Single-Nanoparticle Electrochemical Collision for Monitoring Self-Assembly of Thiol Molecules on Au Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Self-Assembly of Thiol Molecules on Au NPs
2.2. SNEC Tests for Self-Assembled Au NPs
3. Results and Discussion
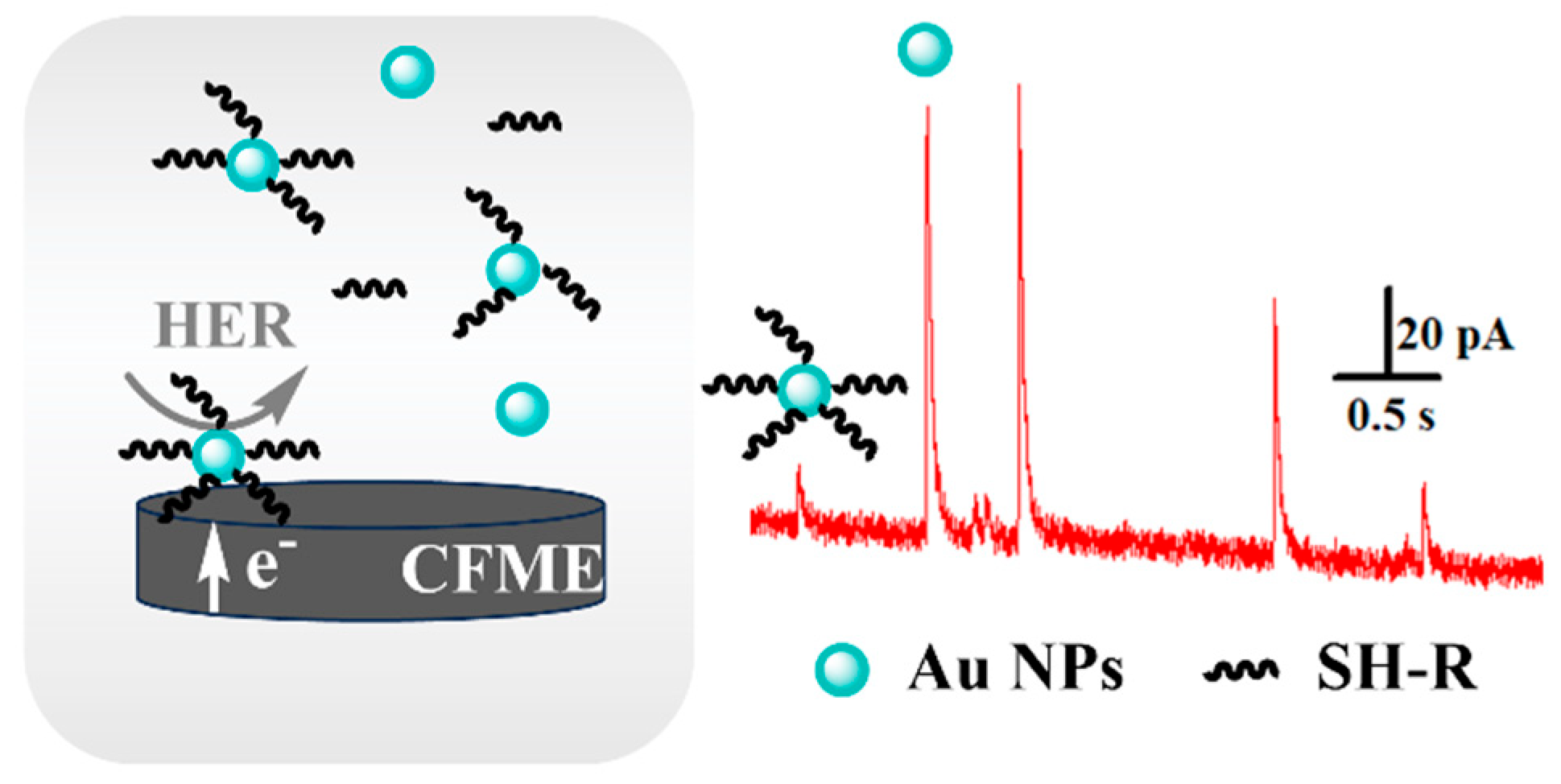
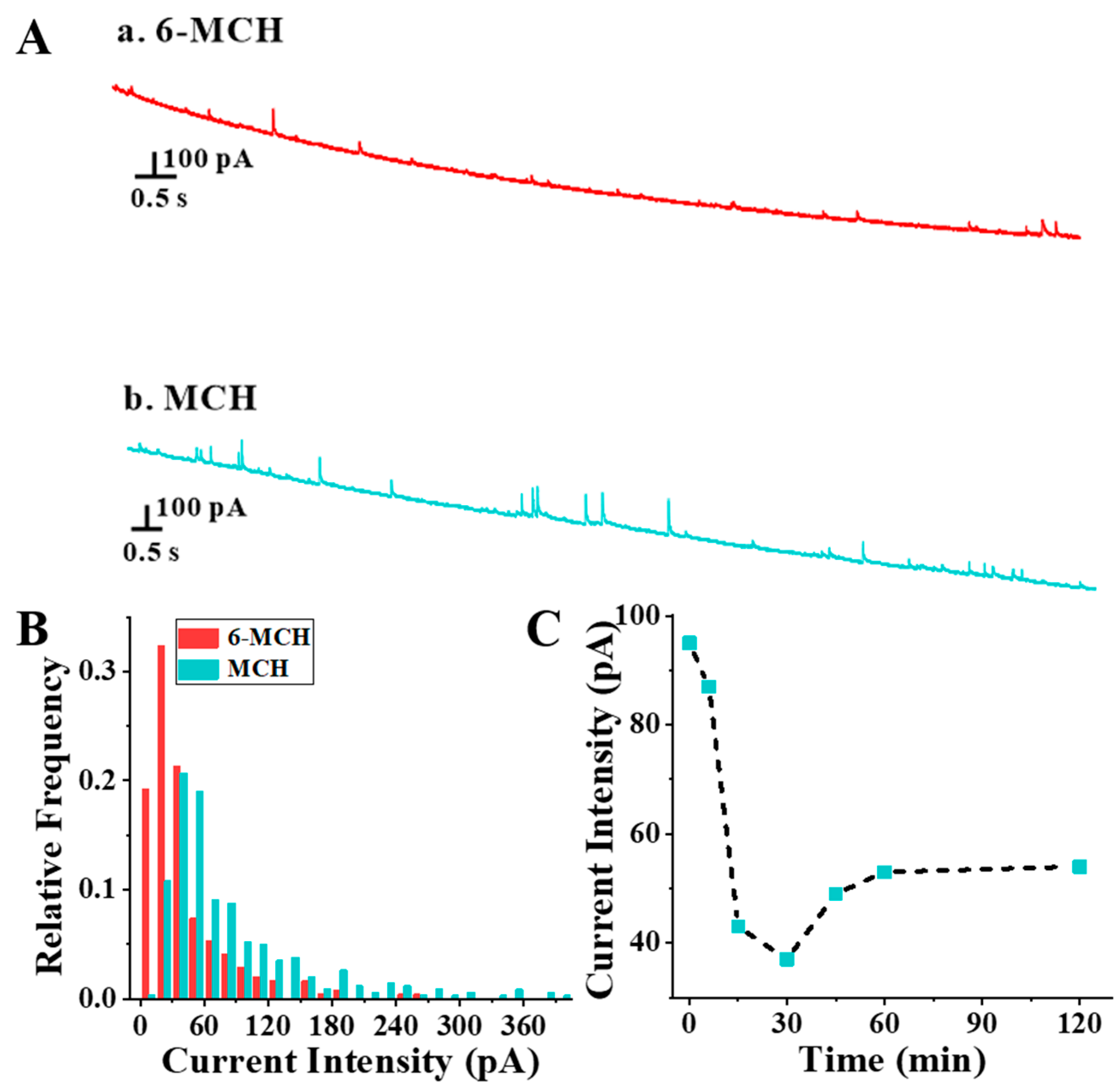
3.1. Feasibility of Self-Assembly Detection of Thiol Molecules on Au NPs Based on SNEC
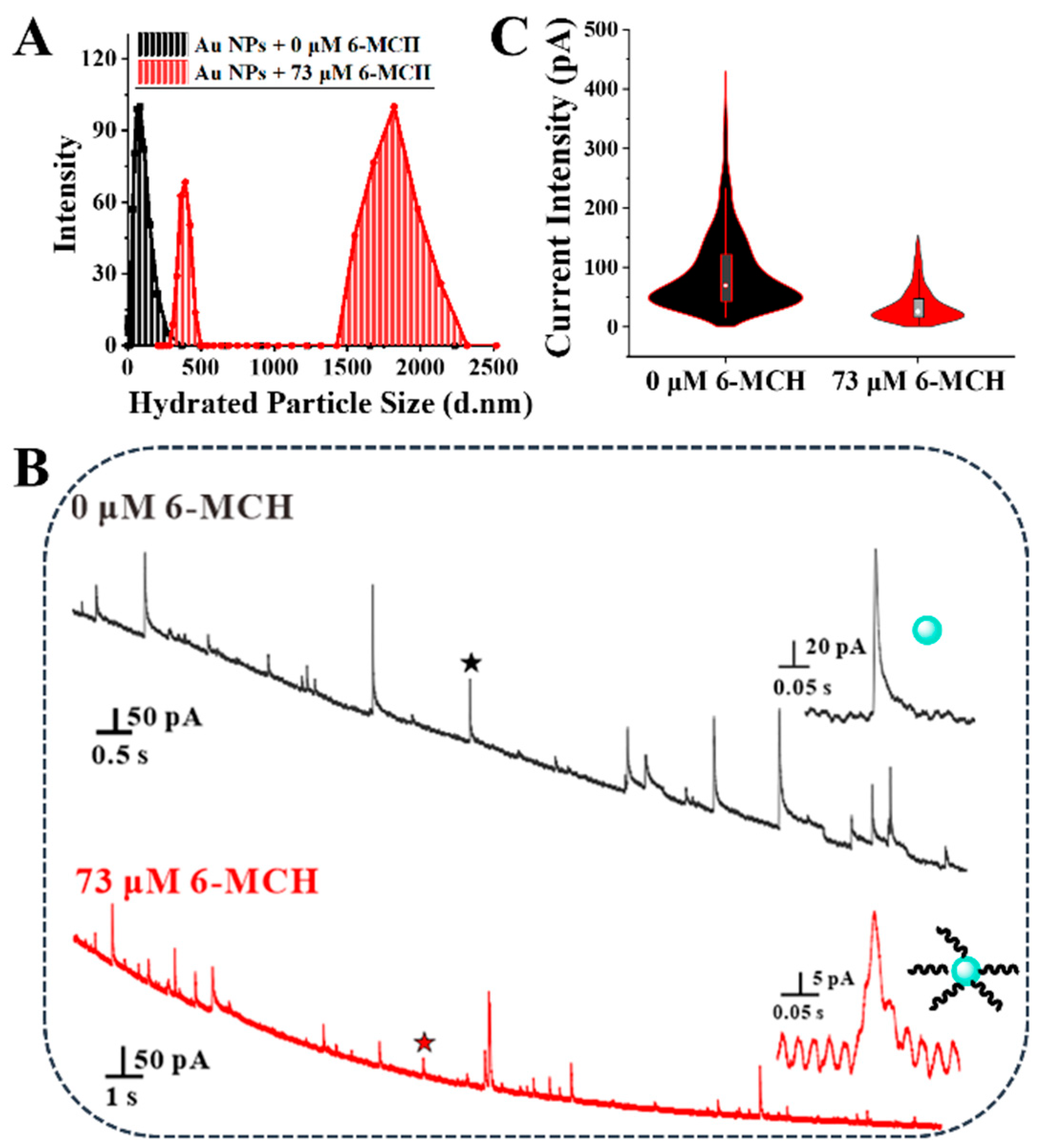
3.2. Effect of the C6-MCH/CAu NPs on the Assembly Efficiency
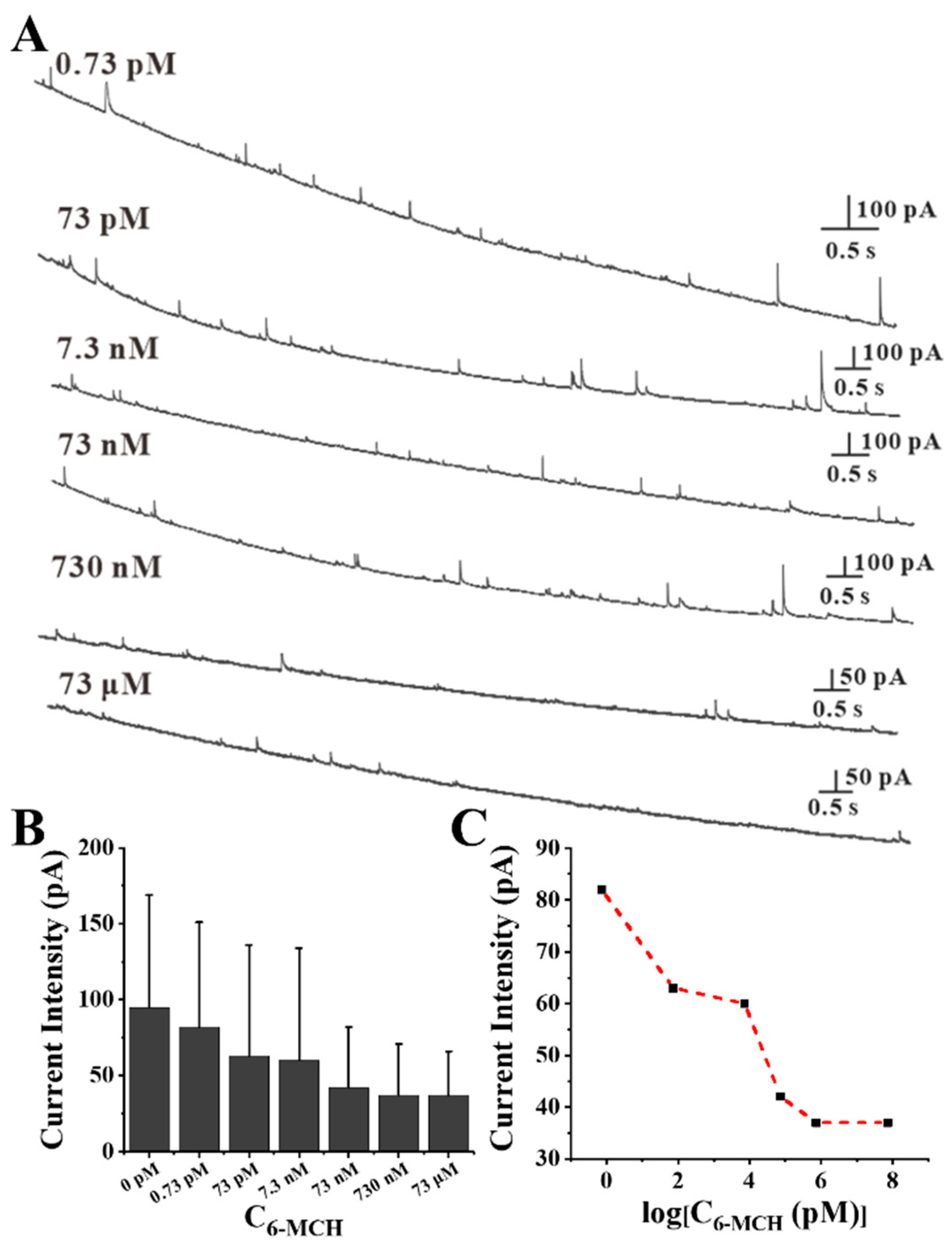
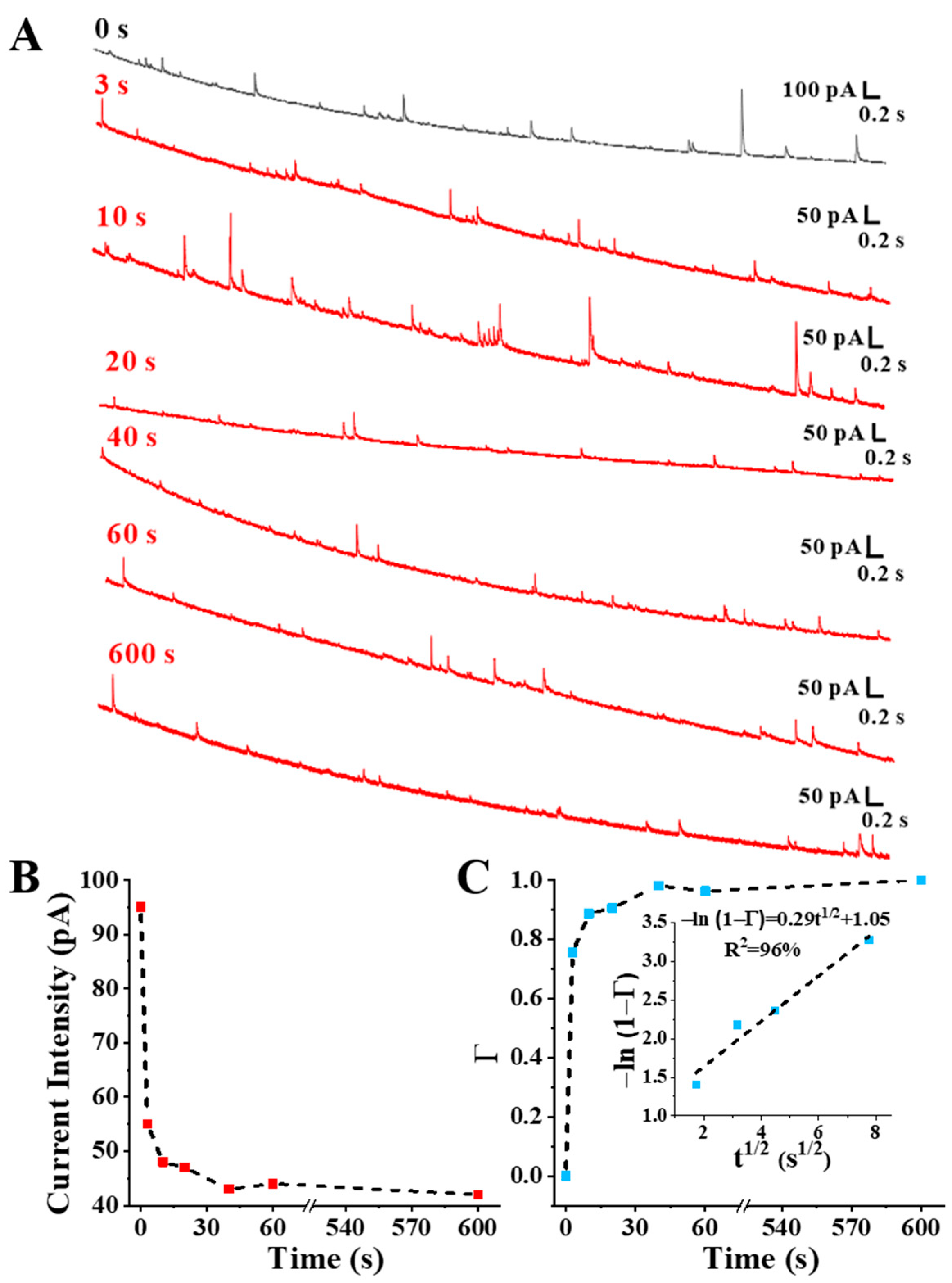
3.3. Self-Assembly Monitoring of Thiol Molecules on Au NPs Based on SNEC
3.4. Effect of Thiol Molecular Structure on the Packing Efficiency of SAMs
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Xianyu, Y.; Jiang, X. Surface modification of gold nanoparticles with small molecules for biochemical analysis. Acc. Chem. Res. 2017, 50, 310–319. [Google Scholar] [CrossRef]
- Watkins, Z.; Karajic, A.; Young, T.; White, R.; Heikenfeld, J. Week-long operation of electrochemical aptamer sensors: New insights into self-assembled monolayer degradation mechanisms and solutions for stability in serum at body temperature. ACS Sens. 2023, 8, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Thiagarajan, S.; Price, E.; Connors, L.; Dettman, A.; Koh, A.S. Study of n-alkanethiol self-assembly behavior on iron particles: Effect of alkyl chain length and adsorption solvent on resulting iron-based magnetorheological fluids. Langmuir 2022, 38, 13506–13521. [Google Scholar] [CrossRef]
- Häkkinen, H. The gold–sulfur interface at the nanoscale. Nat. Chem. 2012, 4, 443–455. [Google Scholar] [CrossRef]
- Guo, Y.; Sun, Y.; Wang, Y.; He, H.; Zhu, Y. Thiol- and alkyne-functionalized copper nanoparticles as electrocatalysts for bisphenol a (BPA) oxidation. J. Solid State Electrochem. 2018, 23, 91–100. [Google Scholar] [CrossRef]
- Kim, J.; Jones, M.R.; Ou, Z.; Chen, Q. In situ electron microscopy imaging and quantitative structural modulation of nanoparticle superlattices. ACS Nano 2016, 10, 9801–9808. [Google Scholar] [CrossRef]
- Woehl, T. Refocusing in situ electron microscopy: Moving beyond visualization of nanoparticle self-assembly to gain practical insights into advanced material fabrication. ACS Nano 2019, 13, 12272–12279. [Google Scholar] [CrossRef]
- Weidman, M.C.; Smilgies, D.M.; Tisdale, W.A. Kinetics of the self-assembly of nanocrystal superlattices measured by real-time in situ x-ray scattering. Nat. Mater. 2016, 15, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Nagaoka, Y.; Hills-Kimball, K.; Tan, R.; Yu, L.; Fang, Y.; Wang, K.; Li, R.; Wang, Z.; Chen, O. Pressure-enabled synthesis of hetero-dimers and hetero-rods through intraparticle coalescence and interparticle fusion of quantum-dot-au satellite nanocrystals. J. Am. Chem. Soc. 2017, 139, 8408–8411. [Google Scholar] [CrossRef]
- Li, Z.; Chen, M.; Chen, Z.; Zhu, Y.L.; Guo, C.; Wang, H.; Qin, Y.; Fang, F.; Wang, D.; Su, C.; et al. Non-equilibrium nanoassemblies constructed by confined coordination on a polymer chain. J. Am. Chem. Soc. 2022, 144, 22651–22661. [Google Scholar] [CrossRef] [PubMed]
- Sannomiya, T.; Voros, J. Single plasmonic nanoparticles for biosensing. Trends Biotechnol. 2011, 29, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Qiu, D.; Wang, S.; Zheng, Y.; Deng, Z. One at a time: Counting single-nanoparticle/electrode collisions for accurate particle sizing by overcoming the instability of gold nanoparticles under electrolytic conditions. Nanotechnology 2013, 24, 505707. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Zhou, Y.G. Nano-impact electrochemistry: Analysis of single bioentities. Trends Anal. Chem. 2020, 123, 115768. [Google Scholar] [CrossRef]
- Davis, C.; Wang, S.X.; Sepunaru, L. What can electrochemistry tell us about individual enzymes? Curr. Opin. Electrochem. 2020, 25, 100643. [Google Scholar] [CrossRef]
- Goines, S.; Dick, J.E. Review—electrochemistry’s potential to reach the ultimate sensitivity in measurement science. J. Electrochem. Soc. 2020, 167, e037505. [Google Scholar] [CrossRef]
- Lu, S.M.; Li, M.Y.; Long, Y.T. Dynamic chemistry interactions: Controlled single-entity electrochemistry. J. Phys. Chem. Lett. 2022, 13, 4653–4659. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.Y.; Yang, Y.J.; Xu, Y.; Yang, X.Y.; Zhang, Z.L. Current lifetime of single-nanoparticle electrochemical collision for in situ monitoring nanoparticles agglomeration and aggregation. Anal. Chem. 2023, 95, 4429–4434. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.Y.; Feng, Z.T.; Yang, Y.J.; Yang, X.Y.; Zhang, Z.L. Current lifetime of single-nanoparticle collision for sizing nanoparticles. Anal. Chem. 2022, 94, 1302–1307. [Google Scholar] [CrossRef]
- Xu, W.; Zheng, J.J.; Li, Y.A.; Gao, X.; Ji, X.; Zhou, Y.G. Nano-impact electrochemistry reveals kinetics information of metal-ion battery materials with multiple redox centers. Angew. Chem. Int. Ed. 2023, 62, e202306185. [Google Scholar] [CrossRef]
- Azimzadeh Sani, M.; Tschulik, K. Unveiling colloidal nanoparticle properties and interactions at a single entity level. Curr. Opin. Electrochem. 2023, 37, 101195. [Google Scholar] [CrossRef]
- Xiao, X.Y.; Bard, A.J. Observing single nanoparticle collisions at an ultramicroelectrode by electrocatalytic amplification. J. Am. Chem. Soc. 2007, 129, 9610–9612. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.G.; Rees, N.V.; Compton, R.G. The electrochemical detection and characterization of silver nanoparticles in aqueous solution. Angew. Chem. Int. Ed. 2011, 50, 4219–4221. [Google Scholar] [CrossRef] [PubMed]
- Quinn, B.M.; Van ’t Hof, P.G.; Lemay, S.G. Time-resolved electrochemical detection of discrete adsorption events. J. Am. Chem. Soc. 2004, 126, 8360–8361. [Google Scholar] [CrossRef] [PubMed]
- Azimzadeh Sani, M.; Pavlopoulos, N.G.; Pezzotti, S.; Serva, A.; Cignoni, P.; Linnemann, J.; Salanne, M.; Gaigeot, M.P.; Tschulik, K. Unexpectedly high capacitance of the metal nanoparticle/water interface: Molecular-level insights into the electrical double layer. Angew. Chem. Int. Ed. 2021, 61, e202112679. [Google Scholar] [CrossRef] [PubMed]
- Bard, A.J. Inner-sphere heterogeneous electrode reactions. Electrocatalysis and photocatalysis: The challenge. J. Am. Chem. Soc. 2010, 132, 7559–7567. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.Y.; Fan, F.R.F.; Zhou, J.P.; Bard, A.J. Current transients in single nanoparticle collision events. J. Am. Chem. Soc. 2008, 130, 16669–16677. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ji, W.; Zhang, S.; Gao, N.; Xu, T.; Wang, X.; Zhang, M. Vitamin d inhibits the early aggregation of alpha-synuclein and modulates exocytosis revealed by electrochemical measurements. Angew. Chem. Int. Ed. 2022, 61, e202111853. [Google Scholar] [CrossRef] [PubMed]
- Tschulik, K.; Compton, R.G. Nanoparticle impacts reveal magnetic field induced agglomeration and reduced dissolution rates. Phys. Chem. Chem. Phys. 2014, 16, 13909–13913. [Google Scholar] [CrossRef] [PubMed]
- Frens, G. Controlled Nucleation for the Regulation of the Particle Size in Monodisperse Gold Suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Bai, Y.Y. Single-nanoparticle electrochemical collision for study of the size-dependent activity of Au nanoparticles during hydrogen evolution reaction. Electroanalysis 2024, 36, e202300354. [Google Scholar] [CrossRef]
- Li, Y.T.; Zhang, S.H.; Wang, L.; Xiao, R.R.; Liu, W.; Zhang, X.W.; Zhou, Z.; Amatore, C.; Huang, W.H. Nanoelectrode for amperometric monitoring of individual vesicular exocytosis inside single synapses. Angew. Chem. Int. Ed. 2014, 53, 12456–12460. [Google Scholar] [CrossRef] [PubMed]
- Bain, C.D.; Troughton, E.B.; Tao, Y.T.; Evall, J.; Whitesides, G.M.; Nuzzo, R.G. Formation of monolayer films by the spontaneous assembly of organic thiols from solution onto gold. J. Am. Chem. Soc. 1989, 111, 321–335. [Google Scholar] [CrossRef]
- Ulman, A. Formation and structure of self-assembled monolayers. Chem. Rev. 1996, 96, 1533–1554. [Google Scholar] [CrossRef]
- Inkpen, M.S.; Liu, Z.F.; Li, H.; Campos, L.M.; Neaton, J.B.; Venkataraman, L. Non-chemisorbed gold–sulfur binding prevails in self-assembled monolayers. Nat. Chem. 2019, 11, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Reimers, J.R.; Ford, M.J.; Marcuccio, S.M.; Ulstrup, J.; Hush, N.S. Competition of van der waals and chemical forces on gold–sulfur surfaces and nanoparticles. Nat. Rev. Chem. 2017, 1, 17–29. [Google Scholar] [CrossRef]
- Dannenberger, O.; Buck, M.; Grunze, M. Self-assembly of n-alkanethiols: A kinetic study by second harmonic generation. J. Phys. Chem. B 1999, 103, 2202–2213. [Google Scholar] [CrossRef]
- Hong, H.G.; Park, W. A study of adsorption kinetics and thermodynamics of Ω-mercaptoalkylhydroquinone self-assembled monolayer on a gold electrode. Electrochim. Acta 2005, 51, 579–587. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Y. Single-Nanoparticle Electrochemical Collision for Monitoring Self-Assembly of Thiol Molecules on Au Nanoparticles. Biosensors 2024, 14, 393. https://doi.org/10.3390/bios14080393
Bai Y. Single-Nanoparticle Electrochemical Collision for Monitoring Self-Assembly of Thiol Molecules on Au Nanoparticles. Biosensors. 2024; 14(8):393. https://doi.org/10.3390/bios14080393
Chicago/Turabian StyleBai, Yiyan. 2024. "Single-Nanoparticle Electrochemical Collision for Monitoring Self-Assembly of Thiol Molecules on Au Nanoparticles" Biosensors 14, no. 8: 393. https://doi.org/10.3390/bios14080393
APA StyleBai, Y. (2024). Single-Nanoparticle Electrochemical Collision for Monitoring Self-Assembly of Thiol Molecules on Au Nanoparticles. Biosensors, 14(8), 393. https://doi.org/10.3390/bios14080393




