of Glutamate and Acetylcholine with Organic Electrochemical Transistors Based on Conducting Polymer/Platinum Nanoparticle Composites. Adv. Mater. 2014, 26, 5658–5664. [Google Scholar] [CrossRef]
- Fenoy, G.E.; Bilderling, C.v.; Knoll, W.; Azzaroni, O.; Marmisollé, W.A. PEDOT: Tosylate-Polyamine-Based Organic Electrochemical Transistors for High-Performance Bioelectronics. Adv. Electron. Mater. 2021, 7, 2100059. [Google Scholar] [CrossRef]
- Du, J.; Cullen, J.J.; Buettner, G.R. Ascorbic Acid: Chemistry, Biology and the Treatment of Cancer. Biochim. Biophys. Acta Bioenerg. 2012, 1826, 443–457. [Google Scholar] [CrossRef]
- Gkoupidenis, P.; Schaefer, N.; Garlan, B.; Malliaras, G.G. Neuromorphic Functions in PEDOT: PSS Organic Electrochemical Transistors. Adv. Mater. 2015, 27, 7176–7180. [Google Scholar] [CrossRef]
- Liao, C.; Mak, C.; Zhang, M.; Chan, H.L.W.; Yan, F. Flexible Organic Electrochemical Transistors for Highly Selective Enzyme Biosensors and Used for Saliva Testing. Adv. Mater. 2015, 27, 676–681. [Google Scholar] [CrossRef]
- Feng, J.; Fang, Y.; Wang, C.; Chen, C.; Tang, C.; Guo, Y.; Wang, L.; Yang, Y.; Zhang, K.; Wang, J.; et al. All-Polymer Fiber Organic Electrochemical Transistor for Chronic Chemical Detection in the Brain. Adv. Funct. Mater. 2023, 33, 2214945. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, G.; Wu, D.; Xiong, C.; Zheng, L.; Ding, Y.; Lu, H.; Zhang, G.; Qiu, L. Highly Selective and Sensitive Sensor Based on an Organic Electrochemical Transistor for the Detection of Ascorbic Acid. Biosens. Bioelectron. 2018, 100, 235–241. [Google Scholar] [CrossRef]
- Becker, B.F. Towards the Physiological Function of Uric Acid. Free Radic. Biol. Med. 1993, 14, 615–631. [Google Scholar] [CrossRef]
- Kutzing, M.K.; Firestein, B.L. Altered Uric Acid Levels and Disease States. J. Pharmacol. Exp. Ther. 2008, 324, 1–7. [Google Scholar] [CrossRef]
- Arcangeli, D.; Gualandi, I.; Mariani, F.; Tessarolo, M.; Ceccardi, F.; Decataldo, F.; Melandr, F.; Tonelli, D.; Fraboni, B.; Scavetta, E. Smart Bandaid Integrated with Fully Textile OECT for Uric Acid Real Time Monitoring in Wound Exudate. ACS Sens. 2023, 8, 1593–1608. [Google Scholar] [CrossRef]
- Tao, Y.; Wang, Y.; Zhu, R.; Chen, Y.; Liu, X.; Li, M.; Yang, L.; Wang, Y.; Wang, D. Fiber Based Organic Electrochemical Transistor Integrated with Molecularly Imprinted Membrane for Uric Acid Detection. Talanta 2022, 238, 123055. [Google Scholar] [CrossRef]
- Varki, N.M.; Varki, A. Diversity in Cell Surface Sialic Acid Presentations: Implications for Biology and Disease. Lab. Investig. 2007, 87, 851–857. [Google Scholar] [CrossRef]
- Chen, L.; Wang, N.; Wu, J.; Yan, F.; Ju, H. Organic Electrochemical Transistor for Sensing of Sialic Acid in Serum Samples. Anal. Chim. Acta 2020, 1128, 231–237. [Google Scholar] [CrossRef]
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical Biosensors: Recommended Definitions and Classification. Biosens. Bioelectron. 1999, 71, 2333–2348. [Google Scholar] [CrossRef]
- Laborda, E.; Molina, A.; Batchelor-McAuley, C.; Compton, R.G. Individual Detection and Characterization of Non-Electrocatalytic, Redox-Inactive Particles in Solution by using Electrochemistry. ChemElectroChem 2018, 5, 410. [Google Scholar] [CrossRef]
- Lichtenstein, A.H.; Schwab, U.S. Relationship of Dietary Fat to Glucose Metabolism. Atherosclerosis 2000, 150, 227–243. [Google Scholar] [CrossRef]
- Kalsbeek, A.; Fleur, S.l.; Flier, E. Circadian control of glucose metabolism. Mol. Metab. 2014, 3, 372–383. [Google Scholar] [CrossRef]
- Liao, C.; Zhang, M.; Niu, L.; Zheng, Z.; Yan, F. Highly Selective and Sensitive Glucose Sensors Based on Organic Electrochemical Transistors with Graphene-modified Gate electrodes. J. Mater. Chem. B 2013, 1, 3820–3829. [Google Scholar] [CrossRef]
- Liao, J.; Lin, S.; Yang, Y.; Liu, K.; Du, W. Highly Selective and Sensitive Glucose Sensors Based on Organic Electrochemical Transistors Using TiO2 Nanotube Arrays-based Gate Electrodes. Sens. Actuators B Chem. 2015, 208, 457–463. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, P.; Wang, R.; Gong, Q.; Fang, C. Smart Sweat Glucose Detection System Based on Organic Electrochemical Transistor and Near Field Communication. Chin. J. Anal. Chem. 2024, 52, 100356. [Google Scholar] [CrossRef]
- Singh, M.; Verma, N.; Garg, A.K.; Redhu, N. Urea Biosensors. Sens. Actuators B-Chem. 2008, 134, 345–351. [Google Scholar] [CrossRef]
- Vanholder, R.; Gryp, T.; Glorieux, G. Urea and Chronic Kidney Disease: The Comeback of the Century? (in Uraemia Research). Nephrol. Dial. Transplant. 2018, 33, 4–12. [Google Scholar] [CrossRef]
- Berto, M.; Diacci, C.; Theuer, L.; Di Lauro, M.; Simon, D.T.; Berggren, M.; Biscarini, F.; Beni, V.; Bortolotti, C.A. Label free urea biosensor based on organic electrochemical transistors. Flex. Print. Electron. 2018, 3, 024001. [Google Scholar] [CrossRef]
- Mohamed, S.J.; Murugasenapathi, N.K.; Murugathas, T.; Gopinath, S.C.B.; Tamilarasan, P. Chapter 15–Organic Electrochemical Transistor-based Advanced Biosensor for Clinical Diagnosis. In Health and Environmental Applications of Biosensing Technologies; Elsevier: Amsterdam, The Netherlands, 2024; pp. 317–340. [Google Scholar] [CrossRef]
- Martinez, F.A.C.; Balciunas, E.M.; Salgado, J.M.; González, J.M.D.; Converti, A.; Oliveira, R.P.d.S. Lactic Acid Properties, Applications and Production: A Review. Trends Food Sci. Technol. 2013, 30, 70–83. [Google Scholar] [CrossRef]
- Gorbach, S.L. Lactic Acid Bacteria and Human Health. Ann. Med. 1990, 22, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Payne, M.E.; Zamarayeva, A.; Pister, V.I.; Yamamoto, N.A.D.; Arias, A.C. Printed, Flexible Lactate Sensors: Design Considerations Before Performing On-Body Measurements. Sci. Rep. 2019, 9, 13720. [Google Scholar] [CrossRef] [PubMed]
- Khodagholy, D.; Curto, V.; Fraser, K.; Gurfinkel, M.; Byrne, R.; Diamond, D.; Malliaras, G.; Benito-Lopez, F.; Owens, R. Organic Electrochemical Transistor Incorporating an Ionogel as Solid State Electolyte for Lactate Sensing. J. Mater. Sci. 2012, 22, 4440–4443. [Google Scholar] [CrossRef]
- Scheiblin, G.; Aliane, A.; Strakosas, X.; Curto, V.F.; Coppard, R.; Marchand, G.; Owens, R.M.; Mailley, P.; Malliaras, G.G. Screen-printed Organic Electrochemical Transistors for Metabolite Sensing. MRS Commun. 2015, 5, 507–511. [Google Scholar] [CrossRef]
- Ji, X.; Lau, H.Y.; Ren, X.; Peng, B.; Zhai, P.; Feng, S.; Chan, P.K.L. Highly Sensitive Metabolite Biosensor Based on Organic Electrochemical Transistor Integrated with Microfluidic Channel and Poly (N-vinyl-2-pyrrolidone)-Capped Platinum Nanoparticles. Adv. Mater. Technol. 2016, 1, 1600042. [Google Scholar] [CrossRef]
- Blanco-López, M.C.; Gutiérrez-Fernández, S.; Lobo-Castañón, M.J.; Miranda-Ordieres, A.J.; Tuñón-Blanco, P. Electrochemical Sensing with Electrodes Modified with Molecularly Imprinted Polymer Films. Anal. Bioanal. Chem. 2004, 378, 1922–1928. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.S.; Dabrowski, M.; D’Souza, F.; Kutner, W. Surface Development of Molecularly Imprinted Polymer Films to Enhance Sensing Signals. Trac-Trends Anal. Chem. 2013, 51, 146–157. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, G.; Xiong, C.; Zheng, L.; He, J.; Ding, Y.; Lu, H.; Zhang, G.; Cho, K.; Qiu, L. Chirality Detection of Amino Acid Enantiomers by Organic Electrochemical Transistor. Biosens. Bioelectron. 2018, 105, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Z.; Xiong, C.; Zheng, L.; Ding, Y.; Lu, H.; Zhang, G.; Qiu, L. Selective Recognition of Histidine Enantiomers Using Novel Molecularly Imprinted Organic Transistor Sensor. Org. Electron. 2018, 61, 254–260. [Google Scholar] [CrossRef]
- O’Connor, L.; Glynn, B. Recent Advances in the Development of Nucleic Acid Diagnostics. Expert Rev. Med. Devices 2010, 7, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Umek, R.; Lin, S.W.; Vielmetter, J.; Terbrueggen, R.; Irvine, B.; Yu, C.; Kayyem, J.; Yowanto, H.; Blackburn, G.; Farkas, D.; et al. Electronic Detection of Nucleic Acids: A Versatile Platform for Molecular Diagnostics. J. Mech. Des. 2001, 3, 74–84. [Google Scholar] [CrossRef]
- Kang, T.; Lu, J.; Yu, T.; Long, Y.; Liu, G. Advances in Nucleic Acid Amplification Techniques (NAATs): COVID-19 Point-of-care Diagnostics as an Example. Biosens. Bioelectron. 2022, 206, 114109. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Song, Q.; Lu, W.; Zhang, Z.; Yu, Y.; Liu, X.; He, R. A Sensitive Platform for DNA Detection Based on Organic Electrochemical Transistor and Nucleic Acid Self-assembly Signal Amplification. R. Soc. Chem. Adv. 2021, 11, 37917–37922. [Google Scholar] [CrossRef]
- Song, J.J.; Lin, P.; Ruan, Y.-F.; Zhao, W.-W.; Wei, W.W.; Hu, J.; Ke, S.M.; Zeng, X.R.; Xu, J.-J.; Chen, H.-Y.; et al. Organic Photo-Electrochemical Transistor-Based Biosensor: A Proof-of-Concept Study toward Highly Sensitive DNA Detection. Adv. Healthc. Mater. 2018, 7, 1800536. [Google Scholar] [CrossRef]
- Li, T.; Fan, Q.; Liu, T.; Zhu, X.; Zhao, J.; Li, G. Detection of Breast Cancer Cells Specially and Accurately by an Electrochemical Method. Biosens. Bioelectron. 2010, 25, 2686–2689. [Google Scholar] [CrossRef]
- Liu, J.; Qin, Y.; Li, D.; Wang, T.; Liu, Y.; Wang, J.; Wang, E. Highly Sensitive and Selective Detection of Cancer Cell with a Label-free Electrochemical Cytosensor. Biosens. Bioelectron. 2013, 41, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Guo, M.; Nie, Z.; Xiao, X.; Yao, S. Aptamer-Based Electrochemical Sensor for Label-Free Recognition and Detection of Cancer Cells. Electroanalysis 2009, 21, 1321–1326. [Google Scholar] [CrossRef]
- Soda, N.; Gonzaga, Z.J.; Chen, S.; Koo, K.M.; Nguyen, N.; Shiddiky, M.J.A.; Rehm, B.H.A. Bioengineered Polymer Nanobeads for Isolation and Electrochemical Detection of Cancer Biomarkers. ACS Appl. Mater. Interfaces 2021, 13, 31418–31430. [Google Scholar] [CrossRef]
- Cantor, J.R.; Sabatini, D.M. Cancer Cell Metabolism: One Hallmark, Many Faces. Cancer Discov. 2012, 2, 881–898. [Google Scholar] [CrossRef] [PubMed]
- Madhav, N.; Shreya, S.; Pranshu, S.; Pallavi, C.; Abbas, Z.M. Tumor Markers: A Diagnostic Tool. Int. J. Oral Maxillofac. Surg. 2016, 7, 17–20. [Google Scholar] [CrossRef]
- Alizadeh, E.; Castle, J.; Quirk, A.; Taylor, C.D.L.; Xu, W.; Prasad, A. Cellular Morphological Features are Predictive Markers of Cancer Cell State. Comput. Biol. Med. 2020, 126, 104044. [Google Scholar] [CrossRef]
- Sali, R.; Jiang, Y.; Attaranzadeh, A.; Holmes, B.; Li, R. Morphological Diversity of Cancer Cells Predicts Prognosis across Tumor Types. JNCI—J. Natl. Cancer Inst. 2024, 116, 555–564. [Google Scholar] [CrossRef]
- Alitalo, K.; Keski-oja, J.; Vaheri, A. Extracellular Matrix Proteins Characterize Human Tumor Cell Lines. Int. J. Cancer 1981, 27, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Tothill, I.E. Biosensors for Cancer Markers Diagnosis. Semin. Cell Dev. Biol. 2009, 20, 55–62. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, N.; Yang, A.; Law, H.K.-w.; Li, L.; Yan, F. Highly Sensitive Detection of Protein Biomarkers with Organic Electrochemical Transistors. Adv. Mater. 2017, 29, 1703787. [Google Scholar] [CrossRef]
- Kailemia, M.J.; Park, D.; Lebrilla, C.B. Glycans and Glycoproteins as Specific Biomarkers for Cancer. Anal. Bioanal. Chem. 2017, 409, 395–410. [Google Scholar] [CrossRef] [PubMed]
- Wolf, G.T.; Chretien, P.B.; Elias, E.G.; Makuch, R.W.; Baskies, A.M.; Spiegel, H.E.; WeisS, J.F. Serum Glycoproteins in Head and Neck Squamous Carcinoma: Correlations with Tumor Extent, Clinical Tumor Stage, and T-cell Levels During Chemotherapy. Am. J. Surg. 1979, 138, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Riley, N.M.; Wen, R.M.; Bertozzi, C.R.; Brooks, J.D.; Pitteri, S.J. Chapter Four-Measuring the Multifaceted Roles of Mucin-domain Glycoproteins in Cancer. Adv. Cancer Res. 2023, 157, 83–121. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Fu, Y.; Wang, N.; Yang, A.; Li, Y.; Wu, J.; Ju, H.; Yan, F. Organic Electrochemical Transistors for the Detection of Cell Surface Glycans. ACS Appl. Mater. Interfaces 2018, 10, 18470–18477. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Wang, W.; Liang, J.; Chen, C.; Cao, Y.; Cai, B.; Chen, B.; He, R. Fabrication of PEDOT: PSS-based Solution Gated Organic Electrochemical Transistor Array for Cancer Cells Detection. R. Soc. Chem. Adv. 2023, 13, 36416–36423. [Google Scholar] [CrossRef]
- Yeung, S.Y.; Gu, X.; Tsang, C.M.; Tsao, S.W.G.; Hsing, I. Organic Electrochemical Transistor Array for Monitoring Barrier Integrity of Epithelial Cells Invaded by Nasopharyngeal Carcinoma. Sens. Actuators B-Chem. 2019, 297, 126761. [Google Scholar] [CrossRef]
- Stamey, T.A.; Yang, N.; Hay, A.R.; McNeal, J.; Freiha, F.S.; Redwine, E.A. Prostate-Specific Antigen as a Serum Marker for Adenocarcinoma of the Prostate. N. Engl. J. Med. 1987, 317, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Lilja, H.; Ulmert, D.; Vickers, A. Prostate-specific Antigen and Prostate Cancer: Prediction, Detection and Monitoring. Nat. Rev. Cancer 2008, 8, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Catalona, W.J.; Smith, D.S.; Ratliff, T.L.; Dodds, K.M.; Coplen, D.E.; Yuan, J.J.; Petros, J.A.; Andriole, G.L. Measurement of Prostate-specific Antigen in Serum as a Screening Test for Prostate Cancer. New Engl. J. Medicine. 1991, 25, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Lee, N.; Park, J.; Park, I.; Kim, J.; Cho, H.J. Organic Electrochemical Transistor Based Immunosensor for Prostate Specific Antigen (PSA) Detection Using Gold Nanoparticles for Signal Amplification. Biosens. Bioelectron. 2010, 25, 2477–2482. [Google Scholar] [CrossRef]
- Pantel, K.; Speicher, M. The Biology of Circulating Tumor Cells. Oncogene 2016, 35, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Ignatiadis, M.; Lee, M.; Jeffrey, S.S. Circulating Tumor Cells and Circulating Tumor DNA: Challenges and Opportunities on the Path to Clinical Utility. Clin. Cancer Res. 2015, 21, 4786–4800. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.A.; Jiang, W.G. Loss of Tight Junction Barrier Function and Its Role in Cancer Metastasis. Biochim. Et Biophys. Acta-Biomembr. 2009, 1788, 872–891. [Google Scholar] [CrossRef] [PubMed]
- Kominsky, S.; Argani, P.; Korz, D.; Evron, E.; Raman, V.; Garrett, E.; Rein, A.; Sauter, G.; Kallioniemi, O.-P.; Sukumar, S. Loss of the Tight Junction Protein Claudin-7 Correlates with Histological Grade in Both Ductal Carcinoma in Situ and Invasive Ductal Carcinoma of the Breast. Oncogene 2003, 22, 2021–2033. [Google Scholar] [CrossRef] [PubMed]
- Utoguchi, N.; Mizuguchi, H.; Dantakean, A.; Makimoto, H.; Wakai, Y.; Tsutsumi, Y.; Nakagawa, S.; Mayumi, T. Effect of Tumour Cell-conditioned Medium on Endothelial Macromolecular Permeability and Its Correlation with Collagen. Br. J. Cancer 1996, 73, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Liu, H.; Wang, W.; Chen, C.; Cao, Y.; Chen, B.; Cai, B.; He, R. Carboxyl Graphene modified PEDOT: PSS organic electrochemical transistor for in situ detection of cancer cell morphology. Nanoscale 2024, 15, 3631–3640. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Liu, J.; Han, H.; Yu, Q.; Yang, L.; Xu, Z.; Jiang, C.; Liu, L.; Xu, W. Visualized in-sensor Computing. Nat. Commun. 2024, 15, 3454. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Yang, L.; Feng, J.; Liu, J.; Sun, L.; Xu, W. Flexible optoelectronic neural transistors with broadband spectrum sensing and instant electrical processing for multimodal neuromorphic computing. SmartMat 2023, 4, e1154. [Google Scholar] [CrossRef]
- Nielsen, C.B.; Giovannitti, A.; Sbircea, D.-T.; Bandiello, E.; Niazi, M.R.; Hanifi, D.A.; Sessolo, M.; Amassian, A.; Malliaras, G.G.; Rivnay, J.; et al. Molecular Design of Semiconducting Polymers for High-Performance Organic Electrochemical Transistors. J. Am. Chem. Soc. 2016, 138, 10252–10259. [Google Scholar] [CrossRef]
- Chen, X.; Marks, A.; Paulsen, B.D.; Wu, R.; Rashid, R.B.; Chen, H.; Alsufyani, M.; Rivnay, J.; McCulloch, I. n-Type Rigid Semiconducting Polymers Bearing Oligo (Ethylene Glycol) Side Chains for High-Performance Organic Electrochemical Transistors. Angew. Chem.-Int. Ed. 2021, 60, 9368–9373. [Google Scholar] [CrossRef]
- Xu, S.; Liu, Y.; Lee, H.; Li, W. Neural Interfaces: Bridging the Brain to the World beyond Healthcare. Exploration 2024, 20230146. [Google Scholar] [CrossRef]
- Xu, M.; Obodo, D.; Yadavall, V.K. The Design, Fabrication, and Applications of Flexible Biosensing Devices. Biosens. Bioelectron. 2019, 124–125, 96–114. [Google Scholar] [CrossRef] [PubMed]
- Bocchetta, P.; Frattini, D.; Ghosh, S.; Mohan, A.M.V.; Kumar, Y.; Kwon, Y. Soft Materials for Wearable/Flexible Electrochemical Energy Conversion, Storage, and Biosensor Devices. Materials 2020, 13, 2733. [Google Scholar] [CrossRef]
- Mitcheson, P.D. Energy harvesting for human wearable and implantable bio-sensors. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 3432–3436. [Google Scholar] [CrossRef]
- Premanode, B.; Toumazou, C. A Novel, Low Power Biosensor for Real Time Monitoring of Creatinine and Urea in Peritoneal Dialysis. Sens. Actuators B Chem. 2007, 120, 732–735. [Google Scholar] [CrossRef]
- Lee, H.; Lee, S.; Lee, W.; Yokota, T.; Fukuda, K.; Someya, T. Ultrathin Organic Electrochemical Transistor with Nonvolatile and Thin Gel Electrolyte for Long-Term Electrophysiological Monitoring. Adv. Funct. Mater. 2019, 29, 1906982. [Google Scholar] [CrossRef]
- Rezali, F.A.M.; Soin, N.; Hatta, S.F.W.M.; Daut, M.H.M.; Nouxman, M.H.A.-H.; Hussin, H. Design Strategies and Prospects in Developing Wearable Glucose Monitoring System Using Printable Organic Transistor and Microneedle: A Review. IEEE Sens. J. 2022, 22, 13785–13799. [Google Scholar] [CrossRef]
- Spooren, A.; Rondou, P.; Debowska, K.; Lintermans, B.; Vermeulen, L.; Samyn, B.; Skieterska, K.; Debyser, G.; Devreese, B.; Vanhoenacker, P.; et al. Resistance of the Dopamine D4 Receptor to Agonist-induced Internalization and Degradation. Cell. Signal. 2010, 22, 600–609. [Google Scholar] [CrossRef]
- Jung, H.H.; Lee, H.; Yea, J.; Jang, K.I. Wearable electrochemical sensors for real-time monitoring in diabetes mellitus and associated complications. Soft Sci. 2024, 4, 15. [Google Scholar] [CrossRef]
- Ni, Y.; Wang, Y.; Xu, W. Recent Process of Flexible Transistor-Structured Memory. Small 2021, 17, 1905332. [Google Scholar] [CrossRef]
- Spanu, A.; Martines, L.; Bonfiglio, A. Interfacing Cells with Organic Transistors: A Review of In Vitro and In Vivo Applications. Lab A Chip 2021, 21, 795–820. [Google Scholar] [CrossRef]
- Saha, T.; Fang, J.; Mukherjee, S.; Dickey, M.D.; Velev, O.D. Wearable Osmotic-Capillary Patch for Prolonged Sweat Harvesting and Sensing. ACS Appl. Mater. Interfaces 2021, 13, 8071–8081. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Min, J.; Yu, Y.; Wang, H.; Yang, Y.; Zhang, H.; Gao, W. Wireless battery-free wearable sweat sensor powered by human motion. Sci. Adv. 2020, 6, eaay9842. [Google Scholar] [CrossRef]
- Janardhanan, J.A.; Chen, Y.; Liu, C.; Tseng, H.; Wu, P.; She, J.; Hsiao, Y.; Yu, H. Sensitive Detection of Sweat Cortisol Using an Organic Electrochemical Transistor Featuring Nanostructured Poly(3,4-Ethylenedioxythiophene) Derivatives in the Channel Layer. Anal. Chem. 2022, 94, 7584–7593. [Google Scholar] [CrossRef]
- Yu, H.; Wei, H.; Gong, J.; Han, H.; Ma, M.; Wang, Y.; Xu, W. Evolution of Bio-Inspired Artificial Synapses: Materials, Structures, and Mechanisms. Small 2021, 17, 2000041. [Google Scholar] [CrossRef]
- Zeng, M.; He, Y.; Zhang, C.; Wan, Q. Neuromorphic Devices for Bionic Sensing and Perception. Front. Neurosci. 2021, 29, 690950. [Google Scholar] [CrossRef]
- Barbarossa, S.; Scutari, G. Bio-Inspired Sensor Network Design. IEEE Signal Process. Mag. 2007, 24, 26–35. [Google Scholar] [CrossRef]
- Tata, U.; Deshmukh, S.; Chiao, J.C.; Carter, R.; Huang, H. Bio-inspired Sensor Skins for Structural Health Monitoring. Smart Mater. Struct. 2009, 18, 104026. [Google Scholar] [CrossRef]
- Yuan, Y.; Gao, R.; Wu, Q.; Fang, S.; Bu, X.; Cui, Y.; Han, C.; Hu, L.; Li, X.; Wang, X.; et al. Artificial Leaky Integrate-and-Fire Sensory Neuron for In-Sensor Computing Neuromorphic Perception at the Edge. ACS Sens. 2023, 8, 2646–2655. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Yao, K.; Huang, N.; Li, H.; Zhou, J.; Shi, R.; Li, J.; Huang, X.; Li, J.; Jia, H.; et al. Ultrathin, Soft, Bioresorbable Organic Electrochemical Transistors for Transient Spatiotemporal Mapping of Brain Activity. Adv. Sci. 2023, 10, 2300504. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Wang, H.; Zhao, Y.; Zhang, X.-D. Neuron Devices: Emerging Prospects in Neural Interfaces and Recognition. Microsyst. Nanoeng. 2022, 8, 128. [Google Scholar] [CrossRef]
- Ni, Y.; Liu, L.; Feng, J.; Yang, L.; Xu, W. Flexible Organic Artificial Synapse with Ultrashort-term Plasticity for Tunable Time-frequency Signal Processing. Chin. Chem. Lett. 2023, 34, 108419. [Google Scholar] [CrossRef]



| Detection Object | Modification | gm | Gain | LOD | Time Response | Ref. |
|---|---|---|---|---|---|---|
| Dopamine | o-MIP/Pt | ~0.11 mS | ~5.5 | 34 nM | - | [60] |
| Nafion/rGO/CSF | ~40 mS | ~1.2 | 1 nM | - | [61] | |
| NOCCs | ~20 mS | ~4.15 | 0.17 µM | - | [62] | |
| FECTs | ~17 mS | ~2.42 | 1 nM | 0.34 s | [63] | |
| Epinephrine | Cotton-OECT | ~10 mS | ~2.36 | 1 µM | <1 s | [66] |
| Nafion and SWNTs | ~3.1 mS | ~1.2 | 0.1 nM | - | [68] | |
| MIP | ~30 mS | ~3 | 10 pM | - | [69] | |
| Acetylcholine | PEDOT–PSS/Pt NPs | ~0.06 mS | ~20 | 5 µM | - | [72] |
| PEDOT–TOS | ~41.7 mS | - | 5 µm | - | [73] | |
| Ascorbic acid | PEDOT–PSS | - | - | 0.1 µM | - | [75] |
| PF-OECT | ~2.73 mS | ~3.5 | 10 µM | - | [77] | |
| MIP | ~17 mS | ~3.5 | 10 nM | - | [78] | |
| Uric acid | PANI/Nafion | ~6 mS | ~1.5 | 0.1 µM | - | [76] |
| PEDOT–PSS | ~4 mS | ~3.5 | 220 µM | 400 s | [81] | |
| MIP | 15.5 mS | ~2.3 | 1 nM | - | [82] | |
| Sialic acid | Carbon Nanotubes | 0.9 mS | ~1.5 | 0.1 mM | - | [83] |
| Glucose | rGO and enzyme | ~5.08 mS | ~1.1 | 10 nM | - | [89] |
| TNTAs | ~5.83 mS | ~1.1 | 100 nM | - | [90] | |
| Prussian Blue | ~2.6 mS | ~1.3 | 0.1 µM | - | [91] | |
| Urea | PET | ~1.83 mS | ~4.7 | 1 µM | 2–3 min | [94] |
| Lactic acid | Iongel | - | - | 10 mM | - | [99] |
| Pt NPs | ~6.5 mS | ~1.9 | 1 µM | 1 min | [101] | |
| Trp Tyr | MIP | ~17 mS | ~8 | 2 nM | - | [104] |
| D-His & L-His | MIP | ~13.5 mS | ~1.2 | 10 nM | - | [105] |
| DNA | HCR | ~2 mS | ~2 | 0.1 pM | - | [109] |
| QDs | ~3.5 mS | ~7 | 1 fM | - | [110] | |
| Tumor Proteins | Catalytic nano-probes. | ~5.3 mS | - | 10 fg mL−1 | - | [121] |
| Tumor Glycoproteins | Nano-probes | ~2.7 mS | - | 10 cells/µL | - | [125] |
| Tumor Prostate-specific antigen | AuNPs–PSA pAb | ~36 mS | - | 1 pg/ml | - | [131] |
| Cortisol | anostructured poly(3,4-Ethylenedioxythiophene) derivatives | - | - | 0.0088 fg/mL | - | [155] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Z.; Hu, Y.; Huang, L.; Zhong, W.; Zhang, J.; Lei, D.; Chen, Y.; Ni, Y.; Liu, Y. Recent Progress in Organic Electrochemical Transistor-Structured Biosensors. Biosensors 2024, 14, 330. https://doi.org/10.3390/bios14070330
Hu Z, Hu Y, Huang L, Zhong W, Zhang J, Lei D, Chen Y, Ni Y, Liu Y. Recent Progress in Organic Electrochemical Transistor-Structured Biosensors. Biosensors. 2024; 14(7):330. https://doi.org/10.3390/bios14070330
Chicago/Turabian StyleHu, Zhuotao, Yingchao Hu, Lu Huang, Wei Zhong, Jianfeng Zhang, Dengyun Lei, Yayi Chen, Yao Ni, and Yuan Liu. 2024. "Recent Progress in Organic Electrochemical Transistor-Structured Biosensors" Biosensors 14, no. 7: 330. https://doi.org/10.3390/bios14070330





