Magnetic Immunoassay Based on Au Pt Bimetallic Nanoparticles/Carbon Nanotube Hybrids for Sensitive Detection of Tetracycline Antibiotics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Development of OVA-TC and OVA-HRP
2.3. Synthesis of Au@Pt/CNTs Nanohybrid Catalyst by Superposition Method
2.4. Synthesis of Au@Pt/CNTs Nanohybrid Catalyst by One-Pot Method
2.5. Characteristic of Au@Pt/CNTs Nanohybrid
2.6. Immobilization of GAM on IMBs
2.7. Combination of Color Probe Based on Au@Pt/CNTs Nanohybrid
2.8. Establishment of Magnetic Immunoassay Assisted by Au@Pt/CNTs Nanohybrid
2.9. Optimization of Magnetic Immunoassay Assisted by Au@Pt/CNTs
2.10. Sample Preparation and Analysis
3. Results
3.1. Construction, Optimization, and Characterization of Au@Pt/CNTs Nanohybrid
3.2. Enzyme Mimetic Activity of Au@Pt/CNTs Nanohybrid
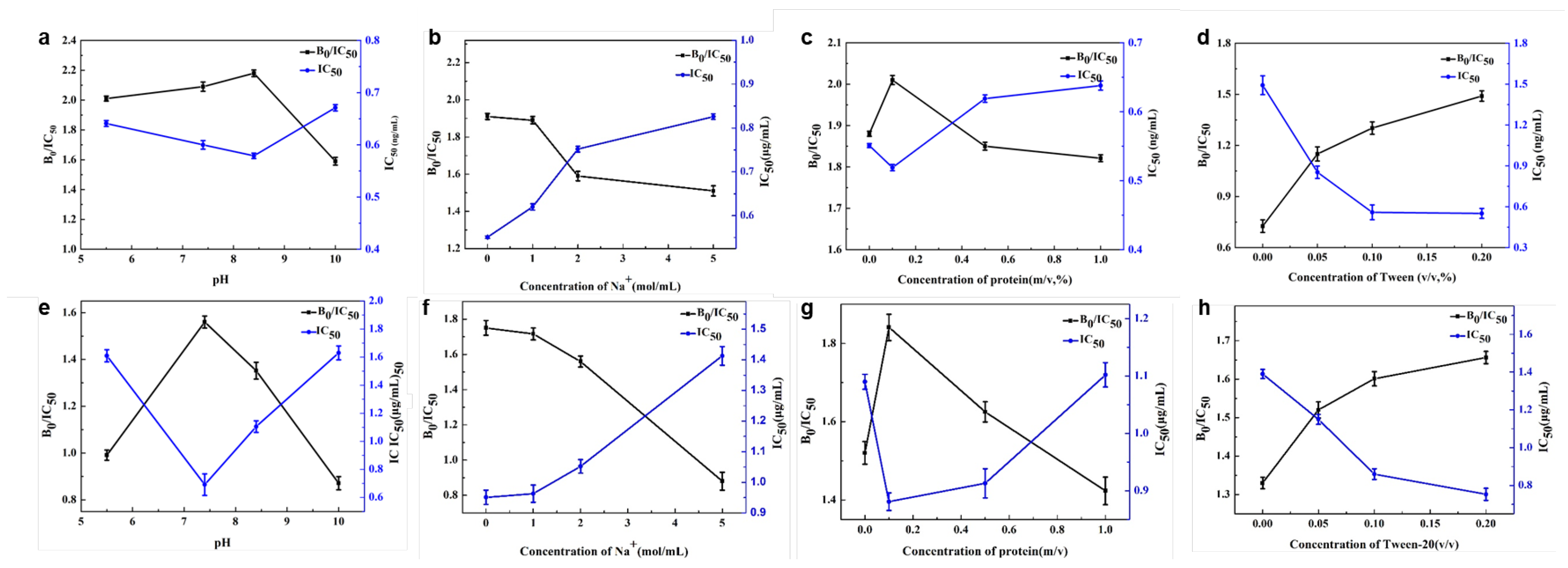
3.3. Optimization of Magnetic Immunoassay Assisted by Au@Pt/CNTs
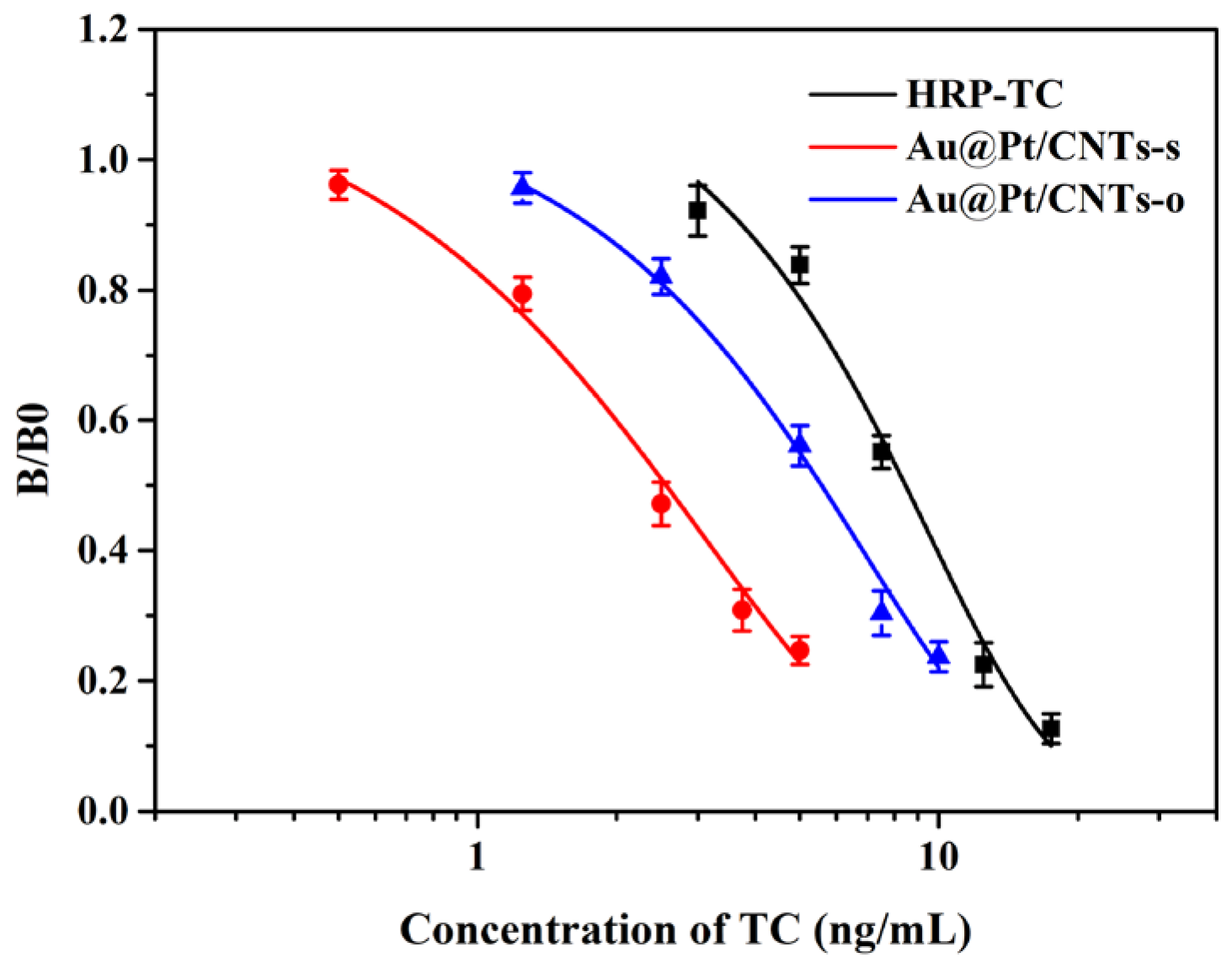
3.4. Analytical Performance of Magnetic Immunoassays Assisted by Au@Pt/CNTs
3.5. Analysis of Samples
4. Discussion
| Nanoparticles | Biosensors | LOD | Targets | Ref. |
|---|---|---|---|---|
| Ni2+-2,3,6,7,10,11-hexahydroxytriphenylene (Ni-HHTP) | electrochemical aptasensor | 1.9 pM | TC | [44] |
| Fe3O4 @MIP | Colorimetric biosensor | 0.4 μM | TC | [45] |
| borophene quantum dots (QDs) | Colorimetric biosensors | 1.02 μM | OTC, TC | [46] |
| Fluorescent biosensors | 1.08 μM | OTC, TC | ||
| non-spherical gold nanoparticle/black phosphorus nanocomposite (BP-nsAu NPs) | Colorimetric biosensor | 90 nM | TC | [48] |
| NH2-MIL-88 B (Fe, Ni) | Colorimetric biosensors | 0.182 μM | TC, OTC, CTC, DC | [49] |
| Fluorescent biosensors | 0.0668 μM | TC, OTC, CTC, DC | ||
| Au@Pt/CNTs-s | Colorimetric immunoassay | 0.74 ng/mL | TC, OTC, CTC, DC | This work |
| Au@Pt/CNTs-o | Colorimetric immunoassay | 1.74 ng/mL | TC, OTC, CTC, DC | This work |
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fuoco, D. Classification framework and chemical biology of tetracycline-structure-based drugs. Antibiotics 2012, 1, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Amangelsin, Y.; Semenova, Y.; Dadar, M.; Aljofan, M.; Bjørklund, G. The impact of tetracycline pollution on the aquatic environment and removal strategies. Antibiotics 2023, 12, 440. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mehedi, H.M.; Rong, Y.; Liu, R.; Li, H.; Ouyang, Q.; Chen, Q. An upconversion nanosensor for rapid and sensitive detection of tetracycline in food based on magnetic-field-assisted separation. Food Chem. 2022, 373, 131497. [Google Scholar] [CrossRef] [PubMed]
- Gan, Z.; Hu, X.; Xu, X.; Zhang, W.; Zou, X.; Shi, J.; Zheng, K.; Arslan, M. A portable test strip based on fluorescent europium-based metal-organic framework for rapid and visual detection of tetracycline in food samples. Food Chem. 2021, 354, 129501. [Google Scholar] [CrossRef] [PubMed]
- Talebi Bezmin Abadi, A.; Rizvanov, A.A.; Haertlé, T.; Blatt, N.L. World health organization report: Current crisis of antibiotic resistance. BioNanoScience 2019, 9, 778–788. [Google Scholar] [CrossRef]
- Chang, Q.; Wang, W.; Regev-Yochay, G.; Lipsitch, M.; Hanage, W.P. Antibiotics in agriculture and the risk to human health: How worried should we be? Evol. Appl. 2015, 8, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Hu, X.; Zhang, X.; Li, W.; Guo, Z.; Huang, X.; Li, Z.; Zhang, R.; Shen, T.; Zou, X.; et al. Ratiometric Sensing for Ultratrace Tetracycline Using Electrochemically Active Metal-Organic Frameworks as Response Signals. J. Agric. Food Chem. 2023, 71, 7584–7592. [Google Scholar] [CrossRef] [PubMed]
- Karageorgou, E.; Armeni, M.; Moschou, I.; Samanidou, V. Ultrasound assisted dispersive extraction for the high-pressure liquid chromatographic determination of tetracycline residues in milk with diode array detection. Food Chem. 2014, 150, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Phomai, K.; Supharoek, S.-A.; Vichapong, J.; Grudpan, K.; Ponhong, K. One-pot co-extraction of dispersive solid phase extraction mploying iron-tannic nanoparticles assisted cloud point extraction for the determination of tetracyclines by high-performance liquid chromatography. Talanta 2023, 252, 123852. [Google Scholar] [CrossRef]
- Moreno-González, D.; García-Campaña, A.M. Salting-out assisted liquid–liquid extraction coupled to ultra-high performance liquid chromatography–tandem mass spectrometry for the determination of tetracycline residues in infant foods. Food Chem. 2017, 221, 1763–1769. [Google Scholar] [CrossRef]
- Susakate, S.; Poapolathep, S.; Chokejaroenrat, C.; Tanhan, P.; Hajslova, J.; Giorgi, M.; Saimek, K.; Zhang, Z.; Poapolathep, A. Multiclass analysis of antimicrobial drugs in shrimp muscle by ultra-high performance liquid chromatography-tandem mass spectrometry. J. Food Drug Anal. 2018, 27, 118–134. [Google Scholar] [CrossRef]
- Sun, Y.; Tian, J.; Wang, L.; Yan, H.; Qiao, F.; Qiao, X. One pot synthesis of magnetic graphene/carbon nanotube composites as magnetic dispersive solid-phase extraction adsorbent for rapid determination of oxytetracycline in sewage water. J. Chromatogr. A 2015, 1422, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Bai, J.; He, P.; Zeng, J. One pot synthesis of nanofiber-coated magnetic composites as magnetic dispersive solid-phase extraction adsorbents for rapid determination of tetracyclines in aquatic food products. Molecules 2023, 28, 7421. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.; Chen, B.; Li, Y.; Meng, H.; Wu, Q.; Yang, J.; Liang, H. Gold nanoparticle-carbon nanotube nanohybrids with peroxidase-like activity for the highly-sensitive immunoassay of kanamycin in milk. Int. J Food Sci. Technol. 2022, 57, 6028–6037. [Google Scholar] [CrossRef]
- Ahmed, S.; Ning, J.; Peng, D.; Chen, T.; Ahmad, I.; Lei, Z.; Chen, G.; Yuan, Z. Current advance in immunoassays for the detection of antibiotics residues: A review. Food Agric. Immunol. 2020, 31, 268–290. [Google Scholar] [CrossRef]
- Ashrafi, A.M.; Bytesnikova, Z.; Barek, J.; Richtera, L.; Adam, V. A critical comparison of natural enzymes and nanozymes in biosensing and bioassays. Biosens. Bioelectron. 2021, 192, 113494. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Ai, Y.J.; Hu, Z.N.; Liang, X.P.; Sun, H.B.; Xin, H.B.; Liang, Q. Recent advances in nanozymes: From matters to bioapplications. Adv. Funct. Mater. 2022, 32, 2110432. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, X.; Shang, J.; Shao, W.; Jin, L.; Quan, C.; Li, J. Emerging nanozyme-based multimodal synergistic therapies in combating bacterial infections. Theranostics 2022, 12, 5995. [Google Scholar] [CrossRef]
- Zandieh, M.; Liu, J. Nanozymes: Definition, Activity, and Mechanisms. Adv. Mater. 2023, 36, 2211041. [Google Scholar] [CrossRef]
- Lu, Z.; Lu, N.; Xiao, Y.; Zhang, Y.; Tang, Z.; Zhang, M. Metal-nanoparticle-supported nanozyme-based colorimetric sensor array for precise identification of proteins and oral bacteria. ACS Appl. Mater. Interfaces 2022, 14, 11156–11166. [Google Scholar] [CrossRef]
- Zhang, L.; Qi, Z.; Yang, Y.; Lu, N.; Tang, Z. Enhanced “electronic tongue” for dental bacterial discrimination and elimination based on a DNA-encoded nanozyme sensor array. ACS Appl. Mater. Interfaces 2024, 16, 11228–11238. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.T.; Li, W.F.; Pan, X.L.; Gadd, G.M. Applications of nanozymes in the environment. Environ. Sci. Nano 2020, 7, 1305–1318. [Google Scholar] [CrossRef]
- Elkomy, H.A.; El-Naggar, S.A.; Elantary, M.A.; Gamea, S.M.; Ragab, M.A.; Basyouni, O.M.; Mouhamed, M.S.; Elnajjar, F.F. Nanozyme as detector and remediator to environmental pollutants: Between current situation and future prospective. Environ. Sci. Pollut. Res. 2024, 31, 3435–3465. [Google Scholar] [CrossRef]
- Morajkar, R.; Fatrekar, A.P.; Vernekar, A.A. single-atom nanozyme cascade for selective tumor-microenvironment-responsive nanocatalytic therapy. ChemMedChem 2023, 18, e202200585. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.X.; Rong, H.T.; Wang, Y.F.; Liu, S.; Xu, P.; Guo, L.M.; Zhu, T.; Rong, H.P.; Wang, D.S.; Zhang, J.T.; et al. Single-atom cobalt nanozymes promote spinal cord injury recovery by anti-oxidation and neuroprotection. Nano Res. 2023, 16, 9752–9759. [Google Scholar] [CrossRef]
- Wu, B.; Hu, D.; Kuang, Y.; Liu, B.; Zhang, X.; Chen, J. Functionalization of carbon nanotubes by an ionic-liquid polymer: Dispersion of Pt and PtRu nanoparticles on carbon nanotubes and their electrocatalytic oxidation of methanol. Angew. Chem. Int. Edit. 2009, 48, 4751–4754. [Google Scholar] [CrossRef]
- Zeng, Z.; Tan, C.; Huang, X.; Bao, S.; Zhang, H. Growth of noblemetal nanoparticles on single-layer TiS2 and TaS2 nanosheets forhydrogen evolution reaction. Energy Environ. Sci. 2014, 7, 797–803. [Google Scholar] [CrossRef]
- Zhang, H.; Jin, M.; Xia, Y. Enhancing the catalytic and electro-catalytic properties of Pt-based catalysts by forming bimetallic nanocrystals with Pd. Chem. Soc. Rev. 2012, 41, 8035–8049. [Google Scholar] [CrossRef]
- You, H.J.; Fang, J.X. Particle-mediated nucleation and growth of solution-synthesized metal nanocrystals: A new story beyond the LaMer curve. Nano Today 2016, 11, 145–167. [Google Scholar] [CrossRef]
- Wei, D.; Zhang, X.; Chen, B.; Zeng, K. Using bimetallic Au@Pt nanozymes as a visual tag and as an enzyme mimic in enhanced sensitive lateral-flow immunoassays: Application for the detection of streptomycin. Anal. Chim. Acta 2020, 1126, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Liu, F.; Abdiryim, T.; Liu, X.; Jamal, R.; Song, Y.; Niyaz, M.; Liu, Y.; Zhang, H.; Tang, X. PEDOT-embellished Ti3C2Tx nanosheet supported Pt-Pd bimetallic nanoparticles as efficient and stable methanol oxidation electrocatalysts. Dalton Trans. 2023, 52, 16345–16355. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Wang, X.; Nie, T.; Yin, L.; Wang, S.; Zhao, Y.; Wu, H.; Mei, H. A novel electrochemical sensing platform for detection of dopamine based on gold nanobipyramid/multi-walled carbon nanotube hybrids. Anal. Bioanal. Chem. 2020, 412, 2433–2441. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Maurya, K.K.; Malviya, M. Electrochemical determination of dopamine using Ni Pd bimetallic nanoparticles decorated on MWCNT: An amperometric sensor. J. Nanopart. Res. 2024, 26, 63. [Google Scholar] [CrossRef]
- Wang, H.; Li, S.; Si, Y.; Zhang, N.; Sun, Z.; Wu, H.; Lin, Y. Platinum nanocatalysts loaded on graphene oxide-dispersed carbon nanotubes with greatly enhanced peroxidase-like catalysis and electrocatalysis activities. Nanoscale 2014, 6, 8107–8116. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Ren, X.F.; Zhang, X.Y.; Wang, X.Z.; Zhang, R.; Bai, P.R.; Du, B.J.; Li, L.P.; Zhao, S.C.; Qin, Y.; et al. Mechanistic and kinetic insights into size-dependent activity in ultra-small Pt/CNTs nanozymes during antibacterial process. Arab. J. Chem. 2022, 15, 101238. [Google Scholar] [CrossRef]
- Chen, Y.; Yuchi, Q.X.; Li, T.; Yang, G.H.; Miao, J.J.; Huang, C.Y.; Liu, J.Y.; Li, A.P.; Qin, Y.; Zhang, L.B. Precise engineering of ultra-thin Fe2O3 decorated Pt-based nanozymes via atomic layer deposition to switch off undesired activity for enhanced sensing performance. Sens. Actuators B-Chem. 2020, 305, 127436. [Google Scholar] [CrossRef]
- Cai, X.L.; Liu, C.H.; Liu, J.; Lu, Y.; Zhong, Y.N.; Nie, K.Q.; Xu, J.L.; Gao, X.; Sun, X.H.; Wang, S.D. Synergistic Effects in CNTs-PdAu/Pt Trimetallic Nanoparticles with High Electrocatalytic Activity and Stability. Nano-Micro. Lett. 2017, 9, 48. [Google Scholar] [CrossRef]
- Ministry of Agriculture and Rural Affairs of the People’s Republic of China. Determination of Tetracyclines Residues in Animal Derived Food by High Performance Liquid Chromatography Method. China Patent GB 31658.6-2021, 16 September 2021. [Google Scholar]
- Kumar, R.; Ahmed, Z.; Rai, R.; Gaur, A.; Kumari, S.; Maruyama, T.; Bagchi, V. Uniformly Decorated Molybdenum Carbide/Nitride Nanostructures on Biomass Templates for Hydrogen Evolution Reaction Applications. ACS Omega 2019, 4, 14155–14161. [Google Scholar] [CrossRef]
- Liu, Y.P.; Zhu, L.Y.; Feng, P.; Dang, C.C.; Li, M.; Lu, H.L.; Gao, L.M. Bimetallic AuPt alloy nanoparticles decorated on ZnO nanowires towards efficient and selective H2S gas sensing. Sens. Actuators B-Chem. 2022, 367, 132024. [Google Scholar] [CrossRef]
- Wang, Y.D.; Xianyu, Y.L. Tuning the plasmonic and catalytic signals of Au@Pt nanoparticles for dual-mode biosensing. Biosens. Bioelectron. 2023, 237, 115553. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, X.; Chang, K.; Zhao, Z.; Huang, J.; Kuang, Q. MOF Encapsulated AuPt bimetallic nanoparticles for improved plasmonic-induced photothermal catalysis of CO2 hydrogenation. Chemistry 2022, 28, e202104514. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Zhao, Y.; Dai, X.; Xu, W.; Zhan, F.; Liu, Y.; Wang, Q. Aptamer tuned nanozyme activity of nickel-metal-organic framework for sensitive electrochemical aptasensing of tetracycline residue. Food Chem. 2024, 430, 137041. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.X.; Zhu, H.J.; Feng, R.L.; Wang, M.Z.; Hu, P.W.; Pan, J.M.; Niu, X.H. Facile molecular imprinting on magnetic nanozyme surface for highly selective colorimetric detection of tetracycline. Sens. Actuators B-Chem. 2022, 370, 132451. [Google Scholar] [CrossRef]
- Gogoi, D.; Hazarika, C.; Neog, G.; Mridha, P.; Bora, H.K.; Das, M.R.; Szunerits, S.; Boukherroub, R. Borophene quantum dots as novel peroxidase-mimicking nanozyme: A dual-mode assay for the detection of oxytetracycline and tetracycline antibiotics. ACS Appl. Mater. Interfaces 2024, 16, 14645–14660. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.N.; Kong, D.Z.; Liu, L.Q.; Song, S.S.; Kuang, H.; Xu, C.L. Development of an ELISA and immunochromatographic assay for tetracycline, oxytetracycline, and chlortetracycline residues in milk and honey based on the class-specific monoclonal antibody. Food Anal. Methods 2016, 9, 905–914. [Google Scholar] [CrossRef]
- Alsulami, T.; Alzahrani, A. Enhanced nanozymatic activity on rough surfaces for H2O2 and tetracycline detection. Biosensors 2024, 14, 106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, M.; Shao, C.; Liu, T.; Sun, M.; Wu, C.; Su, G.; Wang, Y.; Ye, J.; Hu, H.; et al. Nanozyme-induced deep learning-assisted smartphone integrated colorimetric and fluorometric dual-mode for detection of tetracycline analogs. Anal. Chim. Acta. 2024, 1297, 342373. [Google Scholar] [CrossRef]

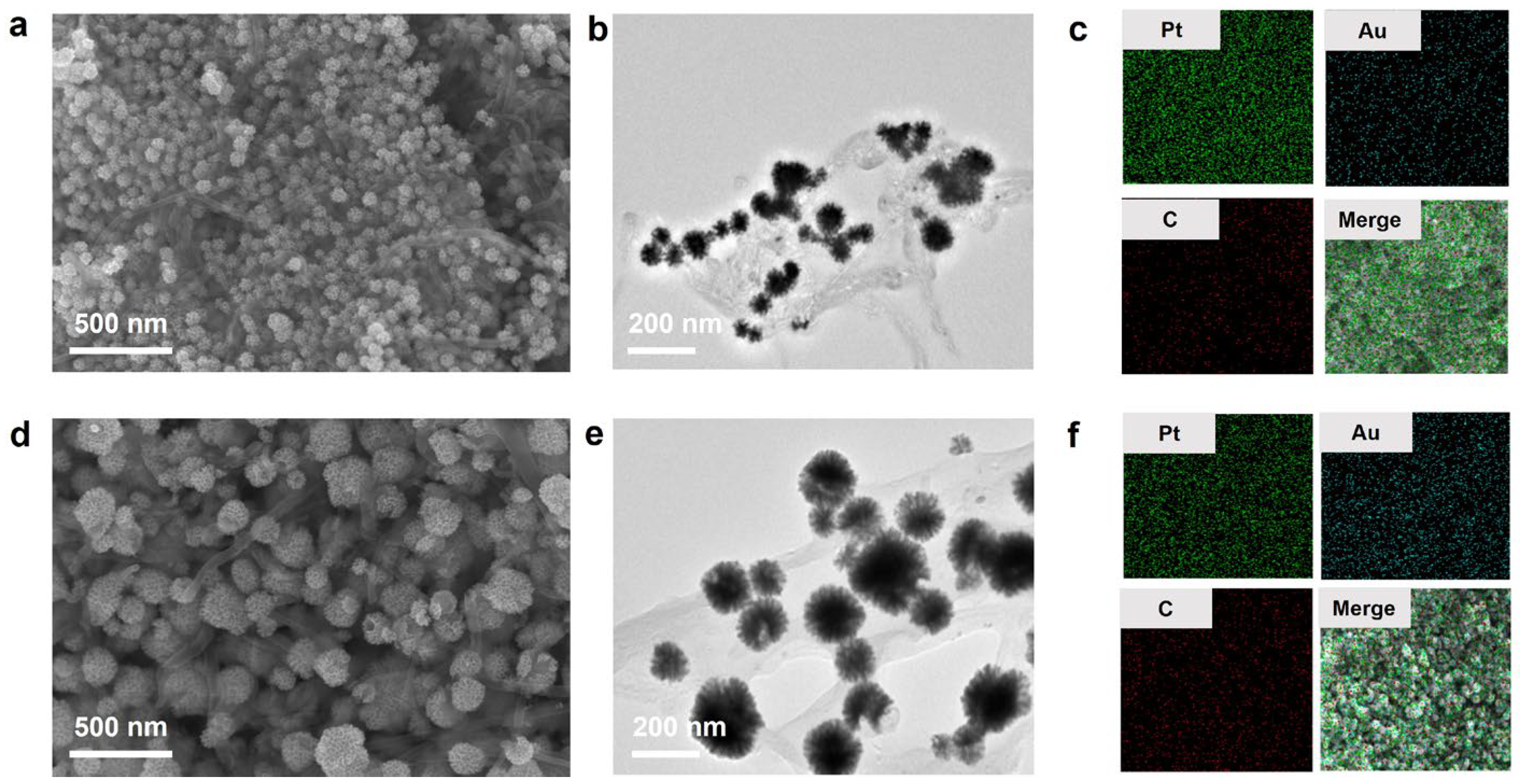



| Catalyst | Substrate | Km (mM) | Vm (10−8 Ms−1) | Reference |
|---|---|---|---|---|
| HRP | TMB | 0.329 | 7.64 | [31] |
| H2O2 | 1.468 | 22.88 | ||
| Au@Pt | TMB | 2.431 × 10−3 | 4.43 | [31] |
| H2O2 | 4.08 × 10−3 | 6.01 | ||
| Au@Pt/CNTs-s | TMB | 1.01 × 10−3 | 3.63 | This work |
| H2O2 | 2.37 × 10−3 | 4.39 | ||
| Au@Pt/CNTs-o | TMB | 3.14 × 10−3 | 4.72 | This work |
| H2O2 | 3.46 × 10−3 | 5.69 | ||
| GOCNT-Pt | TMB | 0.075 | 0.302 | [35] |
| H2O2 | 1.82 | 1.27 | ||
| CNT-Pt | TMB | 0.152 | 0.422 | [35] |
| H2O2 | 6.24 | 2.18 | ||
| 2Fe2O3/30Pt/CNTs | TMB | 0.17 | 18.1 | [36] |
| H2O2 | 0.053 | 6.79 | ||
| 30Pt/CNTs | TMB | 1.15 | 18.9 | [37] |
| H2O2 | 72.91 | 103 |
| Spiked Concentration (ng/mL) | Found Concentration (ng/mL) | Recovery Rate | CV (%) | |
|---|---|---|---|---|
| Milk | 0 | <LOD | − | − |
| 2 | 2.28 ± 0.08 | 114.5% | 7.8% | |
| 5 | 5.32 ± 0.43 | 106.4% | 10.9% | |
| 10 | 9.21 ± 1.39 | 92.1% | 11.5% | |
| pork | 0 | <LOD | − | − |
| 2 | 1.79 ± 0.23 | 89.5% | 11.9% | |
| 5 | 4.43 ± 0.56 | 88.6% | 11.6% | |
| 10 | 9.24 ± 1.34 | 92.4% | 10.5% |
| Sample Number | Results of Milk Samples (ng/mL) | Results of Pork Samples (ng/mL) | ||
|---|---|---|---|---|
| By the Established Method | By UPLC-MS/MS | By the Established Method | By UPLC-MS/MS | |
| 1 | ND 1 | ND | ND | ND |
| 2 | ND | ND | ND | ND |
| 3 | ND | ND | ND | ND |
| 4 | ND | ND | ND | ND |
| 5 | ND | ND | 5.57 | 6.78 |
| 6 | ND | ND | ND | ND |
| 7 | 1.05 | 2.15 | ND | ND |
| 8 | ND | ND | ND | 1.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, J.; Huang, R.; Zeng, K.; Zhang, Z. Magnetic Immunoassay Based on Au Pt Bimetallic Nanoparticles/Carbon Nanotube Hybrids for Sensitive Detection of Tetracycline Antibiotics. Biosensors 2024, 14, 342. https://doi.org/10.3390/bios14070342
Lv J, Huang R, Zeng K, Zhang Z. Magnetic Immunoassay Based on Au Pt Bimetallic Nanoparticles/Carbon Nanotube Hybrids for Sensitive Detection of Tetracycline Antibiotics. Biosensors. 2024; 14(7):342. https://doi.org/10.3390/bios14070342
Chicago/Turabian StyleLv, Jianxia, Rui Huang, Kun Zeng, and Zhen Zhang. 2024. "Magnetic Immunoassay Based on Au Pt Bimetallic Nanoparticles/Carbon Nanotube Hybrids for Sensitive Detection of Tetracycline Antibiotics" Biosensors 14, no. 7: 342. https://doi.org/10.3390/bios14070342






