Evaluation of In Vitro Serotonin-Induced Electrochemical Fouling Performance of Boron Doped Diamond Microelectrode Using Fast-Scan Cyclic Voltammetry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Carbon Fiber Microelectrode (CFME) Fabrication
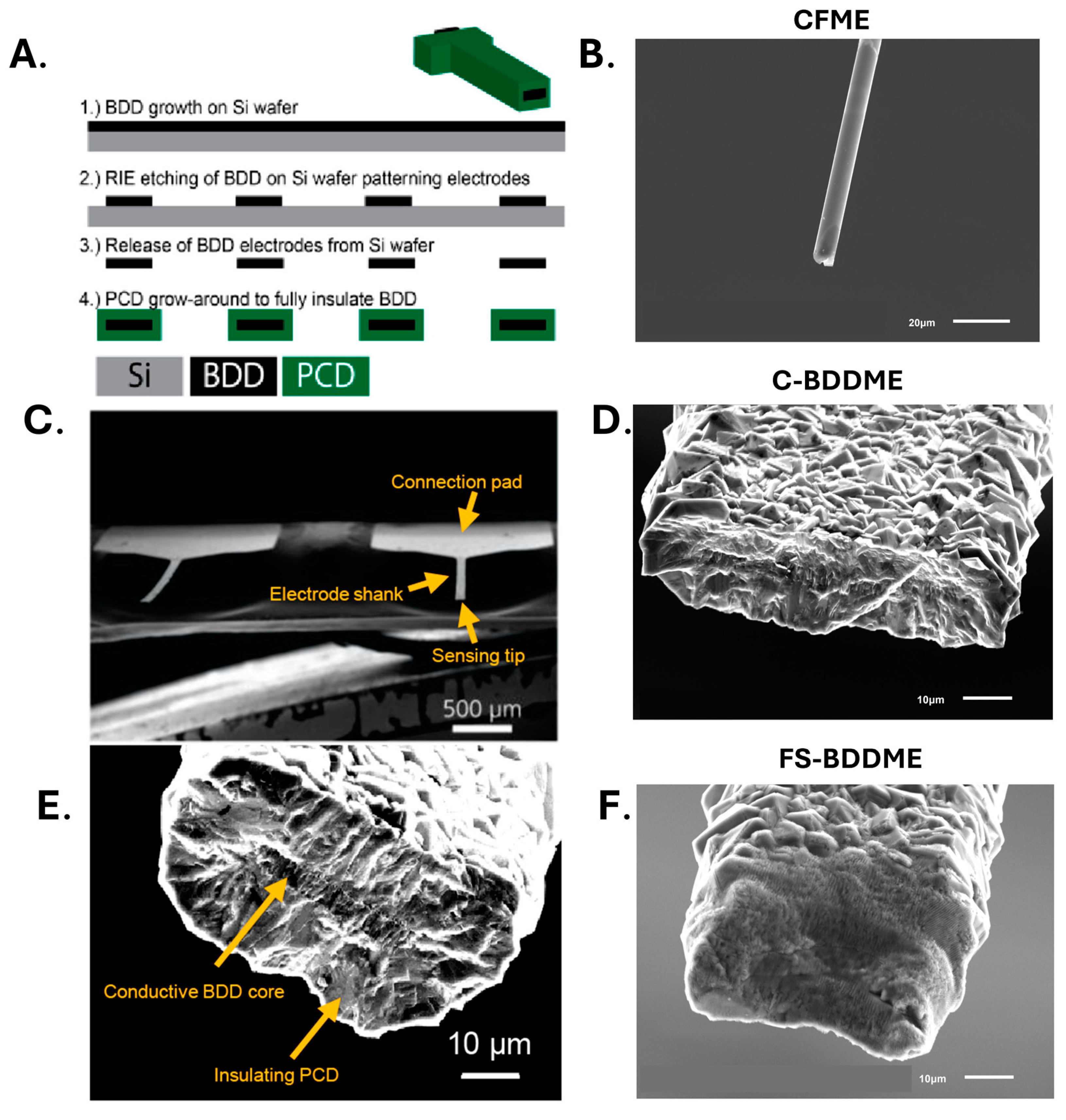
2.3. Boron Doped Diamond Fabrication
2.4. Fast-Scan Cyclic Voltammetry (FSCV)
2.5. Electrochemical Fouling Protocol
2.6. Electrode Stability Analysis
2.7. SEM Imaging
2.8. Statistics
2.9. Hot-Acid Boiling
3. Results
3.1. Serotonin Response
3.2. Electrode Calibrations
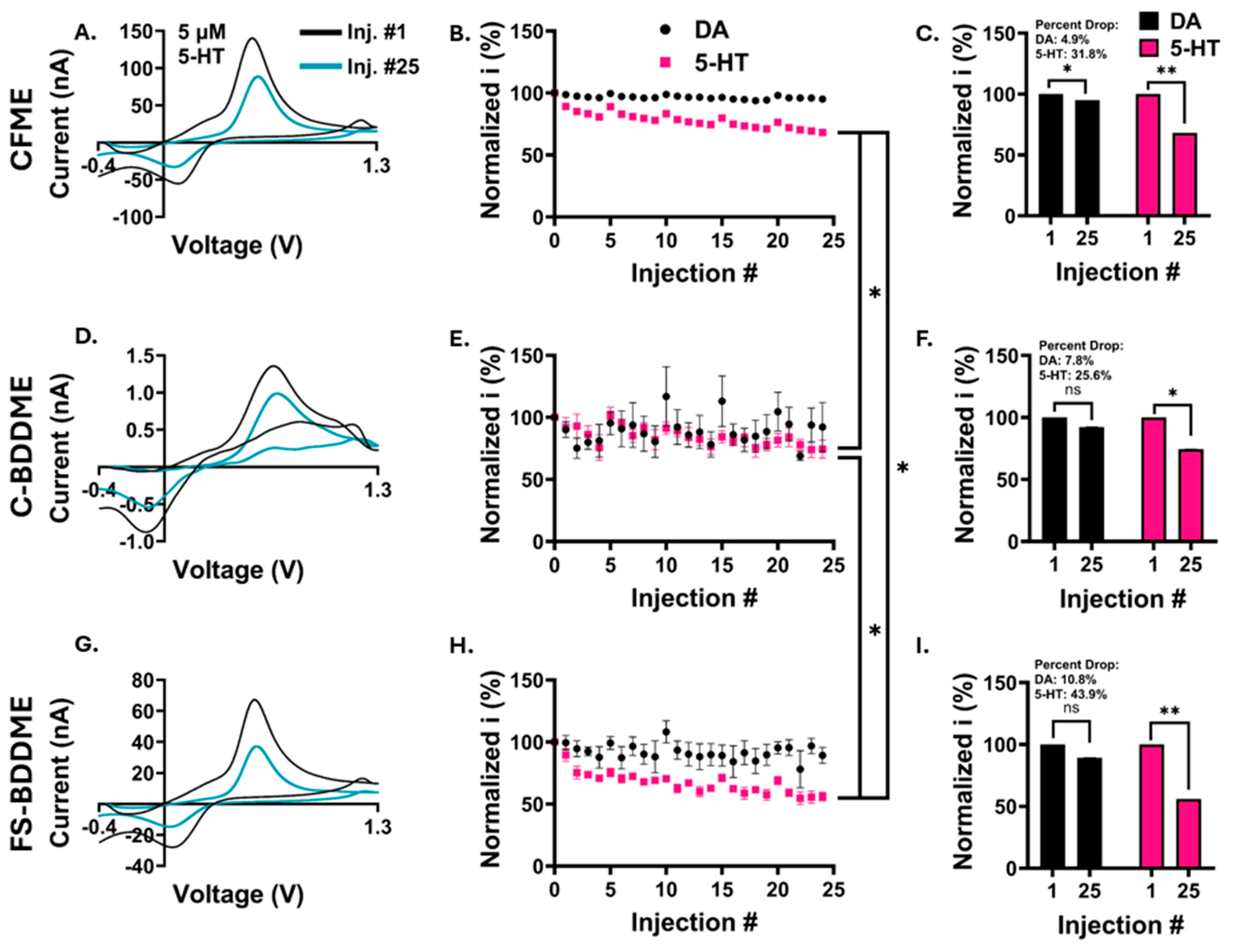
3.3. Electrochemical Fouling with 5 µM DA and 5-HT
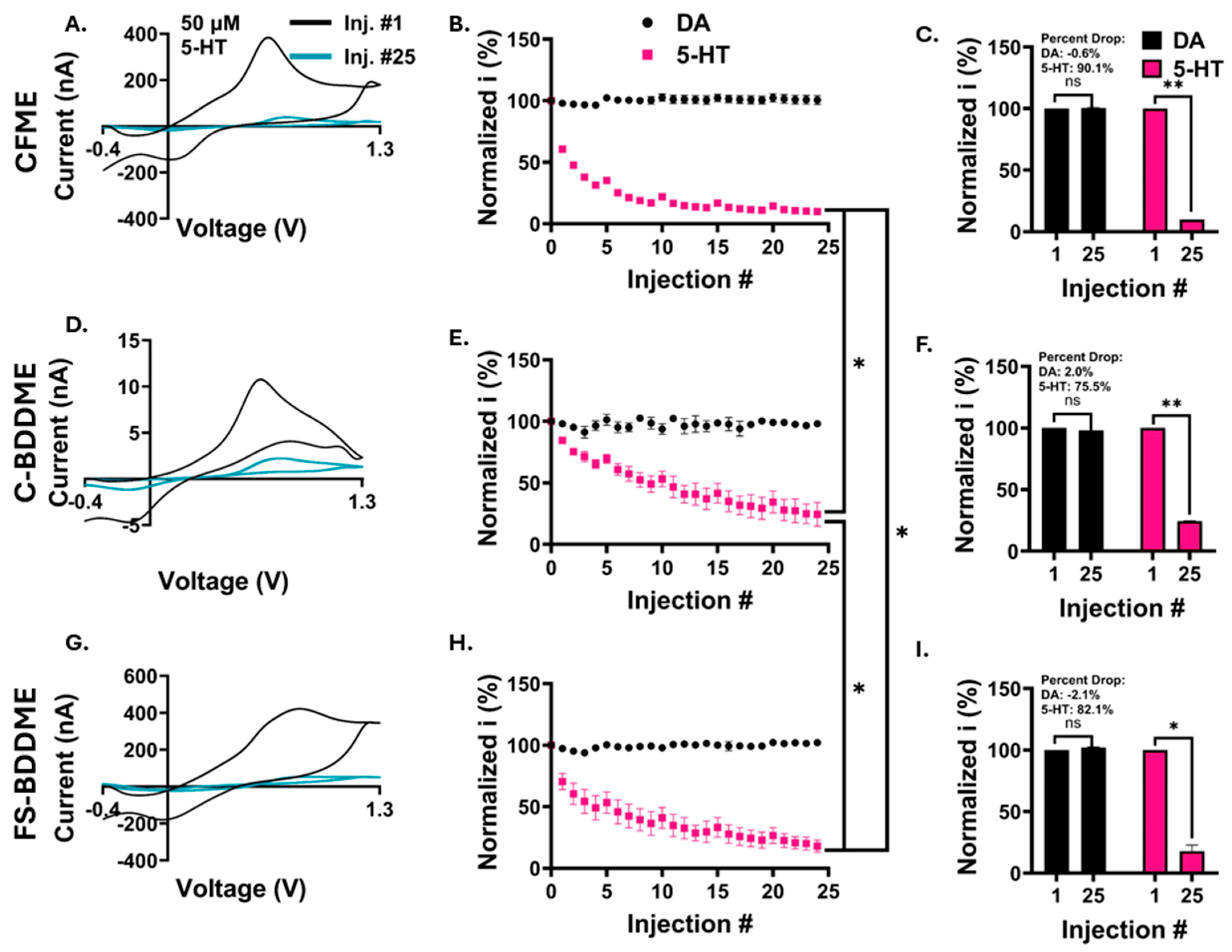
3.4. Electrochemical Fouling with 50 µM DA and 5-HT
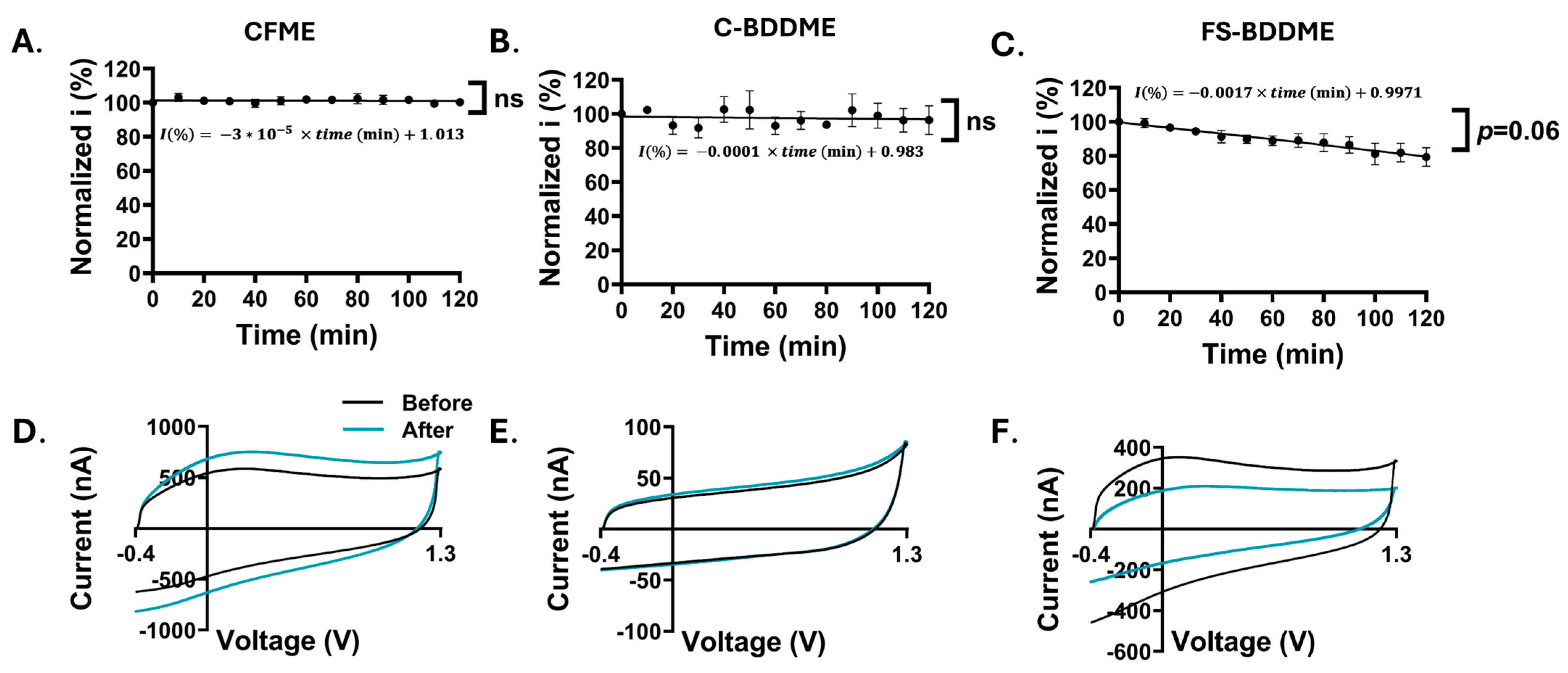
3.5. Electrode Stability Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hyman, S.E. Neurotransmitters. Curr. Biol. 2005, 15, R154–R158. [Google Scholar] [CrossRef] [PubMed]
- Grace, A.A. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat. Rev. Neurosci. 2016, 17, 524–532. [Google Scholar] [CrossRef]
- Wise, R.A.; Jordan, C.J. Dopamine, behavior, and addiction. J. Biomed. Sci. 2021, 28, 83. [Google Scholar] [CrossRef]
- Cooper, S.; Robison, A.J.; Mazei-Robison, M.S. Reward Circuitry in Addiction. Neurotherapeutics 2017, 14, 687–697. [Google Scholar] [CrossRef]
- Stahl, S.M. Beyond the dopamine hypothesis of schizophrenia to three neural networks of psychosis: Dopamine, serotonin, and glutamate. CNS Spectr. 2018, 23, 187–191. [Google Scholar] [CrossRef]
- Kishida, K.T.; Saez, I.; Lohrenz, T.; Witcher, M.R.; Laxton, A.W.; Tatter, S.B.; White, J.P.; Ellis, T.L.; Phillips, P.E.M.; Montague, P.R. Subsecond dopamine fluctuations in human striatum encode superposed error signals about actual and counterfactual reward. Proc. Natl. Acad. Sci. USA 2016, 113, 200–205. [Google Scholar] [CrossRef]
- Saddoris, M.P.; Wang, X.; Sugam, J.A.; Carelli, R.M. Cocaine Self-Administration Experience Induces Pathological Phasic Accumbens Dopamine Signals and Abnormal Incentive Behaviors in Drug-Abstinent Rats. J. Neurosci. 2016, 36, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Aragona, B.J.; Kile, B.M.; Carelli, R.M.; Wightman, R.M. In vivo voltammetric monitoring of catecholamine release in subterritories of the nucleus accumbens shell. Neuroscience 2010, 169, 132–142. [Google Scholar] [CrossRef]
- Buchanan, A.M.; Mena, S.; Choukari, I.; Vasa, A.; Crawford, J.N.; Fadel, J.; Maxwell, N.; Reagan, L.; Cruikshank, A.; Best, J.; et al. Serotonin as a biomarker of toxin-induced Parkinsonism. Mol. Med. Camb. Mass. 2024, 30, 33. [Google Scholar] [CrossRef] [PubMed]
- Venton, B.J.; Cao, Q. Fundamentals of fast-scan cyclic voltammetry for dopamine detection. Analyst 2020, 145, 1158–1168. [Google Scholar] [CrossRef]
- Robinson, D.L.; Venton, B.J.; Heien, M.L.A.V.; Wightman, R.M. Detecting Subsecond Dopamine Release with Fast-Scan Cyclic Voltammetry in Vivo. Clin. Chem. 2003, 49, 1763–1773. [Google Scholar] [CrossRef]
- Park, J.; Takmakov, P.; Wightman, R.M. In vivo comparison of norepinephrine and dopamine release in rat brain by simultaneous measurements with fast-scan cyclic voltammetry. J. Neurochem. 2011, 119, 932–944. [Google Scholar] [CrossRef] [PubMed]
- Samaranayake, S.; Abdalla, A.; Robke, R.; Wood, K.M.; Zeqja, A.; Hashemi, P. In vivo histamine voltammetry in the mouse premammillary nucleus. Analyst 2015, 140, 3759–3765. [Google Scholar] [CrossRef] [PubMed]
- Dunham, K.E.; Venton, B.J. Improving serotonin fast-scan cyclic voltammetry detection: New waveforms to reduce electrode fouling. Analyst 2020, 145, 7437–7446. [Google Scholar] [CrossRef] [PubMed]
- Jackson, B.P.; Dietz, S.M.; Wightman, R.M. Fast-scan cyclic voltammetry of 5-hydroxytryptamine. Anal. Chem. 1995, 67, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Hersey, M.; Woodruff, J.L.; Maxwell, N.; Sadek, A.T.; Bykalo, M.K.; Bain, I.; Grillo, C.A.; Piroli, G.G.; Hashemi, P.; Reagan, L.P. High-fat diet induces neuroinflammation and reduces the serotonergic response to escitalopram in the hippocampus of obese rats. Brain Behav. Immun. 2021, 96, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Weese, M.E.; Krevh, R.A.; Li, Y.; Alvarez, N.T.; Ross, A.E. Defect Sites Modulate Fouling Resistance on Carbon-Nanotube Fiber Electrodes. ACS Sens. 2019, 4, 1001–1007. [Google Scholar] [CrossRef]
- Wrona, M.Z.; Dryhurst, G. Electrochemical oxidation of 5-hydroxytryptamine in aqueous solution at physiological pH. Bioorganic Chem. 1990, 18, 291–317. [Google Scholar] [CrossRef]
- Hashemi, P.; Dankoski, E.C.; Petrovic, J.; Keithley, R.B.; Wightman, R.M. Voltammetric Detection of 5-Hydroxytryptamine Release in the Rat Brain. Anal. Chem. 2009, 81, 9462–9471. [Google Scholar] [CrossRef]
- Singh, Y.S.; Sawarynski, L.E.; Dabiri, P.D.; Choi, W.R.; Andrews, A.M. Head-to-head comparisons of carbon fiber microelectrode coatings for sensitive and selective neurotransmitter detection by voltammetry. Anal. Chem. 2011, 83, 6658–6666. [Google Scholar] [CrossRef]
- Gupta, B.; Perillo, M.L.; Siegenthaler, J.R.; Christensen, I.E.; Welch, M.P.; Rechenberg, R.; Banna, G.M.H.U.; Galstyan, D.; Becker, M.F.; Li, W.; et al. In Vitro Biofouling Performance of Boron-Doped Diamond Microelectrodes for Serotonin Detection Using Fast-Scan Cyclic Voltammetry. Biosensors 2023, 13, 576. [Google Scholar] [CrossRef] [PubMed]
- Seaton, B.T.; Hill, D.F.; Cowen, S.L.; Heien, M.L. Mitigating the Effects of Electrode Biofouling-Induced Impedance for Improved Long-Term Electrochemical Measurements In Vivo. Anal. Chem. 2020, 92, 6334–6340. [Google Scholar] [CrossRef] [PubMed]
- Güell, A.G.; Meadows, K.E.; Unwin, P.R.; Macpherson, J.V. Trace voltammetric detection of serotonin at carbon electrodes: Comparison of glassy carbon, boron doped diamond and carbon nanotube network electrodes. Phys. Chem. Chem. Phys. 2010, 12, 10108. [Google Scholar] [CrossRef] [PubMed]
- Puthongkham, P.; Venton, B.J. Recent advances in fast-scan cyclic voltammetry. Analyst 2020, 145, 1087–1102. [Google Scholar] [CrossRef] [PubMed]
- Puthongkham, P.; Venton, B.J. Nanodiamond Coating Improves the Sensitivity and Antifouling Properties of Carbon Fiber Microelectrodes. ACS Sens. 2019, 4, 2403–2411. [Google Scholar] [CrossRef] [PubMed]
- Melnikov, P.V.; Alexandrovskaya, A.Y.; Naumova, A.O.; Popova, N.M.; Spitsyn, B.V.; Zaitsev, N.K.; Yashtulov, N.A. Modified Nanodiamonds as a Means of Polymer Surface Functionalization. From Fouling Suppression to Biosensor Design. Nanomaterials 2021, 11, 2980. [Google Scholar] [CrossRef] [PubMed]
- Purcell, E.K.; Becker, M.F.; Guo, Y.; Hara, S.A.; Ludwig, K.A.; McKinney, C.J.; Monroe, E.M.; Rechenberg, R.; Rusinek, C.A.; Saxena, A.; et al. Next-Generation Diamond Electrodes for Neurochemical Sensing: Challenges and Opportunities. Micromachines 2021, 12, 128. [Google Scholar] [CrossRef]
- Fan, B.; Rusinek, C.A.; Thompson, C.H.; Setien, M.; Guo, Y.; Rechenberg, R.; Gong, Y.; Weber, A.J.; Becker, M.F.; Purcell, E.; et al. Flexible, diamond-based microelectrodes fabricated using the diamond growth side for neural sensing. Microsyst. Nanoeng. 2020, 6, 42. [Google Scholar] [CrossRef]
- Singh, Y.S.; Sawarynski, L.E.; Michael, H.M.; Ferrell, R.E.; Murphey-Corb, M.A.; Swain, G.M.; Patel, B.A.; Andrews, A.M. Boron-Doped Diamond Microelectrodes Reveal Reduced Serotonin Uptake Rates in Lymphocytes from Adult Rhesus Monkeys Carrying the Short Allele of the 5-HTTLPR. ACS Chem. Neurosci. 2010, 1, 49–64. [Google Scholar] [CrossRef]
- Dong, H.; Wang, S.; Galligan, J.J.; Swain, G.M. Boron-doped diamond nano/microelectrodes for biosensing and in vitro measurements. Front. Biosci. Sch. Ed. 2011, 3, 518–540. [Google Scholar] [CrossRef]
- Bennet, K.E.; Tomshine, J.R.; Min, H.K.; Manciu, F.S.; Marsh, M.P.; Paek, S.B.; Settell, M.L.; Nicolai, E.N.; Blaha, C.D.; Kouzani, A.Z.; et al. A Diamond-Based Electrode for Detection of Neurochemicals in the Human Brain. Front. Hum. Neurosci. 2016, 10, 102. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Quaiserová-Mocko, V.; Pecková, K.; Galligan, J.J.; Fink, G.D.; Swain, G.M. Fabrication, characterization, and application of a diamond microelectrode for electrochemical measurement of norepinephrine release from the sympathetic nervous system. Diam. Relat. Mater. 2006, 15, 761–772. [Google Scholar] [CrossRef]
- Zhao, H.; Bian, X.; Galligan, J.J.; Swain, G.M. Electrochemical measurements of serotonin (5-HT) release from the guinea pig mucosa using continuous amperometry with a boron-doped diamond microelectrode. Diam. Relat. Mater. 2010, 19, 182. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.A.; Bian, X.; Quaiserová-Mocko, V.; Galligan, J.J.; Swain, G.M. In vitro continuous amperometric monitoring of 5-hydroxytryptamine release from enterochromaffin cells of the guinea pig ileum. Analyst 2006, 132, 41–47. [Google Scholar] [CrossRef]
- Sarada, B.V.; Rao, T.N.; Tryk, D.A.; Fujishima, A. Electrochemical oxidation of histamine and serotonin at highly boron-doped diamond electrodes. Anal. Chem. 2000, 72, 1632–1638. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.N.; Unwin, P.R.; Macpherson, J.V. Investigation of film formation properties during electrochemical oxidation of serotonin (5-HT) at polycrystalline boron doped diamond. Phys. Chem. Chem. Phys. 2013, 15, 18085–18092. [Google Scholar] [CrossRef] [PubMed]
- Cooper, S.E.; Venton, B.J. Fast-scan cyclic voltammetry for the detection of tyramine and octopamine. Anal. Bioanal. Chem. 2009, 394, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Pyakurel, P.; Privman Champaloux, E.; Venton, B.J. Fast-Scan Cyclic Voltammetry (FSCV) Detection of Endogenous Octopamine in Drosophila melanogaster Ventral Nerve Cord. ACS Chem. Neurosci. 2016, 7, 1112–1119. [Google Scholar] [CrossRef]
- Gupta, B.; Perillo, M.L.; Christensen, I.E.; Siegenthaler, J.R.; Rechenberg, R.; Becker, M.F.; Li, W.; Purcell, E.K. Waveform Development for Neurotransmitter Detection on Novel Boron-Doped Diamond Microelectrodes. In Proceedings of the 2023 11th International IEEE/EMBS Conference on Neural Engineering (NER), Baltimore, MD, USA, 24–27 April 2023; pp. 1–5. [Google Scholar] [CrossRef]
- Roberts, J.G.; Sombers, L.A. Fast-Scan Cyclic Voltammetry: Chemical Sensing in the Brain and Beyond. Anal. Chem. 2018, 90, 490–504. [Google Scholar] [CrossRef]
- Takmakov, P.; Zachek, M.K.; Keithley, R.B.; Walsh, P.L.; Donley, C.; McCarty, G.S.; Wightman, R.M. Carbon Microelectrodes with a Renewable Surface. Anal. Chem. 2010, 82, 2020–2028. [Google Scholar] [CrossRef]
- Rusinek, C.A.; Guo, Y.; Rechenberg, R.; Becker, M.F.; Purcell, E.; Verber, M.; McKinney, C.; Li, W. All-Diamond Microfiber Electrodes for Neurochemical Analysis. J. Electrochem. Soc. 2018, 165, G3087–G3092. [Google Scholar] [CrossRef]
- Einaga, Y. Diamond electrodes for electrochemical analysis. J. Appl. Electrochem. 2010, 40, 1807–1816. [Google Scholar] [CrossRef]
- Duo, I.; Fujishima, A.; Comninellis, C. Electron transfer kinetics on composite diamond (sp3)–graphite (sp2) electrodes. Electrochem. Commun. 2003, 5, 695–700. [Google Scholar] [CrossRef]
- Callou, T.P.; Garcia, R.; Mukai, A.; Giacomin, N.T.; de Souza, R.G.; Bechara, S.J. Advances in femtosecond laser technology. Clin. Ophthalmol. 2016, 10, 697–703. [Google Scholar] [CrossRef]
- Banna, G.H.U.; Siegenthaler, J.; Benedict, A.; Allen, B.; Martinez, R.M.; Zhang, W.; Li, W. Heavy metal sensing in plant and soil solutions using carbon fiber electrode. Sens. Actuators Phys. 2024, 370, 115232. [Google Scholar] [CrossRef]
- Mitul, A.F.; Zhou, B.; Zhao, H.; Han, M. Micromachining & FBG fabrication using point by point technique utilizing femto-second laser. arXiv 2022, arXiv:2210.07156. [Google Scholar]
- Bucher, E.S.; Brooks, K.; Verber, M.D.; Keithley, R.B.; Owesson-White, C.; Carroll, S.; Takmakov, P.; McKinney, C.J.; Wightman, R.M. Flexible software platform for fast-scan cyclic voltammetry data acquisition and analysis. Anal. Chem. 2013, 85, 10344–10353. [Google Scholar] [CrossRef] [PubMed]
- Abraham, J. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. In Handbook of Transnational Economic Governance Regimes; Tietje, C., Brouder, A., Eds.; Brill: Nijhoff, The Netherlands, 2010; pp. 1041–1053. [Google Scholar] [CrossRef]
- Uhrovčík, J. Strategy for determination of LOD and LOQ values—Some basic aspects. Talanta 2014, 119, 178–180. [Google Scholar] [CrossRef] [PubMed]
- Rodeberg, N.T.; Sandberg, S.G.; Johnson, J.A.; Phillips, P.E.M.; Wightman, R.M. Hitchhiker’s Guide to Voltammetry: Acute and Chronic Electrodes for in Vivo Fast-Scan Cyclic Voltammetry. ACS Chem. Neurosci. 2017, 8, 221–234. [Google Scholar] [CrossRef]
- Heien, M.L.; Khan, A.S.; Ariansen, J.L.; Cheer, J.F.; Phillips, P.E.; Wassum, K.M.; Wightman, R.M. Real-time measurement of dopamine fluctuations after cocaine in the brain of behaving rats. Proc. Natl. Acad. Sci. USA 2005, 102, 10023–10028. [Google Scholar] [CrossRef]
- Roberts, J.G.; Toups, J.V.; Eyualem, E.; McCarty, G.S.; Sombers, L.A. In Situ Electrode Calibration Strategy for Voltammetric Measurements In Vivo. Anal. Chem. 2013, 85, 11568–11575. [Google Scholar] [CrossRef] [PubMed]
- Saylor, R.A.; Hersey, M.; West, A.; Buchanan, A.M.; Berger, S.N.; Nijhout, H.F.; Reed, M.C.; Best, J.; Hashemi, P. In vivo Hippocampal Serotonin Dynamics in Male and Female Mice: Determining Effects of Acute Escitalopram Using Fast Scan Cyclic Voltammetry. Front. Neurosci. 2019, 13, 362. [Google Scholar] [CrossRef] [PubMed]
- Blier, P.; de Montigny, C.; Chaput, Y. A role for the serotonin system in the mechanism of action of antidepressant treatments: Preclinical evidence. J. Clin. Psychiatry 1990, 51, 14–20, discussion 21. [Google Scholar]
- Hrovatin, K.; Kunej, T.; Dolžan, V. Genetic variability of serotonin pathway associated with schizophrenia onset, progression, and treatment. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2020, 183, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Bang, D.; Kishida, K.T.; Lohrenz, T.; White, J.P.; Laxton, A.W.; Tatter, S.B.; Fleming, S.M.; Montague, P.R. Sub-second Dopamine and Serotonin Signaling in Human Striatum during Perceptual Decision-Making. Neuron 2020, 108, 999–1010.e6. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.F.; Parent, K.L.; Atcherley, C.W.; Cowen, S.L.; Heien, M.L. Differential release of dopamine in the nucleus accumbens evoked by low-versus high-frequency medial prefrontal cortex stimulation. Brain Stimulat. 2018, 11, 426–434. [Google Scholar] [CrossRef]
- Hermans, A.; Keithley, R.B.; Kita, J.M.; Sombers, L.A.; Wightman, R.M. Dopamine Detection with Fast-Scan Cyclic Voltammetry Used with Analog Background Subtraction. Anal. Chem. 2008, 80, 4040–4048. [Google Scholar] [CrossRef]
- Clark, J.J.; Sandberg, S.G.; Wanat, M.J.; Gan, J.O.; Horne, E.A.; Hart, A.S.; Akers, C.A.; Parker, J.G.; Willuhn, I.; Martinez, V.; et al. Chronic microsensors for longitudinal, subsecond dopamine detection in behaving animals. Nat. Methods 2010, 7, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Huffman, M.L.; Venton, B.J. Carbon-fiber microelectrodes for in vivo applications. Analyst 2008, 134, 18–24. [Google Scholar] [CrossRef]
- Lin, J.; Peng, Z.; Liu, Y.; Ruiz-Zepeda, F.; Ye, R.; Samuel, E.L.; Yacaman, M.J.; Yakobson, B.I.; Tour, J.M. Laser-induced porous graphene films from commercial polymers. Nat. Commun. 2014, 5, 5714. [Google Scholar] [CrossRef]
- Su, S.; Li, J.; Lee, G.C.B.; Sugden, K.; Webb, D.; Ye, H. Femtosecond laser-induced microstructures on diamond for microfluidic sensing device applications. Appl. Phys. Lett. 2013, 102, 231913. [Google Scholar] [CrossRef]
- Kononenko, V.V.; Kononenko, T.V.; Pimenov, S.M.; Sinyavskii, M.N.; Konov, V.I.; Dausinger, F. Effect of the pulse duration on graphitisation of diamond during laser ablation. Quantum Electron 2005, 35, 252–256. [Google Scholar] [CrossRef]
- Cobb, S.J.; Laidlaw, F.H.; West, G.; Wood, G.; Newton, M.E.; Beanland, R.; Macpherson, J.V. Assessment of acid and thermal oxidation treatments for removing sp2 bonded carbon from the surface of boron doped diamond. Carbon 2020, 167, 1–10. [Google Scholar] [CrossRef]
- Klauser, F.; Ghodbane, S.; Boukherroub, R.; Szunerits, S.; Steinmüller-Nethl, D.; Bertel, E.; Memmel, N. Comparison of different oxidation techniques on single-crystal and nanocrystalline diamond surfaces. Diam. Relat. Mater. 2010, 19, 474–478. [Google Scholar] [CrossRef]
- Bath, B.D.; Michael, D.J.; Trafton, B.J.; Joseph, J.D.; Runnels, P.L.; Wightman, R.M. Subsecond Adsorption and Desorption of Dopamine at Carbon-Fiber Microelectrodes. Anal. Chem. 2000, 72, 5994–6002. [Google Scholar] [CrossRef] [PubMed]
- Heien, M.L.A.V.; Phillips, P.E.M.; Stuber, G.D.; Seipel, A.T.; Wightman, R.M. Overoxidation of carbon-fiber microelectrodes enhances dopamine adsorption and increases sensitivity. Analyst 2003, 128, 1413–1419. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.J.; Collins, A.L.; Sanford, C.A.; Phillips, P.E.M. Dopamine Encoding of Pavlovian Incentive Stimuli Diminishes with Extended Training. J. Neurosci. 2013, 33, 3526–3532. [Google Scholar] [CrossRef]
- Shinoda, M.; Gattass, R.R.; Mazur, E. Femtosecond laser-induced formation of nanometer-width grooves on synthetic single-crystal diamond surfaces. J. Appl. Phys. 2009, 105, 053102. [Google Scholar] [CrossRef]
- Thompson, C.H.; Riggins, T.E.; Patel, P.R.; Chestek, C.A.; Li, W.; Purcell, E. Toward guiding principles for the design of biologically-integrated electrodes for the central nervous system. J. Neural Eng. 2020, 17, 021001. [Google Scholar] [CrossRef]
- Schwerdt, H.N.; Zhang, E.; Kim, M.J.; Yoshida, T.; Stanwicks, L.; Amemori, S.; Dagdeviren, H.E.; Langer, R.; Cima, M.J.; Graybiel, A.M. Cellular-scale probes enable stable chronic subsecond monitoring of dopamine neurochemicals in a rodent model. Commun. Biol. 2018, 1, 144. [Google Scholar] [CrossRef]
- Stiller, A.M.; Black, B.J.; Kung, C.; Ashok, A.; Cogan, S.F.; Varner, V.D.; Pancrazio, J.J. A Meta-Analysis of Intracortical Device Stiffness and Its Correlation with Histological Outcomes. Micromachines 2018, 9, 443. [Google Scholar] [CrossRef] [PubMed]
- Salatino, J.W.; Ludwig, K.A.; Kozai, T.D.Y.; Purcell, E.K. Glial responses to implanted electrodes in the brain. Nat. Biomed. Eng. 2017, 1, 862–877. [Google Scholar] [CrossRef] [PubMed]
- Kozai, T.D.Y.; Langhals, N.B.; Patel, P.R.; Deng, X.; Zhang, H.; Smith, K.L.; Lahann, J.; Kotov, N.A.; Kipke, D.R. Ultrasmall implantable composite microelectrodes with bioactive surfaces for chronic neural interfaces. Nat. Mater. 2012, 11, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Kozai, T.D.Y.; Jaquins-Gerstl, A.S.; Vazquez, A.L.; Michael, A.C.; Cui, X.T. Brain tissue responses to neural implants impact signal sensitivity and intervention strategies. ACS Chem. Neurosci. 2015, 6, 48–67. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perillo, M.L.; Gupta, B.; Siegenthaler, J.R.; Christensen, I.E.; Kepros, B.; Mitul, A.; Han, M.; Rechenberg, R.; Becker, M.F.; Li, W.; et al. Evaluation of In Vitro Serotonin-Induced Electrochemical Fouling Performance of Boron Doped Diamond Microelectrode Using Fast-Scan Cyclic Voltammetry. Biosensors 2024, 14, 352. https://doi.org/10.3390/bios14070352
Perillo ML, Gupta B, Siegenthaler JR, Christensen IE, Kepros B, Mitul A, Han M, Rechenberg R, Becker MF, Li W, et al. Evaluation of In Vitro Serotonin-Induced Electrochemical Fouling Performance of Boron Doped Diamond Microelectrode Using Fast-Scan Cyclic Voltammetry. Biosensors. 2024; 14(7):352. https://doi.org/10.3390/bios14070352
Chicago/Turabian StylePerillo, Mason L., Bhavna Gupta, James R. Siegenthaler, Isabelle E. Christensen, Brandon Kepros, Abu Mitul, Ming Han, Robert Rechenberg, Michael F. Becker, Wen Li, and et al. 2024. "Evaluation of In Vitro Serotonin-Induced Electrochemical Fouling Performance of Boron Doped Diamond Microelectrode Using Fast-Scan Cyclic Voltammetry" Biosensors 14, no. 7: 352. https://doi.org/10.3390/bios14070352




