Development of a “Signal-On” Fluorescent Aptasensor for Highly Selective and Sensitive Detection of ZEN in Cereal Products Using Nitrogen-Doped Carbon Dots Based on the Inner Filter Effect
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Material Characterization
2.3. Preparation of AuNPs
2.4. Synthesis of N-CDs
2.5. Fluorescent Aptasensing of ZEN
2.6. Specificity Test
2.7. Detection of Real Samples
2.8. Statistical Analysis
3. Results and Discussion
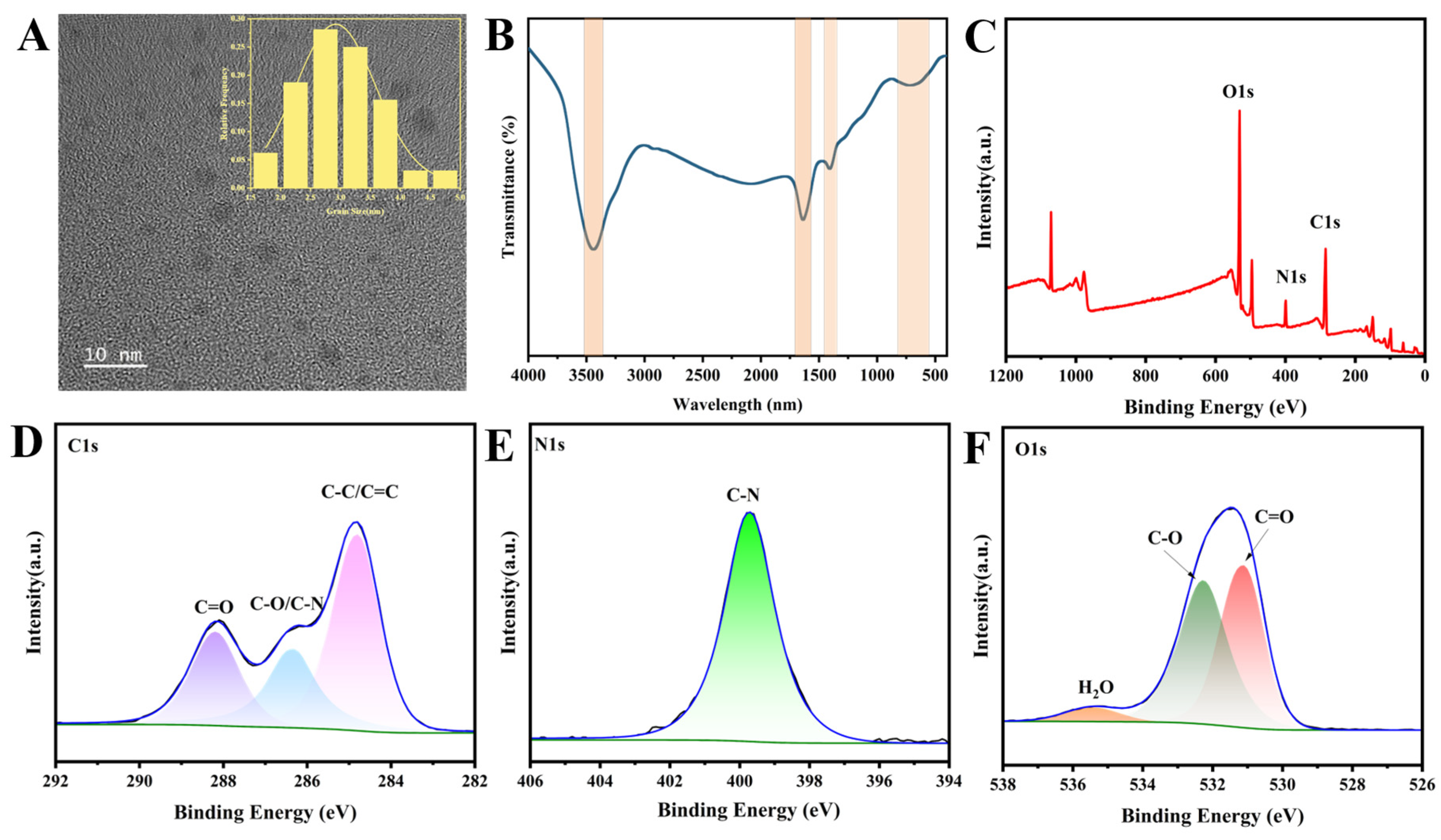
3.1. Morphology and Micro-Structure Characterization of N-CDs
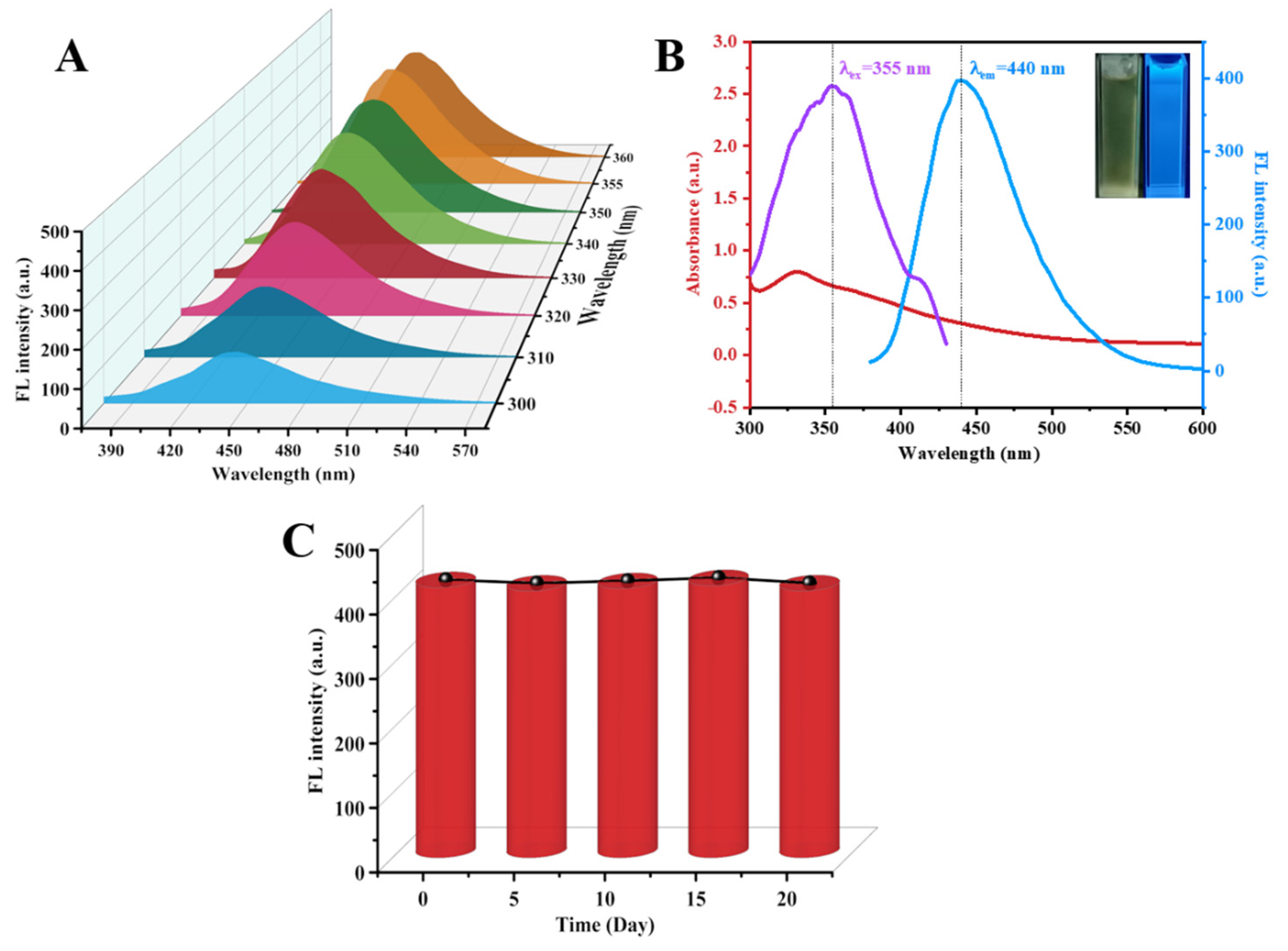
3.2. Optical Properties of As-Synthesized N-CDs
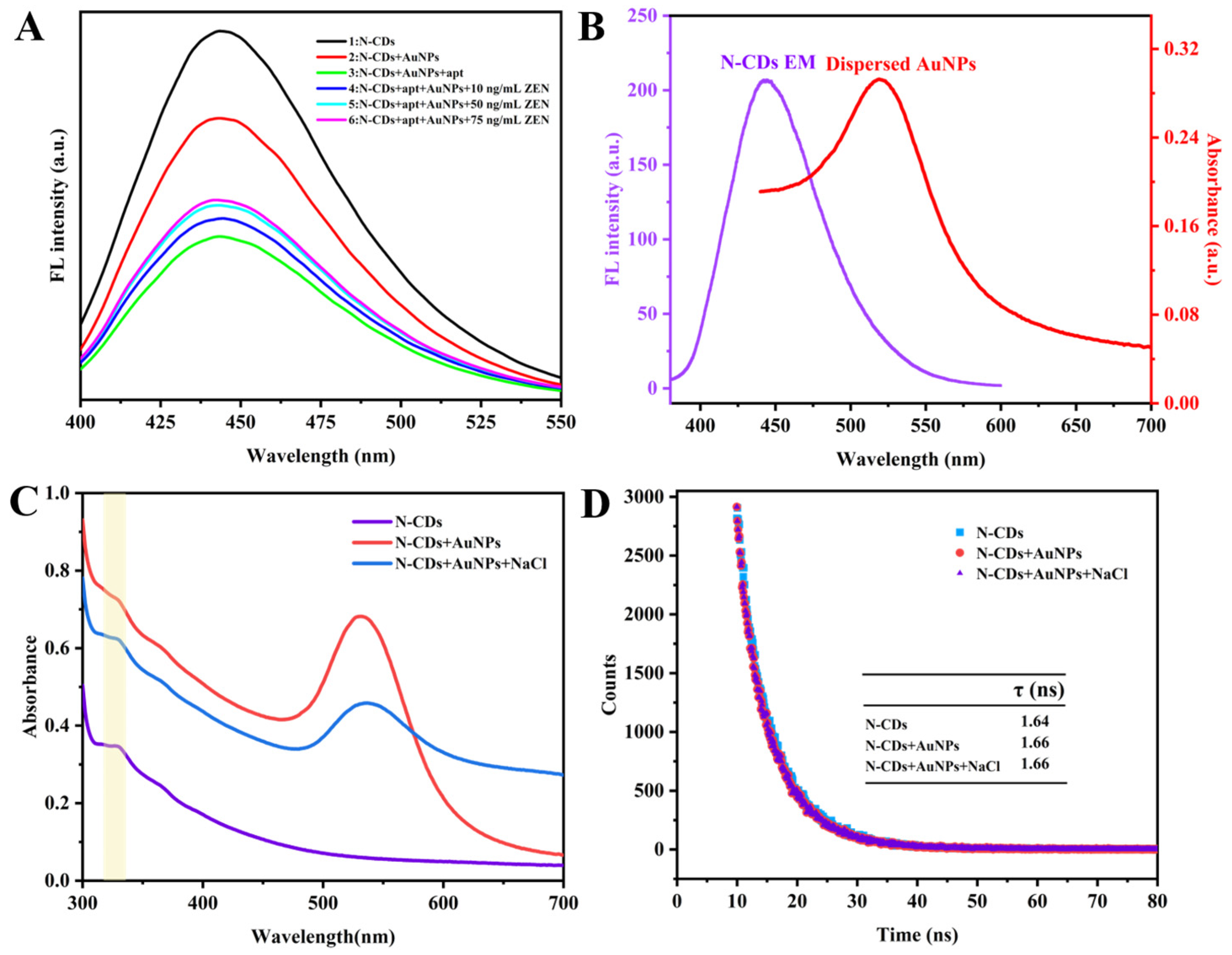
3.3. Fabrication and Detection Principle of Aptasensor
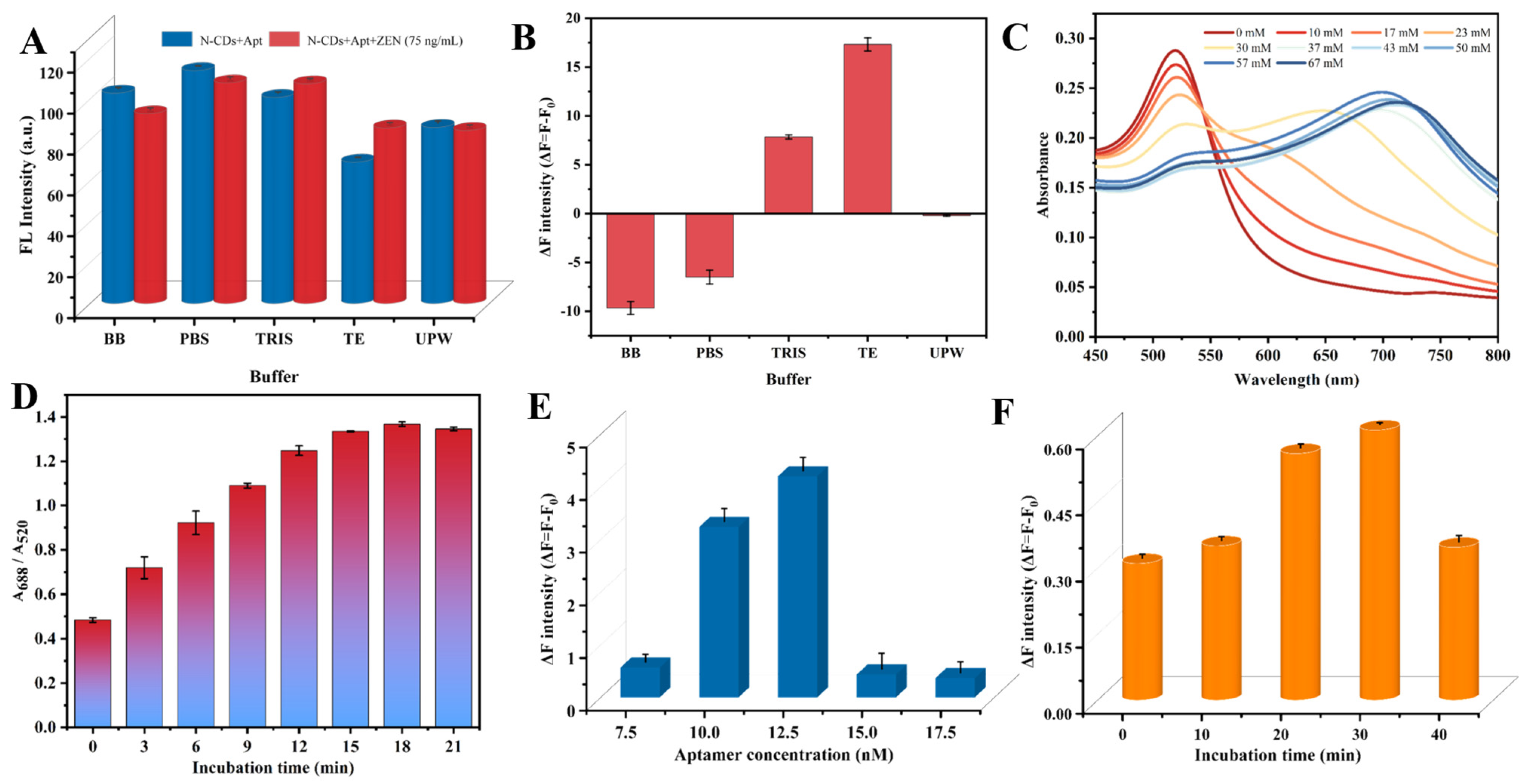
3.4. Optimization of the Detection Conditions
3.5. Aptasensing Performance and Applications
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Yamdeu, J.H.G.; Gong, Y.Y.; Orfila, C. A review of postharvest approaches to reduce fungal and mycotoxin contamination of foods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1521–1560. [Google Scholar] [CrossRef] [PubMed]
- De Rycke, E.; Foubert, A.; Dubruel, P.; Bol, O.I.; De Saeger, S.; Beloglazova, N. Recent advances in electrochemical monitoring of zearalenone in diverse matrices. Food Chem. 2021, 353, 129342. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.; Ash, E.; Gerssen, A.; Van Dam, R.; Franssen, M.C.R.; Nielen, M.W.F. Controlled Production of Zearalenone-Glucopyranoside Standards with Cunninghamella Strains Using Sulphate-Depleted Media. Toxins 2021, 13, 366. [Google Scholar] [CrossRef] [PubMed]
- Murtaza, B.; Jin, B.W.; Wang, L.L.; Li, X.Y.; Saleemi, M.K.; Majeed, S.; Khatoon, A.; Li, G.; Xu, Y.P. Mitigation of zearalenone in vitro using probiotic strains. LWT-Food Sci. Technol. 2023, 186, 115265. [Google Scholar] [CrossRef]
- Kumar, M.; Singh, G.; Kaur, N.; Singh, N. Organic Cation Receptor for Colorimetric Lateral Flow Device: Detection of Zearalenone in Food Samples. ACS Appl. Mater. Interfaces 2022, 14, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Acuña, M.R.; de Pipaón, M.R.P.; Torres-Sánchez, M.J.; Aznar, J. Identification of clinical isolates of Aspergillus, including cryptic species, by matrix assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS). Med. Mycol. 2018, 56, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Kou, Q.M.; Wu, P.; Sun, Q.; Li, C.X.; Zhang, L.; Shi, H.X.; Wu, J.; Wang, Y.R.; Yan, X.L.; Le, T. Selection and truncation of aptamers for ultrasensitive detection of sulfamethazine using a fluorescent biosensor based on graphene oxide. Anal. Bioanal. Chem. 2021, 413, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Kalra, P.; Dhiman, A.; Cho, W.C.; Bruno, J.G.; Sharma, T.K. Simple Methods and Rational Design for Enhancing Aptamer Sensitivity and Specificity. Front. Mol. Biosci. 2018, 5, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Canoura, J.; Yu, H.X.; Alkhamis, O.; Roncancio, D.; Farhana, R.; Xiao, Y. Accelerating Post-SELEX Aptamer Engineering Using Exonuclease Digestion. J. Am. Chem. Soc. 2021, 143, 805–816. [Google Scholar] [CrossRef]
- Fan, Y.T.; Li, J.X.; Amin, K.; Yu, H.S.; Yang, H.H.; Guo, Z.J.; Liu, J.S. Advances in aptamers, and application of mycotoxins detection: A review. Food Res. Int. 2023, 170, 113022. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.L.; Wu, L.J.; Ding, L.H.; Effah, C.Y.; Wu, Y.J.; Xiong, Y.M.; He, L.L. Construction and bioapplications of aptamer-based dual recognition strategy. Biosens. Bioelectron. 2022, 195, 113661. [Google Scholar] [CrossRef]
- Xu, Y.; Zhou, C.Q.; Li, D.M.; Guo, C.X.; Li, Z.Y.; Xing, X.H.; Li, S.X.; Guan, T.; Liu, L.; He, Y.H. A stabilized weak measurement sensor for aptamer detection. Sens. Actuators B-Chem. 2022, 371, 132509. [Google Scholar] [CrossRef]
- Esmaelpourfarkhani, M.; Abnous, K.; Taghdisi, S.M.; Chamsaz, M. A novel turn-off fluorescent aptasensor for ampicillin detection based on perylenetetracarboxylic acid diimide and gold nanoparticles. Biosens. Bioelectron. 2020, 164, 112329. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.H.; Jung, D.H.; Heo, J.H.; Lee, C.Y.; Jeong, S.Y.; Lee, J.H. Gold Nanoparticles as Exquisite Colorimetric Transducers for Water Pollutant Detection. ACS Appl. Mater. Interfaces 2023, 15, 19785. [Google Scholar] [CrossRef]
- Hou, S.L.; Ma, J.J.; Cheng, Y.Q.; Wang, Z.F.; Yan, Y.X. Overview-gold nanoparticles-based sensitive nanosensors in mycotoxins detection. Crit. Rev. Food Sci. Nutr. 2023, 63, 11734. [Google Scholar] [CrossRef]
- Wang, M.J.; Da, Y.; Tian, Y. Fluorescent proteins and genetically encoded biosensors. Chem. Soc. Rev. 2023, 52, 1189–1214. [Google Scholar] [CrossRef]
- Park, J.; Yang, K.A.; Choi, Y.; Choe, J.K. Novel ssDNA aptamer-based fluorescence sensor for perfluorooctanoic acid detection in water. Environ. Int. 2022, 158, 107000. [Google Scholar] [CrossRef]
- Li, J.M.; Li, S.; Li, Z.J.; Zhou, Y.T.; Jin, P.; Zhang, F.Y.; Sun, Q.; Le, T.; Jirimutu. Chromium hydroxide nanoparticles-based fluorescent aptameric sensing for sensitive patulin detection: The significance of nanocrystal and morphology modulation. Talanta 2023, 257, 124296. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Li, Z.J.; Liu, N.X.; Zhou, Y.T.; Zhang, F.Y.; Li, S.; Jin, P.; Xiang, R.; Le, T. Development of a novel fluorescent aptasensor based on the interaction between hexagonal β-Co(OH)2 nanoplates and nitrogen-doped carbon dots for ultrasensitive detection of patulin. Anal. Chim. Acta 2023, 1278, 341710. [Google Scholar] [CrossRef]
- Zhang, X.; Tan, X.Y.; Hu, Y.P. Blue/yellow emissive carbon dots coupled with curcumin: A hybrid sensor toward fluorescence turn-on detection of fluoride ion. J. Hazard. Mater. 2021, 411, 125184. [Google Scholar] [CrossRef]
- Liu, H.X.; Zhong, X.; Pan, Q.; Zhang, Y.; Deng, W.T.; Zou, G.Q.; Hou, H.S.; Ji, X.B. A review of carbon dots in synthesis strategy. Coord. Chem. Rev. 2024, 498, 215468. [Google Scholar] [CrossRef]
- Yin, N.; Yuan, S.; Zhang, M.; Wang, J.Y.; Li, Y.; Peng, Y.; Bai, J.L.; Ning, B.A.; Liang, J.; Gao, Z.X. An aptamer-based fluorometric zearalenone assay using a lighting-up silver nanocluster probe and catalyzed by a hairpin assembly. Microchim. Acta 2020, 187, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Qaddoori, M.H.; Al-Shmgani, H.S. Galangin-Loaded Gold Nanoparticles: Molecular Mechanisms of Antiangiogenesis Properties in Breast Cancer. Int. J. Breast Cancer 2023, 2023, 3251211. [Google Scholar] [CrossRef] [PubMed]
- Li, J.M.; Zhou, Y.T.; Li, Z.J.; Wang, T.; Sun, Q.; Le, T.; Jirimutu. A novel fluorescent sensing platform based on nitrogen-doped carbon quantum dots for rapid and sensitive detection of aflatoxin B1 in corn flour. LWT-Food Sci. Technol. 2023, 185, 115130. [Google Scholar] [CrossRef]
- Tang, X.D.; Yu, H.M.; Bui, B.; Wang, L.Y.; Xing, C.; Wang, S.Y.; Chen, M.L.; Hu, Z.Z.; Chen, W. Nitrogen-doped fluorescence carbon dots as multi-mechanism detection for iodide and curcumin in biological and food samples. Bioact. Mater. 2021, 6, 1541–1554. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Liu, J.H.; Li, R.S.; Li, Y.F.; Huang, C.Z.; Zhen, S.J. Carbon dots synthesized at room temperature for detection of tetracycline hydrochloride. Anal. Chim. Acta 2019, 1063, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.F.; Shen, J.L.; Wang, Z.; Liu, X.; Xu, Y.Y.; Zhao, H.Y.; Astruc, D. Turning waste into wealth: Facile and green synthesis of carbon nanodots from pollutants and applications to bioimaging. Chem. Sci. 2021, 12, 11722. [Google Scholar] [CrossRef]
- Ni, P.; Li, Q.Y.; Xu, C.F.; Lai, H.Q.; Bai, Y.; Chen, T.F. Optical properties of nitrogen and sulfur co-doped carbon dots and their applicability as fluorescent probes for living cell imaging. Appl. Surf. Sci. 2019, 494, 377–383. [Google Scholar] [CrossRef]
- Luo, L.J.; Li, L.B.; Xu, X.X.; Liu, D.; Li, J.Y.; Wang, K.; You, T.Y. Determination of pentachlorophenol by anodic electrochemiluminescence of Ru(bpy)32+ based on nitrogen-doped graphene quantum dots as co-reactant. RSC Adv. 2017, 7, 50634. [Google Scholar] [CrossRef]
- Zhang, W.J.; Liu, S.G.; Han, L.; Luo, H.Q.; Li, N.B. A ratiometric fluorescent and colorimetric dual-signal sensing platform based on N-doped carbon dots for selective and sensitive detection of copper (II) and pyrophosphate ion. Sens. Actuators B-Chem. 2019, 283, 215–221. [Google Scholar] [CrossRef]
- John, G.S.M.; Vuttaradhi, V.K.; Takeuchi, S.; Pitani, R.S.; Venkatraman, G.; Rayala, S.K. Facile synthesis and nanoscale features of a nanostructured nordihydroguaiaretic acid analog for therapeutic applications. J. Nanobiotechnol. 2020, 18, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Lerga, T.M.; Skouridou, V.; Bermudo, M.C.; Bashammakh, A.S.; El-Shahawi, M.S.; Alyoubi, A.O.; O’Sullivan, C.K. Gold nanoparticle aptamer assay for the determination of histamine in foodstuffs. Microchim. Acta 2020, 187, 452–461. [Google Scholar] [CrossRef]
- Kabb, C.; Carmean, R.N.; Sumerlin, B. Probing the surface-localized hyperthermia of gold nanoparticles in a microwave field using polymeric thermometers. Chem. Sci. 2016, 251, 5662–5669. [Google Scholar] [CrossRef]
- Wang, J.L.; Wu, Y.G.; Zhou, P.; Yang, W.P.; Tao, H.; Qiu, S.Y.; Feng, C.W. A novel fluorescent aptasensor for ultrasensitive and selective detection of acetamiprid pesticide based on the inner filter effect between gold nanoparticles and carbon dots. Analyst 2018, 143, 5151–5160. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, J.X.; Liu, S.S.; Li, B.X. A label-free visual aptasensor for zearalenone detection based on target-responsive aptamer-cross-linked hydrogel and color change of gold nanoparticles. Food Chem. 2022, 389, 133078. [Google Scholar] [CrossRef]
- Sun, S.M.; Zhao, R.; Feng, S.M.; Xie, Y.L. Colorimetric zearalenone assay based on the use of an aptamer and of gold nanoparticles with peroxidase-like activity. Microchim. Acta 2018, 185, 14751. [Google Scholar] [CrossRef]
- Zhou, B.B.; Xie, H.; Li, X.Y.; Zhu, Y.B.; Huang, L.J.; Zhong, M.; Chen, L. Construction of a self-reporting molecularly-imprinted electrochemical sensor based on CuHCF modified by rGNR-rGO for the detection of zearalenone. Food Chem. 2024, 448, 139154. [Google Scholar] [CrossRef]
- Zhou, B.B.; Xie, H.; Zhou, S.S.; Sheng, X.X.; Chen, L.; Zhong, M. Construction of AuNPs/reduced graphene nanoribbons co-modified molecularly imprinted electrochemical sensor for the detection of zearalenone. Food Chem. 2023, 423, 136294. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.X.; Wei, Y.W.; Zhou, Q.F.; Wang, H.J.; Wei, Y.N.; Lao, S.B.; Luo, L.H.; Mo, R.F.; Chen, Y.X.; Yang, Y.X.; et al. A sensitive MnO2 nanosheet sensing platform based on a fluorescence aptamer sensor for the detection of zearalenone. Anal. Methods 2022, 14, 4872–4878. [Google Scholar] [CrossRef]
- Liao, X.L.; Liu, Y.M.; Qiu, L.Y.; Cao, L.; Yang, X.X.; Hu, X.G. A quantum dot aptamer fluorescent sensor based on magnetic graphene oxide for the detection of zearalenone. Anal. Methods 2023, 15, 4946–4953. [Google Scholar] [CrossRef]


| Method | Description | Linear Range (ng/mL) | LOD (ng/mL) | Refs. |
|---|---|---|---|---|
| Colorimetric | AuNPs | 2.5~100 | 0.98 | [35] |
| Colorimetric | AuNPs | 10~250 | 10 | [36] |
| Electrochemical | rGNR−rGO | 0.25~500 | 0.09 | [37] |
| Electrochemical | rGNRs/AuNPs | 1~500 | 0.34 | [38] |
| Fluorescence | MnO2 | 1.5~10 | 0.68 | [39] |
| Fluorescence | CdTe QDs/Fe3O4 | 5~120 | 2.9 | [40] |
| Fluorescence | AuNPs/N−CDs | 0.25~200 | 0.0875 | This work |
| Sample | Spiked (ng/mL) | This Work | HPLC | The Correlations (R2) | ||||
|---|---|---|---|---|---|---|---|---|
| Detected (ng/mL) | Recovery (%) | CV (%) | Detected (ng/mL) | Recovery (%) | CV (%) | |||
| Corn flour | 50 | 51.76 | 103.52 | 3.47 | 53.30 | 106.60 | 1.85 | 0.9951 |
| 100 | 90.92 | 90.67 | 6.69 | 96.14 | 96.14 | 1.53 | ||
| 200 | 169.50 | 84.75 | 5.18 | 194.02 | 97.01 | 2.07 | ||
| Corn oil | 50 | 54.28 | 108.56 | 3.44 | 52.28 | 104.56 | 0.55 | 0.9804 |
| 100 | 87.64 | 87.64 | 2.13 | 110.54 | 110.54 | 2.11 | ||
| 200 | 184.15 | 92.08 | 1.24 | 203.54 | 101.77 | 2.53 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Q.; Zhou, Y.; Ma, M.; Zhang, F.; Li, S.; Chen, Z.; Fang, Y.; Le, T.; Xing, F. Development of a “Signal-On” Fluorescent Aptasensor for Highly Selective and Sensitive Detection of ZEN in Cereal Products Using Nitrogen-Doped Carbon Dots Based on the Inner Filter Effect. Biosensors 2024, 14, 347. https://doi.org/10.3390/bios14070347
Sun Q, Zhou Y, Ma M, Zhang F, Li S, Chen Z, Fang Y, Le T, Xing F. Development of a “Signal-On” Fluorescent Aptasensor for Highly Selective and Sensitive Detection of ZEN in Cereal Products Using Nitrogen-Doped Carbon Dots Based on the Inner Filter Effect. Biosensors. 2024; 14(7):347. https://doi.org/10.3390/bios14070347
Chicago/Turabian StyleSun, Qi, Yuting Zhou, Miaomiao Ma, Fuyan Zhang, Shuang Li, Zhuoer Chen, Yu Fang, Tao Le, and Fuguo Xing. 2024. "Development of a “Signal-On” Fluorescent Aptasensor for Highly Selective and Sensitive Detection of ZEN in Cereal Products Using Nitrogen-Doped Carbon Dots Based on the Inner Filter Effect" Biosensors 14, no. 7: 347. https://doi.org/10.3390/bios14070347
APA StyleSun, Q., Zhou, Y., Ma, M., Zhang, F., Li, S., Chen, Z., Fang, Y., Le, T., & Xing, F. (2024). Development of a “Signal-On” Fluorescent Aptasensor for Highly Selective and Sensitive Detection of ZEN in Cereal Products Using Nitrogen-Doped Carbon Dots Based on the Inner Filter Effect. Biosensors, 14(7), 347. https://doi.org/10.3390/bios14070347








