AI-Assisted Detection of Biomarkers by Sensors and Biosensors for Early Diagnosis and Monitoring
Abstract
1. Introduction
2. Challenges in Translational Medicine, Bringing Biomedical Science into Clinical Practice
Criteria for Sensing Platforms in Translational Medicine
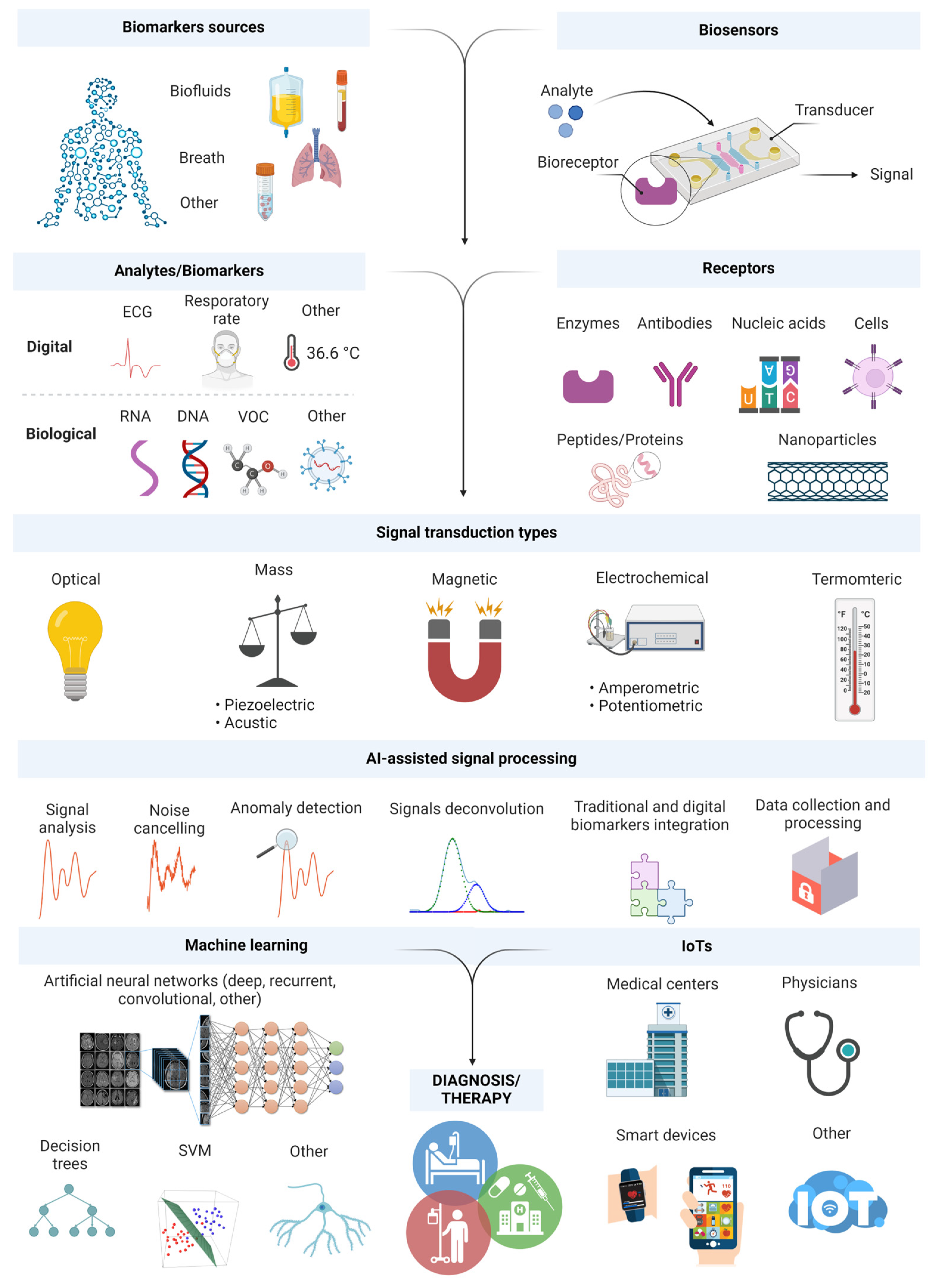
3. Monitoring and Detection of Biomarkers with Sensing Platforms
3.1. Eyes, Contact Lenses
3.2. Teeth and Mouthguard
3.3. Diapers
3.4. Wristbands, Headbands, Directly on Skin, Clothes
3.5. Face Masks
3.6. Smartphones
4. Cancer Biomarker Detection with Biosensors
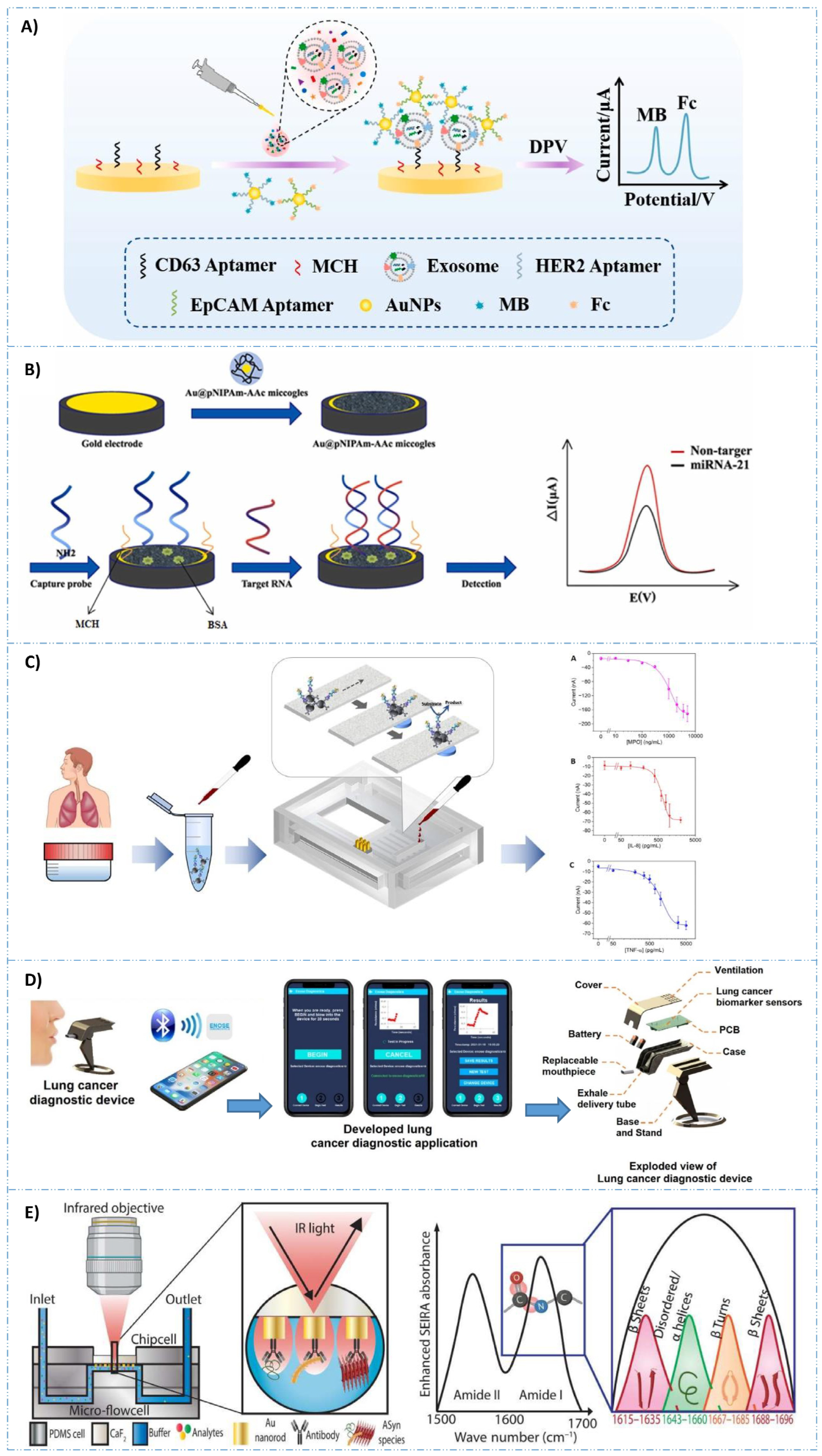
5. AI-Assisted Diagnosis
- data availability;
- model selection;
- reliability;
- deployment alternatives;
- security and privacy;
- utility and user acceptance;
- communication;
- power consumption limitations;
- storage limitations.
6. AI-Assisted Biomarker Discovery
7. Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cagney, D.N.; Sul, J.; Huang, R.Y.; Ligon, K.L.; Wen, P.Y.; Alexander, B.M. The FDA NIH Biomarkers, EndpointS, and other Tools (BEST) resource in neuro-oncology. Neuro. Oncol. 2018, 20, 1162–1172. [Google Scholar] [CrossRef] [PubMed]
- Au, R.; Kolachalama, V.B.; Paschalidis, I.C. Redefining and Validating Digital Biomarkers as Fluid, Dynamic Multi-Dimensional Digital Signal Patterns. Front. Digit. Health 2022, 3, 751629. [Google Scholar] [CrossRef] [PubMed]
- de Miguel-Perez, D.; Russo, A.; Gunasekaran, M.; Buemi, F.; Hester, L.; Fan, X.; Carter-Cooper, B.A.; Lapidus, R.G.; Peleg, A.; Arroyo-Hernández, M.; et al. Baseline extracellular vesicle TGF-β is a predictive biomarker for response to immune checkpoint inhibitors and survival in non–small cell lung cancer. Cancer 2023, 129, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Robin, J.; Harrison, J.E.; Kaufman, L.D.; Rudzicz, F.; Simpson, W.; Yancheva, M. Evaluation of Speech-Based Digital Biomarkers: Review and Recommendations. Digit. Biomark. 2020, 4, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Babrak, L.M.; Menetski, J.; Rebhan, M.; Nisato, G.; Zinggeler, M.; Brasier, N.; Baerenfaller, K.; Brenzikofer, T.; Baltzer, L.; Vogler, C.; et al. Traditional and Digital Biomarkers: Two Worlds Apart? Digit. Biomark. 2019, 3, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Tu, X.; Zhao, H.; Lin, X.; Xie, Y.; Li, S.; Wang, M. A Comparative Study of Levels of Serum Biomarkers in Patients with Different Stages of Asthma. Chin. Gen. Pract. 2022, 25, 1700–1706. [Google Scholar] [CrossRef]
- Janjusevic, M.; Fluca, A.L.; Ferro, F.; Gagno, G.; D’alessandra, Y.; Beltrami, A.P.; Sinagra, G.; Aleksova, A. Traditional and emerging biomarkers in asymptomatic left ventricular dysfunction—Promising non-coding rnas and exosomes as biomarkers in early phases of cardiac damage. Int. J. Mol. Sci. 2021, 22, 4937. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guan, S.; Guan, Y.; Tang, S.; Zhou, Y.; Wang, X.; Bi, H.; Huang, M. Novel Clinical Biomarkers for Drug-Induced Liver InjuryS. Drug Metab. Dispos. 2022, 50, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Hunter, B.; Hindocha, S.; Lee, R.W. The Role of Artificial Intelligence in Early Cancer Diagnosis. Cancers 2022, 14, 1524. [Google Scholar] [CrossRef]
- Wang, L.; Hu, Y.; Jiang, N.; Yetisen, A.K. Biosensors for psychiatric biomarkers in mental health monitoring. Biosens. Bioelectron. 2024, 256, 116242. [Google Scholar] [CrossRef]
- Mahato, K.; Wang, J. Electrochemical sensors: From the bench to the skin. Sens. Actuators B Chem. 2021, 344, 130178. [Google Scholar] [CrossRef]
- Merazzo, K.J.; Totoricaguena-Gorriño, J.; Fernández-Martín, E.; Javier Del Campo, F.; Baldrich, E. Smartphone-enabled personalized diagnostics: Current status and future prospects. Diagnostics 2021, 11, 1067. [Google Scholar] [CrossRef] [PubMed]
- Mueller, B.; Kinoshita, T.; Peebles, A.; Graber, M.A.; Lee, S. Artificial intelligence and machine learning in emergency medicine: A narrative review. Acute Med. Surg. 2022, 9, e740. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S. Artificial Intelligence: A Review of Progress and Prospects in Medicine and Healthcare. J. Electron. Electromed. Eng. Med. Inform. 2022, 4, 1–23. [Google Scholar] [CrossRef]
- Sohrabi, H.; Bolandi, N.; Hemmati, A.; Eyvazi, S.; Ghasemzadeh, S.; Baradaran, B.; Oroojalian, F.; Reza Majidi, M.; de la Guardia, M.; Mokhtarzadeh, A. State-of-the-art cancer biomarker detection by portable (Bio) sensing technology: A critical review. Microchem. J. 2022, 177, 107248. [Google Scholar] [CrossRef]
- Mitchell, K.R.; Esene, J.E.; Woolley, A.T. Advances in multiplex electrical and optical detection of biomarkers using microfluidic devices. Anal. Bioanal. Chem. 2022, 414, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Macovei, D.G.; Irimes, M.B.; Hosu, O.; Cristea, C.; Tertis, M. Point-of-care electrochemical testing of biomarkers involved in inflammatory and inflammatory-associated medical conditions. Anal. Bioanal. Chem. 2023, 415, 1033–1063. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.S.; Dias, S.B.; Jelinek, H.F.; Hadjileontiadis, L.J.; Pappa, A.-M. The convergence of traditional and digital biomarkers through AI-assisted biosensing: A new era in translational diagnostics? Biosens. Bioelectron. 2023, 235, 115387. [Google Scholar] [CrossRef] [PubMed]
- Mikdadi, D.; O’connell, K.A.; Meacham, P.J.; Dugan, M.A.; Ojiere, M.O.; Carlson, T.B.; Klenk, J.A. Applications of artificial intelligence (AI) in ovarian cancer, pancreatic cancer, and image biomarker discovery. Cancer Biomark. 2022, 33, 173–184. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L. Translational Challenges in Psychedelic Medicine. N. Engl. J. Med. 2023, 388, 476–477. [Google Scholar] [CrossRef]
- Maiti, K.S. Non-Invasive Disease Specific Biomarker Detection Using Infrared Spectroscopy: A Review. Molecules 2023, 28, 2320. [Google Scholar] [CrossRef] [PubMed]
- Iwaya, L.H.; Babar, M.A.; Rashid, A.; Wijayarathna, C. On the privacy of mental health apps: An empirical investigation and its implications for app development. Empir. Softw. Eng. 2023, 28, 2. [Google Scholar] [CrossRef]
- Piau, A.; Wild, K.; Mattek, N.; Kaye, J. Current state of digital biomarker technologies for real-life, home-based monitoring of cognitive function for mild cognitive impairment to mild Alzheimer disease and implications for clinical care: Systematic review. J. Med. Internet Res. 2019, 21, e12785. [Google Scholar] [CrossRef]
- Budelier, M.M.; Bateman, R.J. Biomarkers of Alzheimer Disease. J. Appl. Lab. Med. 2020, 5, 194–208. [Google Scholar] [CrossRef] [PubMed]
- Nicosia, J.; Aschenbrenner, A.J.; Balota, D.A.; Sliwinski, M.J.; Tahan, M.; Adams, S.; Stout, S.H.; Wilks, H.M.; Gordon, B.A.; Benzinger, T.L.S.; et al. Reliability, validity, and feasibility of a smartphone-based cognitive assessment for preclinical Alzheimer disease. Alzheimer’s Dement. 2023, 19, e063363. [Google Scholar] [CrossRef]
- van Dam, J.; Wright, J.; Jones, G. The Convergence of Digital Health Technologies: The Role of Digital Therapeutics in the Future Healthcare System. In Digital Therapeutics: Strategic, Scientific, Developmental, and Regulatory Aspects; Routledge: London, UK, 2022; pp. 351–372. ISBN 9781000799231. [Google Scholar]
- Orbay, S. Molecularly Imprinted Polymeric Particles Created Using Droplet-Based Microfluidics: Preparation and Applications. Micromachines 2023, 14, 763. [Google Scholar] [CrossRef] [PubMed]
- Noor, J.; Chaudhry, A.; Batool, S. Microfluidic Technology, Artificial Intelligence, and Biosensors As Advanced Technologies in Cancer Screening: A Review Article. Cureus 2023, 15, e39634. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.H.; Lee, J.K.; Kim, J.H.; Choi, H.S.; Kim, J.; Lee, S.H.; Lee, H.Y. Single Microfluidic Electrochemical Sensor System for Simultaneous Multi-Pulmonary Hypertension Biomarker Analyses. Sci. Rep. 2017, 7, 7545. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, C.; Wang, P.; Wang, C.; Zhang, Y.; Han, L. A high-performance microfluidic detection platform to conduct a novel multiple-biomarker panel for ovarian cancer screening. RSC Adv. 2021, 11, 8124–8133. [Google Scholar] [CrossRef]
- Zhang, H.; Qiu, Y.; Yu, S.; Ding, C.; Hu, J.; Qi, H.; Tian, Y.; Zhang, Z.; Liu, A.; Wu, H. Wearable microfluidic patch with integrated capillary valves and pumps for sweat management and multiple biomarker analysis. Biomicrofluidics 2022, 16, 044104. [Google Scholar] [CrossRef]
- Prabowo, B.A.; Cabral, P.D.; Freitas, P.; Fernandes, E. The challenges of developing biosensors for clinical assessment: A review. Chemosensors 2021, 9, 299. [Google Scholar] [CrossRef]
- Vaz, R.; Frasco, M.F.; Sales, M.G.F. Biosensors: Concept and importance in point-of-care disease diagnosis. In Biosensor Based Advanced Cancer Diagnostics: From Lab to Clinics; Academic Press: Cambridge, MA, USA, 2021; pp. 59–84. ISBN 9780128234242. [Google Scholar]
- Kosack, C.S.; Page, A.L.; Klatser, P.R. A guide to aid the selection of diagnostic tests. Bull. World Health Organ. 2017, 95, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Baryeh, K.; Takalkar, S.; Lund, M.; Liu, G. Introduction to medical biosensors for point of care applications. In Medical Biosensors for Point of Care (POC) Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 3–25. ISBN 9780081000786. [Google Scholar]
- Polizzi, K.M. Biosensors. In Comprehensive Biotechnology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 572–584. ISBN 9780444640475. [Google Scholar]
- Wasilewski, T.; Brito, N.F.; Szulczyński, B.; Wojciechowski, M.; Buda, N.; Melo, A.C.A.; Kamysz, W.; Gębicki, J. Olfactory receptor-based biosensors as potential future tools in medical diagnosis. TrAC Trends Anal. Chem. 2022, 150, 116599. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Mishra, R.K.; Martín, A.; Tang, G.; Nakagawa, T.; Lu, X.; Campbell, A.S.; Lyu, K.M.; Wang, J. Wearable Ring-Based Sensing Platform for Detecting Chemical Threats. ACS Sens. 2017, 2, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Askari, H.; Xu, N.; Groenner Barbosa, B.H.; Huang, Y.; Chen, L.; Khajepour, A.; Chen, H.; Wang, Z.L. Intelligent systems using triboelectric, piezoelectric, and pyroelectric nanogenerators. Mater. Today 2022, 52, 188–206. [Google Scholar] [CrossRef]
- Macchia, E.; Kovács-Vajna, Z.M.; Loconsole, D.; Sarcina, L.; Redolfi, M.; Chironna, M.; Torricelli, F.; Torsi, L. A handheld intelligent single-molecule binary bioelectronic system for fast and reliable immunometric point-of-care testing. Sci. Adv. 2022, 8, eabo0881. [Google Scholar] [CrossRef] [PubMed]
- Al-Dayyeni, W.S.; Al-Yousif, S.; Taher, M.M.; Al-Faouri, A.W.; Tahir, N.M.D.; Jaber, M.M.; Ghabban, F.; Najm, I.A.; Alfadli, I.M.; Ameerbakhsh, O.Z.; et al. A review on electronic nose: Coherent taxonomy, classification, motivations, challenges, recommendations and datasets. IEEE Access 2021, 9, 88535–88551. [Google Scholar] [CrossRef]
- Faham, S.; Salimi, A.; Ghavami, R. Electrochemical-based remote biomarker monitoring: Toward Internet of Wearable Things in telemedicine. Talanta 2023, 253, 123892. [Google Scholar] [CrossRef] [PubMed]
- Phan, D.T.; Nguyen, C.H.; Nguyen, T.D.P.; Tran, L.H.; Park, S.; Choi, J.; Lee, B.; Oh, J. A Flexible, Wearable, and Wireless Biosensor Patch with Internet of Medical Things Applications. Biosensors 2022, 12, 139. [Google Scholar] [CrossRef]
- Polat, E.O.; Cetin, M.M.; Tabak, A.F.; Güven, E.B.; Uysal, B.Ö.; Arsan, T.; Kabbani, A.; Hamed, H.; Gül, S.B. Transducer Technologies for Biosensors and Their Wearable Applications. Biosensors 2022, 12, 385. [Google Scholar] [CrossRef]
- Bhardwaj, V.; Kaushik, A. Biomedical applications of nanotechnology and nanomaterials. Micromachines 2017, 8, 298. [Google Scholar] [CrossRef] [PubMed]
- Medina Cruz, D.; Mostafavi, E.; Vernet-Crua, A.; Barabadi, H.; Shah, V.; Cholula-Díaz, J.L.; Guisbiers, G.; Webster, T.J. Green nanotechnology-based zinc oxide (ZnO) nanomaterials for biomedical applications: A review. J. Phys. Mater. 2020, 3, 34005. [Google Scholar] [CrossRef]
- Palanica, A.; Docktor, M.J.; Lieberman, M.; Fossat, Y. The Need for Artificial Intelligence in Digital Therapeutics. Digit. Biomark. 2020, 4, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Cranfield, B.M.; Koo, M.M.; Abel, G.A.; Swann, R.; McPhail, S.; Rubin, G.P.; Lyratzopoulos, G. Primary care blood tests before cancer diagnosis: National Cancer Diagnosis Audit data. Br. J. Gen. Pract. 2023, 73, E95–E103. [Google Scholar] [CrossRef] [PubMed]
- Baxi, V.; Edwards, R.; Montalto, M.; Saha, S. Digital pathology and artificial intelligence in translational medicine and clinical practice. Mod. Pathol. 2022, 35, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Kather, J.N. Artificial intelligence in oncology: Chances and pitfalls. J. Cancer Res. Clin. Oncol. 2023, 149, 7995–7996. [Google Scholar] [CrossRef] [PubMed]
- Cova, C.M.; Rincón, E.; Espinosa, E.; Serrano, L.; Zuliani, A. Paving the Way for a Green Transition in the Design of Sensors and Biosensors for the Detection of Volatile Organic Compounds (VOCs). Biosensors 2022, 12, 51. [Google Scholar] [CrossRef] [PubMed]
- Rovira, M.; Lafaye, C.; Demuru, S.; Kunnel, B.P.; Aymerich, J.; Cuenca, J.; Serra-Graells, F.; Margarit-Taulé, J.M.; Haque, R.; Saubade, M.; et al. Assessing the performance of a robust multiparametric wearable patch integrating silicon-based sensors for real-time continuous monitoring of sweat biomarkers. Biosens. Bioelectron. 2024, 262, 116560. [Google Scholar] [CrossRef] [PubMed]
- Kukkar, D.; Zhang, D.; Jeon, B.H.; Kim, K.H. Recent advances in wearable biosensors for non-invasive monitoring of specific metabolites and electrolytes associated with chronic kidney disease: Performance evaluation and future challenges. TrAC Trends Anal. Chem. 2022, 150, 116570. [Google Scholar] [CrossRef]
- Nolan, J.K.; Nguyen, T.N.H.; Le, K.V.H.; DeLong, L.E.; Lee, H. Simple Fabrication of Flexible Biosensor Arrays Using Direct Writing for Multianalyte Measurement from Human Astrocytes. SLAS Technol. 2020, 25, 33–46. [Google Scholar] [CrossRef]
- Gao, S.; Li, Q.; Zhang, S.; Sun, X.; Zhou, H.; Wang, Z.; Wu, J. A novel biosensing platform for detection of glaucoma biomarker GDF15 via an integrated BLI-ELASA strategy. Biomaterials 2023, 294, 121997. [Google Scholar] [CrossRef] [PubMed]
- Askarian, B.; Ho, P.; Chong, J.W. Detecting Cataract Using Smartphones. IEEE J. Transl. Eng. Health Med. 2021, 9, 3800110. [Google Scholar] [CrossRef] [PubMed]
- Moreddu, R.; Elsherif, M.; Adams, H.; Moschou, D.; Cordeiro, M.F.; Wolffsohn, J.S.; Vigolo, D.; Butt, H.; Cooper, J.M.; Yetisen, A.K. Integration of paper microfluidic sensors into contact lenses for tear fluid analysis. Lab Chip 2020, 20, 3970–3979. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, S.; Chen, C.; Alifu, N.; Zhang, X.; Du, J.; Li, C.; Xu, L.; Wang, L.; Dong, B. Opal photonic crystal-enhanced upconversion turn-off fluorescent immunoassay for salivary CEA with oral cancer. Talanta 2023, 258, 124435. [Google Scholar] [CrossRef] [PubMed]
- Vellappally, S.; Al Kheraif, A.A.; Anil, S.; Wahba, A.A. IoT medical tooth mounted sensor for monitoring teeth and food level using bacterial optimization along with adaptive deep learning neural network. Meas. J. Int. Meas. Confed. 2019, 135, 672–677. [Google Scholar] [CrossRef]
- Arakawa, T.; Tomoto, K.; Nitta, H.; Toma, K.; Takeuchi, S.; Sekita, T.; Minakuchi, S.; Mitsubayashi, K. A Wearable Cellulose Acetate-Coated Mouthguard Biosensor for in Vivo Salivary Glucose Measurement. Anal. Chem. 2020, 92, 12201–12207. [Google Scholar] [CrossRef]
- Su, H.; Sun, F.; Lu, Z.; Zhang, J.; Zhang, W.; Liu, J. A wearable sensing system based on smartphone and diaper to detect urine in-situ for patients with urinary incontinence. Sens. Actuators B Chem. 2022, 357, 131459. [Google Scholar] [CrossRef]
- Kim, H.; Park, S.; Jeong, I.G.; Song, S.H.; Jeong, Y.; Kim, C.S.; Lee, K.H. Noninvasive Precision Screening of Prostate Cancer by Urinary Multimarker Sensor and Artificial Intelligence Analysis. ACS Nano 2021, 15, 4054–4065. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.P.; Ha, G.; Wright, D.E.; Ma, Y.; Sen-Gupta, E.; Haubrich, N.R.; Branche, P.C.; Li, W.; Huppert, G.L.; Johnson, M.; et al. Highly flexible, wearable, and disposable cardiac biosensors for remote and ambulatory monitoring. npj Digit. Med. 2018, 1, 2. [Google Scholar] [CrossRef]
- Dou, W.; Daoud, A.; Chen, X.; Wang, T.; Malhi, M.; Gong, Z.; Mirshafiei, F.; Zhu, M.; Shan, G.; Huang, X.; et al. Ultrathin and Flexible Bioelectronic Arrays for Functional Measurement of iPSC-Cardiomyocytes under Cardiotropic Drug Administration and Controlled Microenvironments. Nano Lett. 2023, 23, 2321–2331. [Google Scholar] [CrossRef]
- Kim, T.; Park, I. Skin-interfaced Wearable Biosensors: A Mini-Review. J. Sens. Sci. Technol. 2022, 31, 71–78. [Google Scholar] [CrossRef]
- Zeng, X.; Peng, R.; Fan, Z.; Lin, Y. Self-powered and wearable biosensors for healthcare. Mater. Today Energy 2022, 23, 100900. [Google Scholar] [CrossRef]
- Xia, H.Q.; Tang, H.; Zhou, B.; Li, Y.; Zhang, X.; Shi, Z.; Deng, L.; Song, R.; Li, L.; Zhang, Z.; et al. Mediator-free electron-transfer on patternable hierarchical meso/macro porous bienzyme interface for highly-sensitive sweat glucose and surface electromyography monitoring. Sens. Actuators B Chem. 2020, 312, 127962. [Google Scholar] [CrossRef]
- Chidambaram, S.; Maheswaran, Y.; Patel, K.; Sounderajah, V.; Hashimoto, D.A.; Seastedt, K.P.; McGregor, A.H.; Markar, S.R.; Darzi, A. Using Artificial Intelligence-Enhanced Sensing and Wearable Technology in Sports Medicine and Performance Optimisation. Sensors 2022, 22, 6920. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Zhao, W.; Zhang, Y.; Jiang, Q.; He, J.H.; Baeumner, A.J.; Wolfbeis, O.S.; Wang, Z.L.; Salama, K.N.; Alshareef, H.N. A MXene-Based Wearable Biosensor System for High-Performance In Vitro Perspiration Analysis. Small 2019, 15, 1901190. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Haghniaz, R.; Hartel, M.C.; Guan, S.; Bahari, J.; Li, Z.; Baidya, A.; Cao, K.; Gao, X.; Li, J.; et al. A Breathable, Passive-Cooling, Non-Inflammatory, and Biodegradable Aerogel Electronic Skin for Wearable Physical-Electrophysiological-Chemical Analysis. Adv. Mater. 2023, 35, 2209300. [Google Scholar] [CrossRef]
- Jin, X.; Li, G.; Xu, T.; Su, L.; Yan, D.; Zhang, X. Fully integrated flexible biosensor for wearable continuous glucose monitoring. Biosens. Bioelectron. 2022, 196, 113760. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.Q.; Soenksen, L.R.; Donghia, N.M.; Angenent-Mari, N.M.; de Puig, H.; Huang, A.; Lee, R.; Slomovic, S.; Galbersanini, T.; Lansberry, G.; et al. Wearable materials with embedded synthetic biology sensors for biomolecule detection. Nat. Biotechnol. 2021, 39, 1366–1374. [Google Scholar] [CrossRef]
- Daniels, J.; Wadekar, S.; DeCubellis, K.; Jackson, G.W.; Chiu, A.S.; Pagneux, Q.; Saada, H.; Engelmann, I.; Ogiez, J.; Loze-Warot, D.; et al. A mask-based diagnostic platform for point-of-care screening of COVID-19. Biosens. Bioelectron. 2021, 192, 113486. [Google Scholar] [CrossRef]
- Zazzo, L.D.; Magna, G.; Lucentini, M.; Stefanelli, M.; Paolesse, R.; Natale, C. Di Sensor-Embedded Face Masks for Detection of Volatiles in Breath: A Proof of Concept Study. Chemosensors 2021, 9, 356. [Google Scholar] [CrossRef]
- Rezazadeh, M.; Seidi, S.; Lid, M.; Pedersen-Bjergaard, S.; Yamini, Y. The modern role of smartphones in analytical chemistry. TrAC Trends Anal. Chem. 2019, 118, 548–555. [Google Scholar] [CrossRef]
- Torous, J.; Rodriguez, J.; Powell, A. The new digital divide for digital biomarkers. Digit. Biomark. 2017, 1, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Firdaus, M.L.; Saputra, E.; Ginting, S.M.; Wyantuti, S.; Eddy, D.R.; Rahmidar, L.; Yuliarto, B. Smartphone-based digital image colorimetry for non-enzymatic detection of glucose using gold nanoparticles. Sens. Bio-Sens. Res. 2022, 35, 100472. [Google Scholar] [CrossRef]
- Al-Kassawneh, M.; Sadiq, Z.; Jahanshahi-Anbuhi, S. Pullulan-stabilized gold nanoparticles tablet as a nanozyme sensor for point-of-care applications. Sens. Bio-Sens. Res. 2022, 38, 100526. [Google Scholar] [CrossRef]
- Jeon, H.J.; Kim, H.S.; Chung, E.; Lee, D.Y. Nanozyme-based colorimetric biosensor with a systemic quantification algorithm for noninvasive glucose monitoring. Theranostics 2022, 12, 6308–6338. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Gao, W.; Yin, J.; Fan, W.; Wang, Z.; Hu, K.; Mai, Y.; Luan, A.; Xu, B.; Jin, Q. A high-precision thermometry microfluidic chip for real-time monitoring of the physiological process of live tumour cells. Talanta 2021, 226, 122101. [Google Scholar] [CrossRef] [PubMed]
- Ngan Ngo, T.K.; Kuo, C.H.; Tu, T.Y. Recent advances in microfluidic-based cancer immunotherapy-on-a-chip strategies. Biomicrofluidics 2023, 17, 011501. [Google Scholar] [CrossRef] [PubMed]
- Koh, D.-M.; Papanikolaou, N.; Bick, U.; Illing, R.; Kahn, C.E.; Kalpathi-Cramer, J.; Matos, C.; Martí-Bonmatí, L.; Miles, A.; Mun, S.K.; et al. Artificial intelligence and machine learning in cancer imaging. Commun. Med. 2022, 2, 133. [Google Scholar] [CrossRef] [PubMed]
- Raji, H.; Tayyab, M.; Sui, J.; Mahmoodi, S.R.; Javanmard, M. Biosensors and machine learning for enhanced detection, stratification, and classification of cells: A review. Biomed. Microdevices 2022, 24, 26. [Google Scholar] [CrossRef]
- Gupta, A.; Harrison, P.J.; Wieslander, H.; Pielawski, N.; Kartasalo, K.; Partel, G.; Solorzano, L.; Suveer, A.; Klemm, A.H.; Spjuth, O.; et al. Deep Learning in Image Cytometry: A Review. Cytom. Part A 2019, 95, 366–380. [Google Scholar] [CrossRef]
- Riordon, J.; Sovilj, D.; Sanner, S.; Sinton, D.; Young, E.W.K. Deep Learning with Microfluidics for Biotechnology. Trends Biotechnol. 2019, 37, 310–324. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Bhattacharya, S.; Butte, A.J. Application of Machine Learning for Cytometry Data. Front. Immunol. 2022, 12, 787574. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.R.; Ahommed, M.S.; Daizy, M.; Bacchu, M.S.; Ali, M.R.; Al-Mamun, M.R.; Saad Aly, M.A.; Khan, M.Z.H.; Hossain, S.I. Recent development in electrochemical biosensors for cancer biomarkers detection. Biosens. Bioelectron. X 2021, 8, 100075. [Google Scholar] [CrossRef]
- Kaur, B.; Kumar, S.; Kaushik, B.K. Recent advancements in optical biosensors for cancer detection. Biosens. Bioelectron. 2022, 197, 113805. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Xiong, H.; Zhou, Y.; Chen, X.; Yang, W. Tracking epithelial-mesenchymal transition in breast cancer cells based on a multiplex electrochemical immunosensor. Biosens. Bioelectron. 2024, 258, 116372. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Yue, Y.; Zhang, Y.; Zhang, Z.; Zhou, H.S. Advancing Biosensors with Machine Learning. ACS Sens. 2020, 5, 3346–3364. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.A.; Li, R.; Tse, Z.T.H. Reshaping healthcare with wearable biosensors. Sci. Rep. 2023, 13, 4998. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Ta, Q.T.H.; Nguyen, T.K.O.; Nguyen, T.T.D.; Vo, V.G. Role of body-fluid biomarkers in Alzheimer’s disease diagnosis. Diagnostics 2020, 10, 326. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Luna, C.; Torres, C.; Ortiz, R.; Dieguez, C.; Martinez-Galan, J.; Melguizo, C.; Prados, J.C.; Caba, O. Proteomic biomarkers in body fluids associated with pancreatic cancer. Oncotarget 2018, 9, 16573–16587. [Google Scholar] [CrossRef]
- Mukherjee, A.; Pednekar, C.B.; Kolke, S.S.; Kattimani, M.; Duraisamy, S.; Burli, A.R.; Gupta, S.; Srivastava, S. Insights on Proteomics-Driven Body Fluid-Based Biomarkers of Cervical Cancer. Proteomes 2022, 10, 13. [Google Scholar] [CrossRef]
- Yang, T.; Yang, Q.; Zhou, Y.; Wen, C. Glucose trend prediction model based on improved wavelet transform and gated recurrent unit. Math. Biosci. Eng. 2023, 20, 17037–17056. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Cai, A.; Xu, T.; Zhang, X. Artificial intelligence biosensors for continuous glucose monitoring. Interdiscip. Mater. 2023, 2, 290–307. [Google Scholar] [CrossRef]
- Rollo, F.; Bachechi, C.; Po, L. Anomaly Detection and Repairing for Improving Air Quality Monitoring. Sensors 2023, 23, 640. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; He, T.; Zhou, H.; Zhang, Z.; Lee, C. Artificial intelligence enhanced sensors—Enabling technologies to next-generation healthcare and biomedical platform. Bioelectron. Med. 2023, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Khatib, O.; Ren, S.; Malof, J.; Padilla, W.J. Learning the Physics of All-Dielectric Metamaterials with Deep Lorentz Neural Networks. Adv. Opt. Mater. 2022, 10, 202200097. [Google Scholar] [CrossRef]
- Hollon, T.C.; Pandian, B.; Adapa, A.R.; Urias, E.; Save, A.V.; Khalsa, S.S.S.; Eichberg, D.G.; D’Amico, R.S.; Farooq, Z.U.; Lewis, S.; et al. Near real-time intraoperative brain tumor diagnosis using stimulated Raman histology and deep neural networks. Nat. Med. 2020, 26, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Lussier, F.; Missirlis, D.; Spatz, J.P.; Masson, J.F. Machine-Learning-Driven Surface-Enhanced Raman Scattering Optophysiology Reveals Multiplexed Metabolite Gradients Near Cells. ACS Nano 2019, 13, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yao, T.; Zhu, S.; El Saddik, A. Deep learning-based multimedia analytics: A review. ACM Trans. Multimed. Comput. Commun. Appl. 2019, 15, 1–26. [Google Scholar] [CrossRef]
- Augustine, P. The Industry Use Cases for the Digital Twin Idea. In Advances in Computers; Elsevier: Amsterdam, The Netherlands, 2020; Volume 117, pp. 79–105. ISBN 9780128187562. [Google Scholar]
- Angulo, C.; Gonzalez-Abril, L.; Raya, C.; Ortega, J.A. A Proposal to Evolving Towards Digital Twins in Healthcare. In Lecture Notes in Computer Science (Including Subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics); LNBI; Springer: Cham, Switzerland, 2020; Volume 12108, pp. 418–426. ISBN 9783030453848. [Google Scholar]
- Laubenbacher, R.; Sluka, J.P.; Glazier, J.A. Using digital twins in viral infection. Science 2021, 371, 1105–1106. [Google Scholar] [CrossRef]
- Kim, J.H.; Suh, Y.J.; Park, D.; Yim, H.; Kim, H.; Kim, H.J.; Yoon, D.S.; Hwang, K.S. Technological advances in electrochemical biosensors for the detection of disease biomarkers. Biomed. Eng. Lett. 2021, 11, 309–334. [Google Scholar] [CrossRef]
- Jarahi Khameneh, A.; Rahimi, S.H.; Abbas, M.; Rahimi, S.; Mehmandoust, S.; Rastgoo, A.; Heydarian, A.; Eskandari, V. Trends in electrochemical biosensors for the early diagnosis of breast cancer through the detection of relevant biomarkers. Chem. Phys. Impact 2024, 8, 100425. [Google Scholar] [CrossRef]
- Sinha, K.; Uddin, Z.; Kawsar, H.I.; Islam, S.; Deen, M.J.; Howlader, M.M.R. Analyzing chronic disease biomarkers using electrochemical sensors and artificial neural networks. TrAC Trends Anal. Chem. 2023, 158, 116861. [Google Scholar] [CrossRef]
- Chu, S.S.; Nguyen, H.A.; Zhang, J.; Tabassum, S.; Cao, H. Towards Multiplexed and Multimodal Biosensor Platforms in Real-Time Monitoring of Metabolic Disorders. Sensors 2022, 22, 5200. [Google Scholar] [CrossRef]
- Beduk, T.; Beduk, D.; Hasan, M.R.; Guler Celik, E.; Kosel, J.; Narang, J.; Salama, K.N.; Timur, S. Smartphone-Based Multiplexed Biosensing Tools for Health Monitoring. Biosensors 2022, 12, 583. [Google Scholar] [CrossRef]
- Hou, Z.; Zheng, J.; Zhang, C.; Li, T.; Chen, D.; Hu, L.; Hu, J.; Xiong, B.; Ye, H.; Jaffrezic-Renault, N.; et al. Direct ultrasensitive electrochemical detection of breast cancer biomarker-miRNA-21 employing an aptasensor based on a microgel nanoparticle composite. Sens. Actuators B Chem. 2022, 367, 132067. [Google Scholar] [CrossRef]
- Zhang, M.; Xia, L.; Mei, W.; Zou, Q.; Liu, H.; Wang, H.; Zou, L.; Wang, Q.; Yang, X.; Wang, K. One-step multiplex analysis of breast cancer exosomes using an electrochemical strategy assisted by gold nanoparticles. Anal. Chim. Acta 2023, 1254, 341130. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Capitán, M.; Sanchís, A.; Carvalho, E.O.; Baldi, A.; Vilaplana, L.; Cardoso, V.F.; Calleja, Á.; Wei, M.; de la Rica, R.; Hoyo, J.; et al. Engineering a Point-of-Care Paper-Microfluidic Electrochemical Device Applied to the Multiplexed Quantitative Detection of Biomarkers in Sputum. ACS Sens. 2023, 8, 3032–3042. [Google Scholar] [CrossRef]
- Emam, S.; Nasrollahpour, M.; Allen, J.P.; He, Y.; Hussein, H.; Shah, H.S.; Tavangarian, F.; Sun, N.X. A handheld electronic device with the potential to detect lung cancer biomarkers from exhaled breath. Biomed. Microdevices 2022, 24, 41. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Lee, K.K.; Kang, M.S.; Shin, D.M.; Oh, J.W.; Lee, C.S.; Han, D.W. Artificial olfactory sensor technology that mimics the olfactory mechanism: A comprehensive review. Biomater. Res. 2022, 26, 40. [Google Scholar] [CrossRef]
- Sarhadi, V.K.; Armengol, G. Molecular Biomarkers in Cancer. Biomolecules 2022, 12, 1021. [Google Scholar] [CrossRef]
- Hu, J.; Hu, N.; Pan, D.; Zhu, Y.; Jin, X.; Wu, S.; Lu, Y. Smell cancer by machine learning-assisted peptide/MXene bioelectronic array. Biosens. Bioelectron. 2024, 262, 116562. [Google Scholar] [CrossRef] [PubMed]
- Keith, J.A.; Vassilev-Galindo, V.; Cheng, B.; Chmiela, S.; Gastegger, M.; Müller, K.R.; Tkatchenko, A. Combining Machine Learning and Computational Chemistry for Predictive Insights into Chemical Systems. Chem. Rev. 2021, 121, 9816–9872. [Google Scholar] [CrossRef] [PubMed]
- Lansford, J.L.; Vlachos, D.G. Infrared spectroscopy data- and physics-driven machine learning for characterizing surface microstructure of complex materials. Nat. Commun. 2020, 11, 1513. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Xu, Z.; Kamran, M.; Zinchik, S.; Paheding, S.; McDonald, A.G.; Bar-Ziv, E.; Zavala, V.M. Using ATR-FTIR spectra and convolutional neural networks for characterizing mixed plastic waste. Comput. Chem. Eng. 2021, 155, 107547. [Google Scholar] [CrossRef]
- Lowe, M.; Qin, R.; Mao, X. A Review on Machine Learning, Artificial Intelligence, and Smart Technology in Water Treatment and Monitoring. Water 2022, 14, 1384. [Google Scholar] [CrossRef]
- Tseng, Y.J.; Chuang, P.J.; Appell, M. When Machine Learning and Deep Learning Come to the Big Data in Food Chemistry. ACS Omega 2023, 8, 15854–15864. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Zhang, Z.; Wei, J.; Dong, B.; Lee, C. Wavelength-multiplexed hook nanoantennas for machine learning enabled mid-infrared spectroscopy. Nat. Commun. 2022, 13, 3859. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Cadusch, J.J.; Crozier, K.B. Plasmonic Mid-Infrared Filter Array-Detector Array Chemical Classifier Based on Machine Learning. ACS Photonics 2021, 8, 648–657. [Google Scholar] [CrossRef]
- Shimizu, F.M.; de Barros, A.; Braunger, M.L.; Gaal, G.; Riul, A. Information visualization and machine learning driven methods for impedimetric biosensing. TrAC Trends Anal. Chem. 2023, 165, 117115. [Google Scholar] [CrossRef]
- Feldmann, C.; Yonchev, D.; Bajorath, J. Analysis of biological screening compounds with single-or multi-target activity via diagnostic machine learning. Biomolecules 2020, 10, 1605. [Google Scholar] [CrossRef]
- Kelp, G.; Arju, N.; Lee, A.; Esquivel, E.; Delgado, R.; Yu, Y.; Dutta-Gupta, S.; Sokolov, K.; Shvets, G. Application of metasurface-enhanced infra-red spectroscopy to distinguish between normal and cancerous cell types. Analyst 2019, 144, 1115–1127. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, X.; Cao, X.; Huang, C.; Liu, E.; Qian, S.; Liu, X.; Wu, Y.; Dong, F.; Qiu, C.W.; et al. Artificial intelligence: A powerful paradigm for scientific research. Innovation 2021, 2, 100179. [Google Scholar] [CrossRef] [PubMed]
- John-Herpin, A.; Kavungal, D.; von Mücke, L.; Altug, H. Infrared Metasurface Augmented by Deep Learning for Monitoring Dynamics between All Major Classes of Biomolecules. Adv. Mater. 2021, 33, 2006054. [Google Scholar] [CrossRef] [PubMed]
- Kavungal, D.; Magalhães, P.; Kumar, S.T.; Kolla, R.; Lashuel, H.A.; Altug, H. Artificial intelligence-coupled plasmonic infrared sensor for detection of structural protein biomarkers in neurodegenerative diseases. Sci. Adv. 2023, 9, eadg9644. [Google Scholar] [CrossRef] [PubMed]
- Talens, J.B.; Pelegri-Sebastia, J.; Sogorb, T.; Ruiz, J.L. Prostate cancer detection using e-nose and AI for high probability assessment. BMC Med. Inform. Decis. Mak. 2023, 23, 205. [Google Scholar] [CrossRef] [PubMed]
- Wojnowski, W.; Kalinowska, K. Machine Learning and Electronic Noses for Medical Diagnostics. In Artificial Intelligence in Medicine; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–17. ISBN 9783030580803. [Google Scholar]
- Liu, T.; Guo, L.; Wang, M.; Su, C.; Wang, D.; Dong, H.; Chen, J.; Wu, W. Review on Algorithm Design in Electronic Noses: Challenges, Status, and Trends. Intell. Comput. 2023, 2, 12. [Google Scholar] [CrossRef]
- Zniber, M.; Vahdatiyekta, P.; Huynh, T.P. Analysis of urine using electronic tongue towards non-invasive cancer diagnosis. Biosens. Bioelectron. 2023, 219, 114810. [Google Scholar] [CrossRef] [PubMed]
- Olivier, M.; Asmis, R.; Hawkins, G.A.; Howard, T.D.; Cox, L.A. The Need for Multi-Omics Biomarker Signatures in Precision Medicine. Int. J. Mol. Sci. 2019, 20, 4781. [Google Scholar] [CrossRef] [PubMed]
- Campuzano, S.; Barderas, R.; Yáñez-Sedeño, P.; Pingarrón, J.M. Electrochemical biosensing to assist multiomics analysis in precision medicine. Curr. Opin. Electrochem. 2021, 28, 100703. [Google Scholar] [CrossRef]
- Kokabi, M.; Tahir, M.N.; Singh, D.; Javanmard, M. Advancing Healthcare: Synergizing Biosensors and Machine Learning for Early Cancer Diagnosis. Biosensors 2023, 13, 884. [Google Scholar] [CrossRef]
- Taheri, M.; Deen, I.A.; Packirisamy, M.; Deen, M.J. Metal Oxide -Based Electrical/electrochemical Sensors for Health Monitoring Systems. TrAC Trends Anal. Chem. 2024, 171, 117509. [Google Scholar] [CrossRef]
- Yu, A.; Zhu, M.; Chen, C.; Li, Y.; Cui, H.; Liu, S.; Zhao, Q. Implantable Flexible Sensors for Health Monitoring. Adv. Healthc. Mater. 2024, 13, 2302460. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, Y.; Jiang, N.; Yetisen, A.K. Wearable artificial intelligence biosensor networks. Biosens. Bioelectron. 2023, 219, 114825. [Google Scholar] [CrossRef] [PubMed]
- Dai, B.; Gao, C.; Xie, Y. Flexible wearable devices for intelligent health monitoring. View 2022, 3, 20220027. [Google Scholar] [CrossRef]
- Göndöcs, D.; Dörfler, V. AI in medical diagnosis: AI prediction & human judgment. Artif. Intell. Med. 2024, 149, 102769. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Cheng, F.; Xu, Y.; Wen, Q.; Liu, Y. Probabilistic Representation and Inverse Design of Metamaterials Based on a Deep Generative Model with Semi-Supervised Learning Strategy. Adv. Mater. 2019, 31, 1901111. [Google Scholar] [CrossRef] [PubMed]
- Schackart, K.E., III; Yoon, J.Y. Machine learning enhances the performance of bioreceptor-free biosensors. Sensors 2021, 21, 5519. [Google Scholar] [CrossRef] [PubMed]
- Sabry, F.; Eltaras, T.; Labda, W.; Alzoubi, K.; Malluhi, Q. Machine Learning for Healthcare Wearable Devices: The Big Picture. J. Healthc. Eng. 2022, 2022, 4653923. [Google Scholar] [CrossRef]
- Chen, P.H.C.; Liu, Y.; Peng, L. How to develop machine learning models for healthcare. Nat. Mater. 2019, 18, 410–414. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, J.; Guo, C.; Xu, H. Dynamic power optimization for secondary wearable biosensors in e-healthcare leveraging cognitive WBSNs with imperfect spectrum sensing. Future Gener. Comput. Syst. 2020, 112, 67–92. [Google Scholar] [CrossRef]
- Sivapalan, G.; Nundy, K.K.; Dev, S.; Cardiff, B.; John, D. ANNet: A Lightweight Neural Network for ECG Anomaly Detection in IoT Edge Sensors. IEEE Trans. Biomed. Circuits Syst. 2022, 16, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, C. An ultra-low power turning angle based biomedical signal compression engine with adaptive threshold tuning. Sensors 2017, 17, 1809. [Google Scholar] [CrossRef]
- Parrilla, M.; De Wael, K. Wearable Self-Powered Electrochemical Devices for Continuous Health Management. Adv. Funct. Mater. 2021, 31, 2107042. [Google Scholar] [CrossRef]
- Boubin, M.; Shrestha, S. Microcontroller Implementation of Support Vector Machine for Detecting Blood Glucose Levels Using Breath Volatile Organic Compounds. Sensors 2019, 19, 2283. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zheng, B.; Yoon, S.W.; Ko, H.S. A support vector machine-based ensemble algorithm for breast cancer diagnosis. Eur. J. Oper. Res. 2018, 267, 687–699. [Google Scholar] [CrossRef]
- Usama, M.; Qadir, J.; Raza, A.; Arif, H.; Yau, K.L.A.; Elkhatib, Y.; Hussain, A.; Al-Fuqaha, A. Unsupervised Machine Learning for Networking: Techniques, Applications and Research Challenges. IEEE Access 2019, 7, 65579–65615. [Google Scholar] [CrossRef]
- Vakilian, K.A. Optimization Methods Can Increase the Durability of Smart Electrochemical Biosensors. In Proceedings of the Proceedings—2022 8th International Iranian Conference on Signal Processing and Intelligent Systems, ICSPIS 2022, Mazandaran, Iran, 28–29 December 2022. [Google Scholar]
- Wang, J.; Xu, B.; Shi, L.; Zhu, L.; Wei, X. Prospects and Challenges of AI and Neural Network Algorithms in MEMS Microcantilever Biosensors. Processes 2022, 10, 1658. [Google Scholar] [CrossRef]
- Sui, J.; Xie, P.; Lin, Z.; Javanmard, M. Electronic classification of barcoded particles for multiplexed detection using supervised machine learning analysis. Talanta 2020, 215, 120791. [Google Scholar] [CrossRef] [PubMed]
- Kumar Sharma, P.; Ruotolo, A.; Khan, R.; Mishra, Y.K.; Kumar Kaushik, N.; Kim, N.Y.; Kumar Kaushik, A. Perspectives on 2D-borophene flatland for smart bio-sensing. Mater. Lett. 2022, 308, 131089. [Google Scholar] [CrossRef]
- Lee, H.J.; Yang, J.C.; Choi, J.; Kim, J.; Lee, G.S.; Sasikala, S.P.; Lee, G.H.; Park, S.H.K.; Lee, H.M.; Sim, J.Y.; et al. Hetero-Dimensional 2D Ti3C2TxMXene and 1D Graphene Nanoribbon Hybrids for Machine Learning-Assisted Pressure Sensors. ACS Nano 2021, 15, 10347–10356. [Google Scholar] [CrossRef]
- Shi, L.; Tang, P.; Hu, J.; Zhang, Y. A Strategy for Multigas Identification Using Multielectrical Parameters Extracted from a Single Carbon-Based Field-Effect Transistor Sensor. ACS Sens. 2024, 9, 3126–3136. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, W.; Chen, J.; Li, H.; Han, L.; Li, X.; Wang, J.; Song, W.; Xu, C.; Cai, X.; et al. Sensitivity-Enhancing Strategies of Graphene Field-Effect Transistor Biosensors for Biomarker Detection. ACS Sens. 2024, 9, 2705–2727. [Google Scholar] [CrossRef]
- Sadighbayan, D.; Hasanzadeh, M.; Ghafar-Zadeh, E. Biosensing based on field-effect transistors (FET): Recent progress and challenges. TrAC Trends Anal. Chem. 2020, 133, 116067. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.; Park, C.; Sunwoo, S.H.; Kim, M.; Lee, H.; Lee, M.; Kim, D.H. Soft conductive nanocomposites for recording biosignals on skin. Soft Sci. 2023, 3, 28. [Google Scholar] [CrossRef]
- Yang, Y.; Cui, J.; Luo, D.; Murray, J.; Chen, X.; Hülck, S.; Tripp, R.A.; Zhao, Y. Rapid Detection of SARS-CoV-2 Variants Using an Angiotensin-Converting Enzyme 2-Based Surface-Enhanced Raman Spectroscopy Sensor Enhanced by CoVari Deep Learning Algorithms. ACS Sens. 2024, 9, 3158–3169. [Google Scholar] [CrossRef]
- Qureshi, R.; Irfan, M.; Ali, H.; Khan, A.; Nittala, A.S.; Ali, S.; Shah, A.; Gondal, T.M.; Sadak, F.; Shah, Z.; et al. Artificial Intelligence and Biosensors in Healthcare and Its Clinical Relevance: A Review. IEEE Access 2023, 11, 61600–61620. [Google Scholar] [CrossRef]
- Wang, T.; Lu, Y.; Cao, Z.; Shu, L.; Zheng, X.; Liu, A.; Xie, M. When sensor-cloud meets mobile edge computing. Sensors 2019, 19, 5324. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Liu, J.; Liu, Y.; Fu, H.; Hu, Y.; Cheng, J.; Qi, H.; Wu, Y.; Zhang, J.; Zhao, Y. Structure and Illumination Constrained GAN for Medical Image Enhancement. IEEE Trans. Med. Imaging 2021, 40, 3955–3967. [Google Scholar] [CrossRef] [PubMed]
- Dave, T.; Athaluri, S.A.; Singh, S. ChatGPT in medicine: An overview of its applications, advantages, limitations, future prospects, and ethical considerations. Front. Artif. Intell. 2023, 6, 1169595. [Google Scholar] [CrossRef]
- Singh, S.; Varma, P.; Sreelekha, G.; Adak, C.; Shukla, R.P.; Kamble, V.B. Metal oxide-based gas sensor array for VOCs determination in complex mixtures using machine learning. Microchim. Acta 2024, 191, 196. [Google Scholar] [CrossRef]
- Sukor, A.S.A.; Zakaria, A.; Rahim, N.A. Activity recognition using accelerometer sensor and machine learning classifiers. In Proceedings of the 2018 IEEE 14th International Colloquium on Signal Processing & Its Applications (CSPA), Penang, Malaysia, 9–10 March 2018; pp. 233–238. [Google Scholar]
- Sundararajan, K.; Georgievska, S.; te Lindert, B.H.W.; Gehrman, P.R.; Ramautar, J.; Mazzotti, D.R.; Sabia, S.; Weedon, M.N.; van Someren, E.J.W.; Ridder, L.; et al. Sleep classification from wrist-worn accelerometer data using random forests. Sci. Rep. 2021, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Delmastro, F.; Martino, F.D.; Dolciotti, C. Cognitive Training and Stress Detection in MCI Frail Older People through Wearable Sensors and Machine Learning. IEEE Access 2020, 8, 65573–65590. [Google Scholar] [CrossRef]
- Patlar Akbulut, F.; Ikitimur, B.; Akan, A. Wearable sensor-based evaluation of psychosocial stress in patients with metabolic syndrome. Artif. Intell. Med. 2020, 104, 101824. [Google Scholar] [CrossRef]
- Balamurugan, G.; Annadurai, C.; Nelson, I.; Nirmala Devi, K.; Oliver, A.S.; Gomathi, S. Optical bio sensor based cancer cell detection using optimized machine learning model with quantum computing. Opt. Quantum Electron. 2024, 56, 97. [Google Scholar] [CrossRef]
- Posada-Quintero, H.F.; Reljin, N.; Moutran, A.; Georgopalis, D.; Lee, E.C.H.; Giersch, G.E.W.; Casa, D.J.; Chon, K.H. Mild dehydration identification using machine learning to assess autonomic responses to cognitive stress. Nutrients 2020, 12, 42. [Google Scholar] [CrossRef]
- Wong, C.K.; Ho, D.T.Y.; Tam, A.R.; Zhou, M.; Lau, Y.M.; Tang, M.O.Y.; Tong, R.C.F.; Rajput, K.S.; Chen, G.; Chan, S.C.; et al. Artificial intelligence mobile health platform for early detection of COVID-19 in quarantine subjects using a wearable biosensor: Protocol for a randomised controlled trial. BMJ Open 2020, 10, e038555. [Google Scholar] [CrossRef]
- Miao, F.; Wen, B.; Hu, Z.; Fortino, G.; Wang, X.P.; Liu, Z.D.; Tang, M.; Li, Y. Continuous blood pressure measurement from one-channel electrocardiogram signal using deep-learning techniques. Artif. Intell. Med. 2020, 108, 101919. [Google Scholar] [CrossRef] [PubMed]
- Un, K.C.; Wong, C.K.; Lau, Y.M.; Lee, J.C.Y.; Tam, F.C.C.; Lai, W.H.; Lau, Y.M.; Chen, H.; Wibowo, S.; Zhang, X.; et al. Observational study on wearable biosensors and machine learning-based remote monitoring of COVID-19 patients. Sci. Rep. 2021, 11, 4388. [Google Scholar] [CrossRef]
- Potluri, S.; Chandran, A.B.; Diedrich, C.; Schega, L. Machine Learning based Human Gait Segmentation with Wearable Sensor Platform. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, Berlin, Germany, 23–27 July 2019; pp. 588–594. [Google Scholar]
- Kwon, S.; Hong, J.; Choi, E.K.; Lee, B.; Baik, C.; Lee, E.; Jeong, E.R.; Koo, B.K.; Oh, S.; Yi, Y. Detection of atrial fibrillation using a ring-type wearable device (CardioTracker) and deep learning analysis of photoplethysmography signals: Prospective observational proof-of-concept study. J. Med. Internet Res. 2020, 22, e16443. [Google Scholar] [CrossRef]
- Jafrasteh, F.; Farmani, A.; Mohamadi, J. Meticulous research for design of plasmonics sensors for cancer detection and food contaminants analysis via machine learning and artificial intelligence. Sci. Rep. 2023, 13, 15349. [Google Scholar] [CrossRef]
- Marom, O.; Nakhoul, F.; Tisch, U.; Shiban, A.; Abassi, Z.; Haick, H. Gold nanoparticle sensors for detecting chronic kidney disease and disease progression. Nanomedicine 2012, 7, 639–650. [Google Scholar] [CrossRef] [PubMed]
- van de Goor, R.; van Hooren, M.; Dingemans, A.M.; Kremer, B.; Kross, K. Training and Validating a Portable Electronic Nose for Lung Cancer Screening. J. Thorac. Oncol. 2018, 13, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Regalia, G.; Onorati, F.; Lai, M.; Caborni, C.; Picard, R.W. Multimodal wrist-worn devices for seizure detection and advancing research: Focus on the Empatica wristbands. Epilepsy Res. 2019, 153, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wen, J.; Abdulkadir, A.; Cui, Y.; Erus, G.; Mamourian, E.; Melhem, R.; Srinivasan, D.; Govindarajan, S.T.; Chen, J.; et al. Gene-SGAN: Discovering disease subtypes with imaging and genetic signatures via multi-view weakly-supervised deep clustering. Nat. Commun. 2024, 15, 354. [Google Scholar] [CrossRef] [PubMed]
- Shiammala, P.N.; Duraimutharasan, N.K.B.; Vaseeharan, B.; Alothaim, A.S.; Al-Malki, E.S.; Snekaa, B.; Safi, S.Z.; Singh, S.K.; Velmurugan, D.; Selvaraj, C. Exploring the artificial intelligence and machine learning models in the context of drug design difficulties and future potential for the pharmaceutical sectors. Methods 2023, 219, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Copley, S.J.; Viola, P.; Lu, H.; Aboagye, E.O. Radiomics and artificial intelligence for precision medicine in lung cancer treatment. Semin. Cancer Biol. 2023, 93, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Rabaan, A.A.; Bakhrebah, M.A.; Alotaibi, J.; Natto, Z.S.; Alkhaibari, R.S.; Alawad, E.; Alshammari, H.M.; Alwarthan, S.; Alhajri, M.; Almogbel, M.S.; et al. Unleashing the power of artificial intelligence for diagnosing and treating infectious diseases: A comprehensive review. J. Infect. Public Health 2023, 16, 1837–1847. [Google Scholar] [CrossRef] [PubMed]
- Sarvesh Raikar, G.; Raikar, A.S.; Somnache, S.N. Advancements in artificial intelligence and machine learning in revolutionising biomarker discovery. Braz. J. Pharm. Sci. 2023, 59, e23146. [Google Scholar] [CrossRef]
- FDANIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and Other Tools) Resource; FDA: Silver Spring, MD, USA, 2018; p. 61. [Google Scholar]
- Shah, A.; Grimberg, D.C.; Inman, B.A. Classification of Molecular Biomarkers. Soc. Int. Urol. J. 2020, 1, 8–15. [Google Scholar] [CrossRef]
- Liu, X.; Cui, B.; Wang, Q.; Ma, Y.; Li, L.; Chen, Z. Biomarkers for respiratory diseases: Present applications and future discoveries. Clin. Transl. Discov. 2021, 1, e11. [Google Scholar] [CrossRef]
- Pham, Y.L.; Beauchamp, J. Breath Biomarkers in Diagnostic Applications. Molecules 2021, 26, 5514. [Google Scholar] [CrossRef] [PubMed]
- Belizário, J.E.; Faintuch, J.; Malpartida, M.G. Breath Biopsy and Discovery of Exclusive Volatile Organic Compounds for Diagnosis of Infectious Diseases. Front. Cell. Infect. Microbiol. 2021, 10, 564194. [Google Scholar] [CrossRef] [PubMed]
- Adeoye, J.; Su, Y.X. Artificial intelligence in salivary biomarker discovery and validation for oral diseases. Oral Dis. 2024, 30, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Winchester, L.M.; Harshfield, E.L.; Shi, L.; Badhwar, A.P.; Khleifat, A.A.; Clarke, N.; Dehsarvi, A.; Lengyel, I.; Lourida, I.; Madan, C.R.; et al. Artificial intelligence for biomarker discovery in Alzheimer’s disease and dementia. Alzheimer’s Dement. 2023, 19, 5860–5871. [Google Scholar] [CrossRef] [PubMed]
- Prelaj, A.; Miskovic, V.; Zanitti, M.; Trovo, F.; Genova, C.; Viscardi, G.; Rebuzzi, S.E.; Mazzeo, L.; Provenzano, L.; Kosta, S.; et al. Artificial intelligence for predictive biomarker discovery in immuno-oncology: A systematic review. Ann. Oncol. 2024, 35, 29–65. [Google Scholar] [CrossRef] [PubMed]
- Delavari, P.; Ozturan, G.; Yuan, L.; Yilmaz, Ö.; Oruc, I. Artificial intelligence, explainability, and the scientific method: A proof-of-concept study on novel retinal biomarker discovery. PNAS Nexus 2023, 2, pgad290. [Google Scholar] [CrossRef] [PubMed]
- Kyriazakos, S.; Pnevmatikakis, A.; Cesario, A.; Kostopoulou, K.; Boldrini, L.; Valentini, V.; Scambia, G. Discovering Composite Lifestyle Biomarkers with Artificial Intelligence From Clinical Studies to Enable Smart eHealth and Digital Therapeutic Services. Front. Digit. Health 2021, 3, 648190. [Google Scholar] [CrossRef] [PubMed]
- Echle, A.; Rindtorff, N.T.; Brinker, T.J.; Luedde, T.; Pearson, A.T.; Kather, J.N. Deep learning in cancer pathology: A new generation of clinical biomarkers. Br. J. Cancer 2021, 124, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Jeong, Y.W.; Park, Y.; Kim, K.; Park, J.; Kang, D.R. Applications of deep learning methods in digital biomarker research using noninvasive sensing data. Digit. Health 2022, 8, 20552076221136642. [Google Scholar] [CrossRef]
- Satam, H.; Joshi, K.; Mangrolia, U.; Waghoo, S.; Zaidi, G.; Rawool, S.; Thakare, R.P.; Banday, S.; Mishra, A.K.; Das, G.; et al. Next-Generation Sequencing Technology: Current Trends and Advancements. Biology 2023, 12, 997. [Google Scholar] [CrossRef]
- Cabello-Aguilar, S.; Vendrell, J.A.; Solassol, J. A Bioinformatics Toolkit for Next-Generation Sequencing in Clinical Oncology. Curr. Issues Mol. Biol. 2023, 45, 9737–9752. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.K.; Liu, W.H.; Wang, C.Y.; Lu, J.J.; Chen, C.H.; Wu-Chou, Y.H.; Chang, P.Y.; Chang, S.C.; Yang, C.H.; Tsai, M.L.; et al. Targeted next generation sequencing for genetic mutations of dilated cardiomyopathy. Acta Cardiol. Sin. 2019, 35, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Cascini, F.; Beccia, F.; Causio, F.A.; Melnyk, A.; Zaino, A.; Ricciardi, W. Scoping review of the current landscape of AI-based applications in clinical trials. Front. Public Health 2022, 10, 949377. [Google Scholar] [CrossRef] [PubMed]
- Tenchov, R.; Sapra, A.K.; Sasso, J.; Ralhan, K.; Tummala, A.; Azoulay, N.; Zhou, Q.A. Biomarkers for Early Cancer Detection: A Landscape View of Recent Advancements, Spotlighting Pancreatic and Liver Cancers. ACS Pharmacol. Transl. Sci. 2024, 7, 586–613. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Lasalde-Ramírez, J.A.; Mahato, K.; Wang, J.; Gao, W. Wearable chemical sensors for biomarker discovery in the omics era. Nat. Rev. Chem. 2022, 6, 899–915. [Google Scholar] [CrossRef]
- Beltrán, J.F.; Wahba, B.M.; Hose, N.; Shasha, D.; Kline, R.P. Inexpensive, non-invasive biomarkers predict Alzheimer transition using machine learning analysis of the Alzheimer’s Disease Neuroimaging (ADNI) database. PLoS ONE 2020, 15, e0235663. [Google Scholar] [CrossRef] [PubMed]
- Eren, A.M.; Kiefl, E.; Shaiber, A.; Veseli, I.; Miller, S.E.; Schechter, M.S.; Fink, I.; Pan, J.N.; Yousef, M.; Fogarty, E.C.; et al. Community-led, integrated, reproducible multi-omics with anvi’o. Nat. Microbiol. 2021, 6, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Kucherenko, I.S.; Soldatkin, O.O.; Topolnikova, Y.V.; Dzyadevych, S.V.; Soldatkin, A.P. Novel Multiplexed Biosensor System for the Determination of Lactate and Pyruvate in Blood Serum. Electroanalysis 2019, 31, 1625–1631. [Google Scholar] [CrossRef]
- Ni, X.; Ouyang, W.; Jeong, H.; Kim, J.T.; Tzaveils, A.; Mirzazadeh, A.; Wu, C.; Lee, J.Y.; Keller, M.; Mummidisetty, C.K.; et al. Automated, multiparametric monitoring of respiratory biomarkers and vital signs in clinical and home settings for COVID-19 patients. Proc. Natl. Acad. Sci. USA 2021, 118, e2026610118. [Google Scholar] [CrossRef]
- Mann, M.; Kumar, C.; Zeng, W.F.; Strauss, M.T. Artificial intelligence for proteomics and biomarker discovery. Cell Syst. 2021, 12, 759–770. [Google Scholar] [CrossRef]
- Meyer, J.G. Deep learning neural network tools for proteomics. Cell Rep. Methods 2021, 1, 100003. [Google Scholar] [CrossRef] [PubMed]
- Hartman, E.; Scott, A.M.; Karlsson, C.; Mohanty, T.; Vaara, S.T.; Linder, A.; Malmström, L.; Malmström, J. Interpreting biologically informed neural networks for enhanced proteomic biomarker discovery and pathway analysis. Nat. Commun. 2023, 14, 5359. [Google Scholar] [CrossRef] [PubMed]
- Acosta, J.N.; Falcone, G.J.; Rajpurkar, P.; Topol, E.J. Multimodal biomedical AI. Nat. Med. 2022, 28, 1773–1784. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Rahman, S.; Zhou, J.; Kang, J.J. A Comprehensive Review on Machine Learning in Healthcare Industry: Classification, Restrictions, Opportunities and Challenges. Sensors 2023, 23, 4178. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Liu, C.; Xu, T.; Su, L.; Zhang, X. Artificial intelligence biosensors: Challenges and prospects. Biosens. Bioelectron. 2020, 165, 112412. [Google Scholar] [CrossRef] [PubMed]
- Bruno, E.; Viana, P.F.; Sperling, M.R.; Richardson, M.P. Seizure detection at home: Do devices on the market match the needs of people living with epilepsy and their caregivers? Epilepsia 2020, 61, S11–S24. [Google Scholar] [CrossRef] [PubMed]
- Franciotti, R.; Nardini, D.; Russo, M.; Onofrj, M.; Sensi, S.L. Comparison of Machine Learning-based Approaches to Predict the Conversion to Alzheimer’s Disease from Mild Cognitive Impairment. Neuroscience 2023, 514, 143–152. [Google Scholar] [CrossRef]
- Li, Y.; Luo, L.; Kong, Y.; George, S.; Li, Y.; Guo, X.; Li, X.; Yeatman, E.; Davenport, A.; Li, Y.; et al. A Point-of-Care Sensing Platform for Multiplexed Detection of Chronic Kidney Disease Biomarkers Using Molecularly Imprinted Polymers. Adv. Funct. Mater. 2024, 34, 2316865. [Google Scholar] [CrossRef]
- Hassan, R.Y.A. Advances in Electrochemical Nano-Biosensors for Biomedical and Environmental Applications: From Current Work to Future Perspectives. Sensors 2022, 22, 7539. [Google Scholar] [CrossRef]
- Shi, L.; Li, Y.; Li, Z. Early cancer detection by SERS spectroscopy and machine learning. Light Sci. Appl. 2023, 12, 234. [Google Scholar] [CrossRef]
- Stranieri, A.; Venkatraman, S.; Minicz, J.; Zarnegar, A.; Firmin, S.; Balasubramanian, V.; Jelinek, H.F. Emerging point of care devices and artificial intelligence: Prospects and challenges for public health. Smart Health 2022, 24, 100279. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Lin, M.; Yin, L.; De la paz, E.; Pei, K.; Sonsa-ard, T.; de Loyola Silva, A.N.; Khorshed, A.A.; Zhang, F.; Tostado, N.; et al. An epidermal patch for the simultaneous monitoring of haemodynamic and metabolic biomarkers. Nat. Biomed. Eng. 2021, 5, 737–748. [Google Scholar] [CrossRef] [PubMed]





| Sensing Device | Type of ML Algorithm | Application | Ref. |
|---|---|---|---|
| Metal oxide-based gas sensor array | RF, K-Nearest Neighbor (KNN), DT, Linear regression, Logistic Regression, Naïve Bayes, LDA, ANN, SVM | Detection, classification, and prediction of concentrations of the four gases simultaneously for disease diagnosis and treatment monitoring. | [168] |
| Accelerometer sensor embedded in a smartphone | Several ML classifiers | Medical diagnostic, monitoring of users’ daily routine, and detection of abnormal cases | [169] |
| Accelerometer in wristband | RF | Sleep monitoring | [170] |
| Zephyr BioHarness for Electrocardiography (ECG) | Batch normalization, SVM, KNN | Cognitive training and stress detection | [171] |
| ECG, galvanic skin response (GSR), body temperature, SpO2, glucose level, and blood pressure | Neural network model | Psychosocial stress detection | [172] |
| Optical biosensor | DL | Cancer cell detection | [173] |
| Electrodermal activity (EDA) and Photoplethysmogram (PPG) | LDA, quadratic discriminant analysis, logistic regression, SVM, Gaussian kernel, KNN, DTs | Hydration monitoring | [174] |
| Skin temperature, respiratory rate, blood pressure, pulse rate, blood oxygen saturation, and daily activities | Multiple ML techniques | Early detection of COVID-19 | [175] |
| ECG, PPG, and blood pressure (BP) | ResNet with Long short-term memory for hypertension detection | Blood pressure measurement | [176] |
| Heart rate, heart rate variability, respiration rate, oxygen saturation, blood pulse wave, skin temperature sensors | Multivariate regression for case deterioration | COVID-19 detection | [177] |
| Inertial measurement unit (IMU) sensor module and plantar pressure | K-means clustering, ANN, SVM | Rehabilitation | [178] |
| PPG sensor in a ring-type device | DL | Arrhythmia detection | [179] |
| Plasmonics sensors | Logistic regression, SVM, ANN, CNN, KMM | Cancer detection | [180] |
| Au nanoparticle-based sensor | SVM | Chronic kidney disease detection | [181] |
| Electronic nose | ANN | Differentiating lung cancer patients | [182] |
| Accelerometer and electrodermal activity | SVM | Seizure detection | [183] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wasilewski, T.; Kamysz, W.; Gębicki, J. AI-Assisted Detection of Biomarkers by Sensors and Biosensors for Early Diagnosis and Monitoring. Biosensors 2024, 14, 356. https://doi.org/10.3390/bios14070356
Wasilewski T, Kamysz W, Gębicki J. AI-Assisted Detection of Biomarkers by Sensors and Biosensors for Early Diagnosis and Monitoring. Biosensors. 2024; 14(7):356. https://doi.org/10.3390/bios14070356
Chicago/Turabian StyleWasilewski, Tomasz, Wojciech Kamysz, and Jacek Gębicki. 2024. "AI-Assisted Detection of Biomarkers by Sensors and Biosensors for Early Diagnosis and Monitoring" Biosensors 14, no. 7: 356. https://doi.org/10.3390/bios14070356
APA StyleWasilewski, T., Kamysz, W., & Gębicki, J. (2024). AI-Assisted Detection of Biomarkers by Sensors and Biosensors for Early Diagnosis and Monitoring. Biosensors, 14(7), 356. https://doi.org/10.3390/bios14070356








