Label-Free Electrochemical Dopamine Biosensor Based on Electrospun Nanofibers of Polyaniline/Carbon Nanotube Composites
Abstract
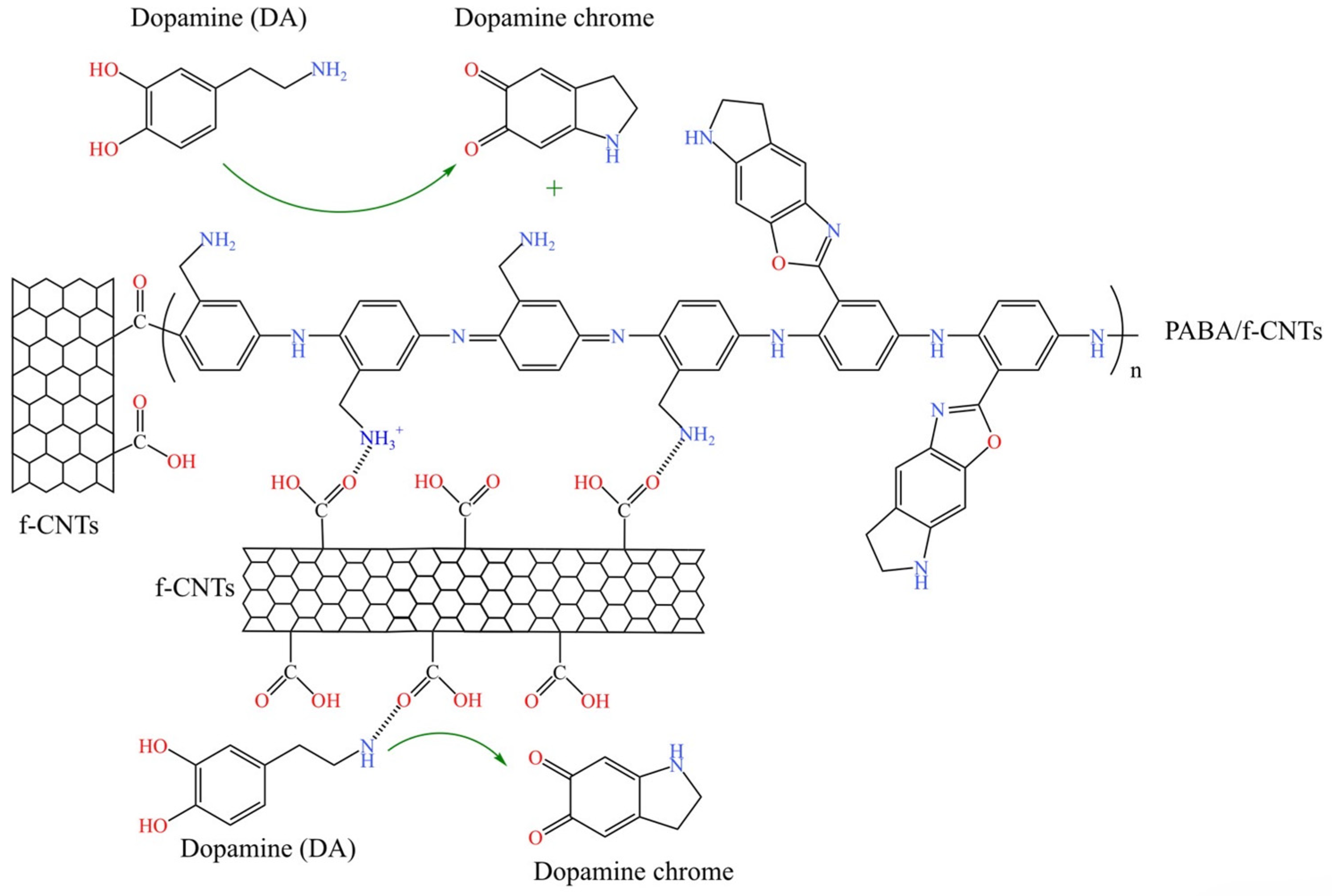
:1. Introduction
2. Experimental Section
2.1. Reagents and Materials
2.2. Instrumentation
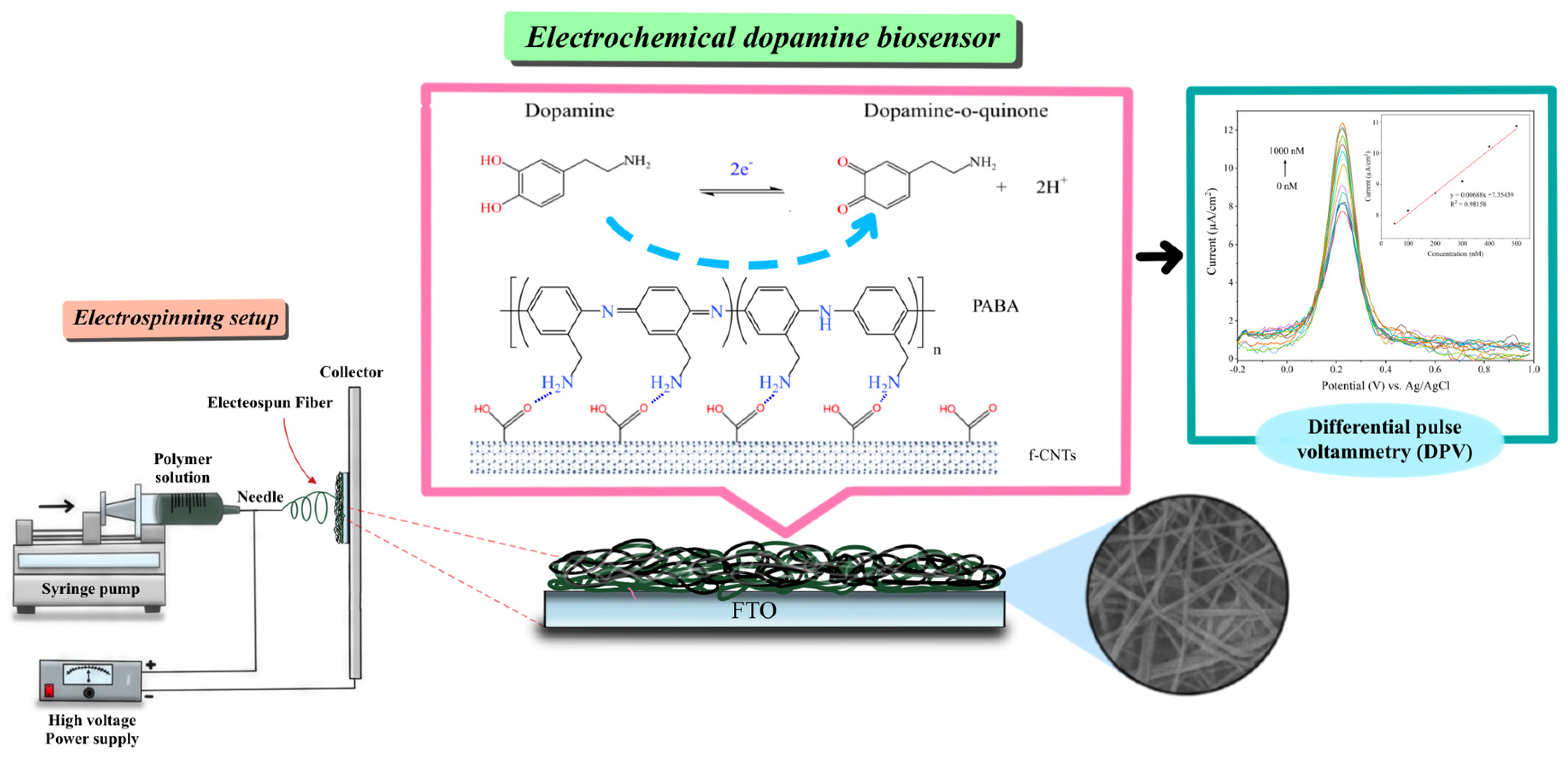
2.3. Fabrication of PANI/f-CNTs and PABA/f-CNTs Electrospun Nanofiber Films
2.4. Electrochemical Detection of Dopamine
2.5. Application of the Obtained Label-Free DA Biosensor in Artificial Urine
3. Results and Discussion
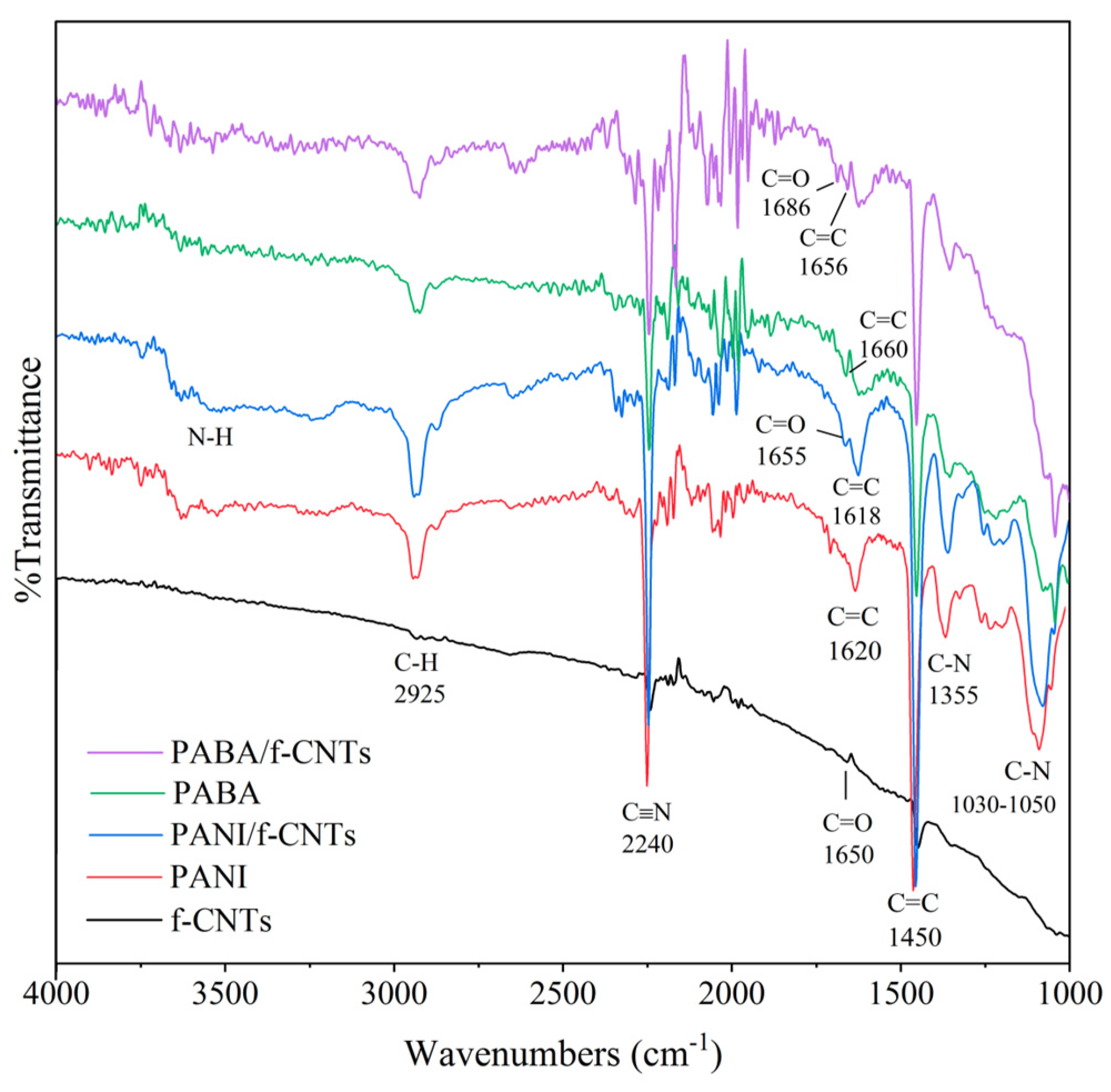
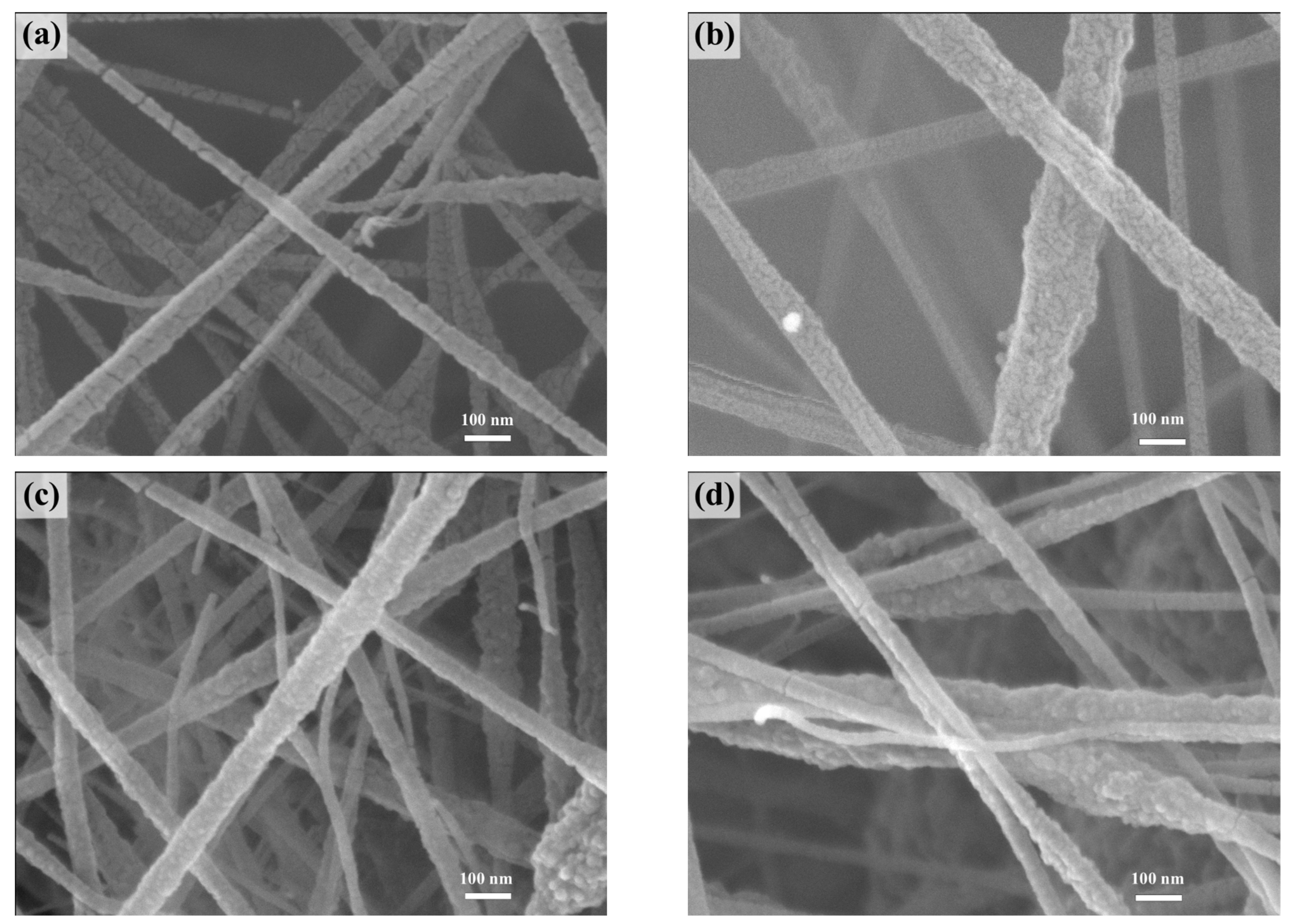
3.1. Characterization of Electrospun Nanofiber Films
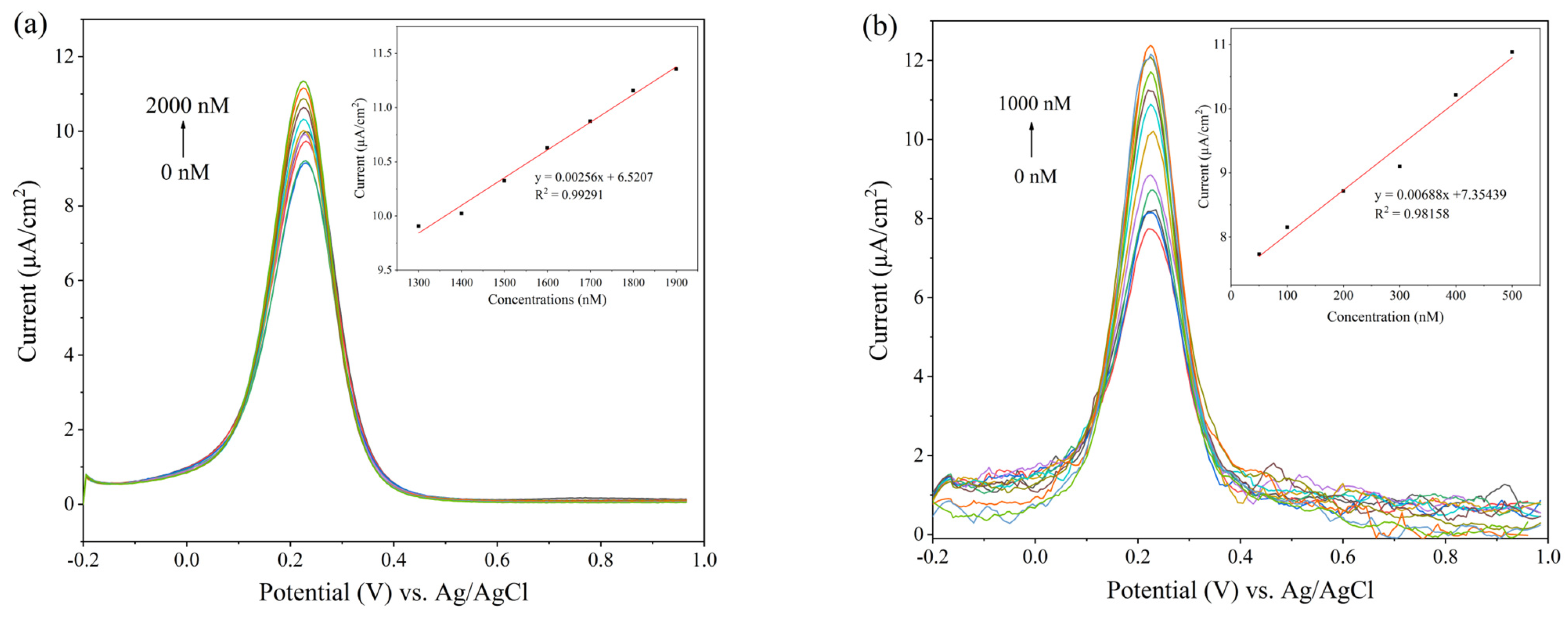
3.2. Electrochemical Detection of Dopamine
3.3. Selectivity, Reproducibility, Repeatability and Stability Studies
3.4. Application of the Obtained Label-Free DA Biosensor in Artificial Urine
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rhee, Y.H.; Ko, J.Y.; Chang, M.Y.; Yi, S.H.; Kim, D.; Kim, C.H.; Shim, J.W.; Jo, A.Y.; Kim, B.W.; Lee, H.; et al. Protein-Based Human Ips Cells Efficiently Generate Functional Dopamine Neurons and Can Treat a Rat Model of Parkinson Disease. J. Clin. Investig. 2011, 121, 2326–2335. [Google Scholar] [CrossRef] [PubMed]
- Dobryakova, E.; Genova, H.M.; DeLuca, J.; Wylie, G.R. The Dopamine Imbalance Hypothesis of Fatigue in Multiple Sclerosis and Other Neurological Disorders. Front. Neurol. 2015, 6, 52. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, D.S.; MacKie, P.J.; Kareken, D.A.; Hutchins, G.D.; Chumin, E.J.; Christian, B.T.; Yoder, K.K. Differential Dopamine Function in Fibromyalgia. Brain Imaging Behav. 2016, 10, 829–839. [Google Scholar] [CrossRef]
- Moon, J.M.; Thapliyal, N.; Hussain, K.K.; Goyal, R.N.; Shima, Y.B. Conducting Polymer-Based Electrochemical Biosensors for Neurotransmitters: A review. Biosens. Bioelectron. 2018, 102, 540–552. [Google Scholar] [CrossRef]
- Klanker, M.; Feenstra, M.; Denys, D. Dopaminergic Control of Cognitive Flexibility in Humans and Animals. Front. Neurosci. 2013, 7, 00201. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, J.; Wei, X. A Sensitive and Selective Sensor for Dopamine Determination Based on a Molecularly Imprinted Electropolymer of o-Aminophenol. Sens. Actuators B 2009, 140, 663–669. [Google Scholar] [CrossRef]
- Li, B.R.; Hsieh, Y.J.; Chen, Y.X.; Chung, Y.T.; Pan, C.Y.; Chen, Y.T. An Ultrasensitive Nanowire-Transistor Biosensor for Detecting Dopamine Release from Living PC12 Cells under Hypoxic Stimulation. J. Am. Chem. Soc. 2013, 135, 16034–16037. [Google Scholar] [CrossRef]
- Yang, C.; Cao, Q.; Puthongkham, P.; Lee, S.T.; Ganesana, M.; Lavrik, N.V.; Venton, B.J. 3D-Printed Carbon Electrodes for Neurotransmitter Detection. Angew. Chem. Int. Ed. 2018, 57, 14255–14259. [Google Scholar] [CrossRef]
- Zhao, T.; Wang, J.W.; Zhang, H.S.; Zheng, X.; Chen, Y.P.; Tang, H.; Jiang, J.H. Development of Dual-Nanopore Biosensors for Detection of Intracellular Dopamine and Dopamine Efflux from Single PC12 Cell. Anal. Chem. 2022, 94, 15541–15545. [Google Scholar] [CrossRef]
- Li, W.; Jin, J.; Xiong, T.; Yu, P.; Mao, L. Fast-Scanning Potential-Gated Organic Electrochemical Transistors for Highly Sensitive Sensing of Dopamine in Living Rat Brain. Angew. Chem. Int. Ed. 2022, 61, e202204134. [Google Scholar] [CrossRef]
- Fathi, F.; Sueoka, B.; Zhao, F.; Zeng, X. Nitrogen-Doped 4H Silicon Carbide Single-Crystal Electrode for Selective Electrochemical Sensing of Dopamine. Anal. Chem. 2023, 95, 4855–4862. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.Y.; Liu, R.; Jiang, J.C.; Meng, L.Y. Villous 3D Nanoconfined Flexible Carbon Fibers-Based Electrode Toward Dopamine Electrochemical Detection. Diam. Relat. Mat. 2024, 141, 110695. [Google Scholar] [CrossRef]
- Ratlam, C.; Phanichphant, S.; Sriwichai, S. Development of Dopamine Biosensor Based on Polyaniline/Carbon Quantum Dots Composite. J. Polym. Res. 2020, 27, 183. [Google Scholar] [CrossRef]
- Capella, P.; Ghasemzadeh, B.; Mitchell, K.; Adams, R.N. Nafion-coated Carbon Fiber Electrodes for Neurochemical Studies in Brain Tissue. Electroanalysis 1990, 2, 175–182. [Google Scholar] [CrossRef]
- Granero, A.M.; Pierini, G.D.; Robledo, S.N.; Di Nezio, M.S.; Fernández, H.; Zon, M.A. Simultaneous Determination of Ascorbic and Uric Acids and Dopamine in Human Serum Samples Using Three-way Calibration with Data from Square Wave Voltammetry. Microchem. J. 2016, 129, 205–212. [Google Scholar] [CrossRef]
- Chang, Y.H.; Woi, P.M.; Alias, Y. The Selective Electrochemical Detection of Dopamine in the Presence of Ascorbic Acid and Uric Acid Using Electro-Polymerised-β-Cyclodextrin Incorporated f-MWCNTs/Polyaniline Modified Glassy Carbon Electrode. Microchem. J. 2019, 148, 322–330. [Google Scholar] [CrossRef]
- Runsewe, D.O.; Haya, G.; Betancourt, T.; Irvin, J.A. Conducting Polymer-Based Electrochemical Aptasensor for the Detection of Adenosine. ACS Appl. Polym. Mater. 2021, 3, 6674–6683. [Google Scholar] [CrossRef]
- Xue, C.; Wang, X.; Zhu, W.; Han, Q.; Zhu, C.; Hong, J.; Zhou, X.; Jiang, H. Electrochemical Serotonin Sensing Interface Based on Double-Layered Membrane of Reduced Graphene Oxide/Polyaniline Nanocomposites and Molecularly Imprinted Polymers Embedded with Gold Nanoparticles. Sens. Actuators B 2014, 196, 57–63. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, X.T.; Zhou, J.P.; Liu, G.Z.; Zhang, S.Y. Electrochemical Preparation of Surface Molecularly Imprinted Poly(3-Aminophenylboronic Acid)/MWCNTs Nanocomposite for Sensitive Sensing of Epinephrine. Mater. Sci. Eng. C-Mater. Biol. Appl. 2018, 91, 696–704. [Google Scholar] [CrossRef]
- Ko, S.H.; Kim, S.W.; Lee, Y.J. Flexible Sensor with Electrophoretic Polymerized Graphene Oxide/PEDOT:PSS Composite for Voltammetric Determination of Dopamine Concentration. Sci. Rep. 2021, 11, 21101. [Google Scholar] [CrossRef]
- Sriwichai, S.; Janmanee, R.; Phanichphant, S.; Shinbo, K.; Kato, K.; Kaneko, F.; Yamamoto, T.; Baba, A. Development of an Electrochemical-Surface Plasmon Dual Biosensor Based on Carboxylated Conducting Polymer Thin Films. J. Appl. Polym. Sci. 2018, 135, 45641. [Google Scholar] [CrossRef]
- Haya, G.; Runsewe, D.O.; Otakpor, M.U.; Pohlman, G.E.; Towne, A.; Betancourt, T.; Irvin, J.A. Functionalized Thiophene-Based Aptasensors for the Electrochemical Detection of Mucin-1. ACS Appl. Polym. Mater. 2023, 5, 1208–1218. [Google Scholar] [CrossRef]
- Baba, A.; Mannen, T.; Ohdaira, Y.; Shinbo, K.; Kato, K.; Kaneko, F.; Fukuda, N.; Ushijima, H. Detection of Adrenaline on Poly(3-aminobenzylamine) Ultrathin Film by Electrochemical-Surface Plasmon Resonance Spectroscopy. Langmuir 2010, 26, 18476–18482. [Google Scholar] [CrossRef] [PubMed]
- Sriwichai, S.; Phanichphant, S. Fabrication and Characterization of Electrospun Poly(3-Aminobenzylamine)/Functionalized Multi-Walled Carbon Nanotubes Composite Film for Electrochemical Glucose Biosensor. Express Polym. Lett. 2022, 16, 439–450. [Google Scholar] [CrossRef]
- Fu, Y.; Sheng, Q.; Zheng, J. The Novel Sulfonated Polyaniline-Decorated Carbon Nanosphere Nanocomposites for Electrochemical Sensing of Dopamine. New J. Chem. 2017, 41, 15439–15446. [Google Scholar] [CrossRef]
- Kushwaha, C.S.; Shukla, S.K. Electrochemical Sensing of Paracetamol Using Iron Oxide Encapsulated in Chitosan-Grafted-Polyaniline. ACS Appl. Polym. Mater. 2020, 2, 2252–2259. [Google Scholar] [CrossRef]
- Suzuki, I.; Fukuda, M.; Shirakawa, K.; Jiko, H.; Gotoh, M. Carbon Nanotube Multi-Electrode Array Chips for Noninvasive Real-Time Measurement of Dopamine, Action Potentials, and Postsynaptic Potentials. Biosens. Bioelectron. 2013, 49, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Greiner, A.; Wendorff, J.H. Electrospinning: A Fascinating Method for the Preparation of Ultrathin Fibers. Angew. Chem. Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Ding, J.; Wei, G. Electrospinning: A Facile Technique for Fabricating Polymeric Nanofibers Doped with Carbon Nanotubes and Metallic Nanoparticles for Sensor Applications. RSC Adv. 2014, 4, 52598–52610. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Majumder, S.; Sagor, M.M.H.; Arafat, M.T. Functional Electrospun Polymeric Materials for Bioelectronic Devices: A Review. Mater. Adv. 2022, 3, 6753–6772. [Google Scholar] [CrossRef]
- Abidian, M.R.; Kim, D.H.; Martin, D.C. Conducting-Polymer Nanotubes for Controlled Drug Release. Adv. Mater. 2006, 18, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Bashur, C.A.; Goldstein, A.S.; Schmidt, C.E. Polypyrrole-Coated Electrospun PLGA Nanofibers for Neural Tissue Applications. Biomaterials 2009, 30, 4325–4335. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.J.; Kameoka, J. Amperometric Cholesterol Biosensor Using Layer-By-Layer Adsorption Technique onto Electrospun Polyaniline Nanofibers. J. Ind. Eng. Chem. 2012, 18, 193–197. [Google Scholar] [CrossRef]
- Kilic, N.M.; Gelen, S.S.; Zeybekler, S.E.; Odaci, D. Carbon-Based Nanomaterials Decorated Electrospun Nanofibers in Biosensors: A Review. ACS Omega 2024, 9, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Drew, C.; Lee, S.H.; Senecal, K.J.; Kumar, J.; Samuelson, L.A. Electrospun Nanofibrous Membranes for Highly Sensitive Optical Sensors. Nano Lett. 2002, 2, 1273–1275. [Google Scholar] [CrossRef]
- Lee, M.; Kim, T.I.; Kim, K.H.; Kim, J.H.; Choi, M.S.; Choi, H.J.; Koh, K. Formation of a Self-Assembled Phenylboronic Acid Monolayer and Its Application Toward Developing a Surface Plasmon Resonance-Based Monosaccharide Sensor. Anal. Biochem. 2002, 310, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Panapimonlawat, T.; Phanichphant, S.; Sriwichai, S. Electrochemical Dopamine Biosensor Based on Poly(3-aminobenzylamine) Layer-by-Layer Self-Assembled Multilayer Thin Film. Polymers 2021, 13, 1488. [Google Scholar] [CrossRef]
- Alero-Koroma, D.; Abbas, Z.K.; Barton, S.J.; Foot, P.J.S. The Synthesis and Properties of Novel Conducting Polyaniline and Poly[(nitrile butadiene-co-acrylonitrile)/Polyvinyl Chloride] Blends. Polym. Polym. Compos. 2013, 21, 403–412. [Google Scholar] [CrossRef]
- Choudhury, A.; Kar, P. Doping Effect of Carboxylic Acid Group Functionalized Multi-Walled Carbon Nanotube on Polyaniline. Compos. Pt. B-Eng. 2011, 42, 1641–1647. [Google Scholar] [CrossRef]
- Deng, J.; Wang, T.; Guo, J.; Liu, P. Electrochemical Capacity Fading of Polyaniline Electrode in Supercapacitor: An XPS analysis. Prog. Nat. Sci. Mater. Int. 2017, 27, 257–260. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, Z.; Li, P.; Zong, X.; Dong, G.; Zhang, Y. Facile Fabrication of Polyaniline/Multi-Walled Carbon Nanotubes/Molybdenum Disulfide Ternary Nanocomposite and Its High Performance Ammonia-Sensing at Room Temperature. Sens. Actuators B 2018, 258, 895–905. [Google Scholar] [CrossRef]
- Goyanes, S.; Rubiolo, G.R.; Salazar, A.; Jimeno, A.; Corcuera, M.A.; Mondragon, I. Carboxylation Treatment of Multiwalled Carbon Nanotubes Monitored by Infrared and Ultraviolet Spectroscopies and Scanning Probe Microscopy. Diam. Relat. Mat. 2007, 16, 412–417. [Google Scholar] [CrossRef]
- Wong, K.C.; Goh, P.S.; Ng, B.C.; Ismail, A.F. Thin Film Nanocomposite Embedded with Polymethyl Methacrylate Modified Multi-Walled Carbon Nanotubes for CO2 Removal. RSC Adv. 2015, 5, 31683–31690. [Google Scholar] [CrossRef]
- Karacan, I.; Erdogan, G. The Influence of Thermal Stabilization Stage on the Molecular Structure of Polyacrylonitrile Fibers Prior to the Carbonization Stage. Fiber. Polym. 2012, 13, 295–302. [Google Scholar] [CrossRef]
- Ahuja, P.; Ujjain, S.K.; Arora, I.; Samim, M. Hierarchically Grown NiO-Decorated Polyaniline-Reduced Graphene Oxide Composite for Ultrafast Sunlight-Driven Photocatalysis. ACS Omega 2018, 3, 7846–7855. [Google Scholar] [CrossRef]
- Niu, H.; Qin, S.; Mao, X.; Zhang, S.; Wang, R.; Wan, L.; Xu, J.; Miao, S. Axle-Sleeve Structured MWCNTs/Polyaniline Composite Film as Cost-effective Counter Electrodes for High Efficient Dye-Sensitized Solar Cells. Electrochim. Acta 2014, 121, 285–293. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, L.L.; Zhao, X.S.; Wu, J. Graphene/Polyaniline Nanofiber Composites as Supercapacitor Electrodes. Chem. Mater. 2010, 22, 1392–1401. [Google Scholar] [CrossRef]
- Marmisollé, W.A.; Gregurec, D.; Moya, S.; Azzaroni, O. Polyanilines with Pendant Amino Groups as Electrochemically Active Copolymers at Neutral pH. ChemElectroChem 2015, 2, 2011–2019. [Google Scholar] [CrossRef]
- Fenoy, G.E.; Giussi, J.M.; Bilderling, C.; Maza, E.M.; Pietrasanta, L.I.; Knoll, W.; Marmisollé, W.A.; Azzaroni, O. Reversible Modulation of the Redox Activity in Conducting Polymer Nanofilms Induced by Hydrophobic Collapse of a Surface-Grafted Polyelectrolyte. J. Colloid Interface Sci. 2018, 518, 92–101. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Xu, C.; Jiang, H.; Li, C.; Zhang, L.; Lin, J.; Shen, Z.X. Advanced Energy Storage Devices: Basic Principles. Analytical Methods, and Rational Materials Design. Adv. Sci. 2018, 5, 1700322. [Google Scholar] [CrossRef] [PubMed]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A Practical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Liu, Y.C.; Hsu, W.F.; Wu, T.M. Electrochemical Determination of Dopamine Using a Conductive Polypyrrole/Carbon-Coated Mesoporous Silica Composite Electrode. J. Appl. Electrochem. 2020, 50, 311–319. [Google Scholar] [CrossRef]
- Manivel, P.; Dhakshnamoorthy, M.; Balamurugan, A.; Ponpandian, N.; Mangalaraja, D.; Viswanathan, C. Conducting Polyaniline-Graphene Oxide Fibrous Nanocomposites: Preparation, Characterization and Simultaneous Electrochemical Detection of Ascorbic Acid, Dopamine and Uric Acid. RSC Adv. 2013, 3, 14428–14438. [Google Scholar] [CrossRef]
- Sebastian, J.; Daniel, M.; Neppolian, B.; Samuel, J.M. Electroactive Modified Poly(2-Aminobenzoic Acid)-Blend-Aloe Vera/GCE as an Efficient Dopamine Sensor. J. Polym. Res. 2023, 30, 356. [Google Scholar] [CrossRef]
- Abbaspour, A.; Noori, A. A Cyclodextrin Host–Guest Recognition Approach to an Electrochemical Sensor for Simultaneous Quantification of Serotonin and Dopamine. Biosens. Bioelectron. 2011, 26, 4674–4680. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.P.; Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Biological and Psychological Markers of Stress in Humans: Focus on the Trier Social Stress Test. Neurosci. Biobehav. Rev. 2014, 38, 94–124. [Google Scholar] [CrossRef] [PubMed]
- Dalirirad, S.; Steckl, A.J. Lateral Flow Assay Using Aptamer-Based Sensing for On-Site Detection of Dopamine in Urine. Anal. Biochem. 2020, 596, 113637. [Google Scholar] [CrossRef] [PubMed]
- Sami, A.S.; Barani, S.S.I.; Rashid, R.F.; Zeebaree, S.Y.S.; Zeebaree, A.Y.S.; Zebari, O.I.H.; Ali, A.M.; Ilyas, K.S.; Biso, F.H.; Issa, M.M. Novel Biosensor for Highly Sensitive Detection of Serum Albumin in Artificial Human Urine Using CuNPs@AG. Sens. Bio-Sens. Res. 2024, 43, 100633. [Google Scholar] [CrossRef]
- Ulubay, S.; Dursun, Z. Cu Nanoparticles Incorporated Polypyrrole Modified GCE for Sensitive Simultaneous Determination of Dopamine and Uric acid. Talanta 2010, 80, 1461–1466. [Google Scholar] [CrossRef]
- Rezaei, B.; Boroujeni, M.K.; Ensaf, A.A. Fabrication of DNA, o-Phenylenediamine, and Gold Nanoparticle Bioimprinted Polymer Electrochemical Sensor for the Determination of Dopamine. Biosens. Bioelectron. 2015, 66, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Guo, Y.; Zheng, J.; Zhou, X.; Li, Q.; Lin, R. Simultaneous Determination of Ascorbic Acid, Dopamine and Uric Acid using Poly(L-leucine)/DNA Composite Film Modified Electrode. Sens. Actuator B-Chem. 2015, 213, 188–194. [Google Scholar] [CrossRef]
- Atta, N.F.; Galal, A.; El-Ads, E.H. Gold Nanoparticles-Coated Poly(3,4-Ethylene-Dioxythiophene) for the Selective Determination of Sub-Nano Concentrations of Dopamine in Presence of Sodium Dodecyl Sulfate. Electrochim. Acta 2012, 69, 102–111. [Google Scholar] [CrossRef]
- Atta, N.F.; Galal, A.; Ahmed, R.A. Poly(3,4-Ethylene-Dioxythiophene) Electrode for the Selective Determination of Dopamine in Presence of Sodium Dodecyl Sulfate. Bioelectrochemistry 2011, 80, 132–141. [Google Scholar] [CrossRef] [PubMed]






| PANI | PANI/f-CNT | PABA | PABA/f-CNT | |
|---|---|---|---|---|
| Aanodic | 0.0104 | 0.0218 | 0.0096 | 0.0154 |
| Acathodic | −0.0374 | −0.0392 | −0.0334 | −0.0372 |
| ΔEa,c | 0.279 | 0.266 | 0.346 | 0.297 |
| Sensitivity (µA·cm−2·µM−1) | LOD (µM) | LOQ (µM) | Linear Range (µM) | |
|---|---|---|---|---|
| PANI | 2.560 | 0.4542 | 1.5140 | 1.3–1.9 |
| PANI/f-CNTs | 6.876 | 0.0974 | 0.3245 | 0.05–0.5 |
| PABA | 2.809 | 0.4178 | 1.3926 | 1.1–1.5 |
| PABA/f-CNTs | 7.269 | 0.1554 | 0.5179 | 0.05–0.5 |
| Electrode | Linear Range (µM) | Sensitivity (µA·cm−2·µM−1) | LOD (µM) | References |
|---|---|---|---|---|
| PANI/CQDs | 10–90 | 0.00802 | 0.1013 | [13] |
| poly-β-CD(f-MWCNTs)/PANI | 2–24 | 26.79 | 0.0164 | [16] |
| GO/PEDOT:PSS | - | 69.3 | 0.008 | [20] |
| SPANI/CNSs | 0.50–1780 | 1.139 | 0.0152 | [25] |
| PANI-GO/GCE | 2–18 | - | 0.5 | [54] |
| CD/PNAANI/CNT modified CPE | 2–200 | - | sub-µM | [56] |
| PANI/f-CNT | 0.05–0.5 | 6.876 | 0.0974 | This work |
| PABA/f-CNT | 0.05–0.5 | 7.269 | 0.1554 | This work |
| Electrode | Added Concentration (µM) | Measured Concentration (µM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| PANI/f-CNTs | 0.15 | 152.88 | 101.92 | 0.634 |
| 0.25 | 247.52 | 99.01 | 0.691 | |
| 0.35 | 346.51 | 99.00 | 0.806 | |
| PABA/f-CNTs | 0.15 | 150.67 | 100.45 | 0.968 |
| 0.25 | 248.01 | 99.20 | 1.829 | |
| 0.35 | 348.91 | 99.69 | 0.801 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaewda, C.; Sriwichai, S. Label-Free Electrochemical Dopamine Biosensor Based on Electrospun Nanofibers of Polyaniline/Carbon Nanotube Composites. Biosensors 2024, 14, 349. https://doi.org/10.3390/bios14070349
Kaewda C, Sriwichai S. Label-Free Electrochemical Dopamine Biosensor Based on Electrospun Nanofibers of Polyaniline/Carbon Nanotube Composites. Biosensors. 2024; 14(7):349. https://doi.org/10.3390/bios14070349
Chicago/Turabian StyleKaewda, Chanaporn, and Saengrawee Sriwichai. 2024. "Label-Free Electrochemical Dopamine Biosensor Based on Electrospun Nanofibers of Polyaniline/Carbon Nanotube Composites" Biosensors 14, no. 7: 349. https://doi.org/10.3390/bios14070349






