Digitalization of Enzyme-Linked Immunosorbent Assay with Graphene Field-Effect Transistors (G-ELISA) for Portable Ferritin Determination
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Buffers
2.3. Protein Solutions
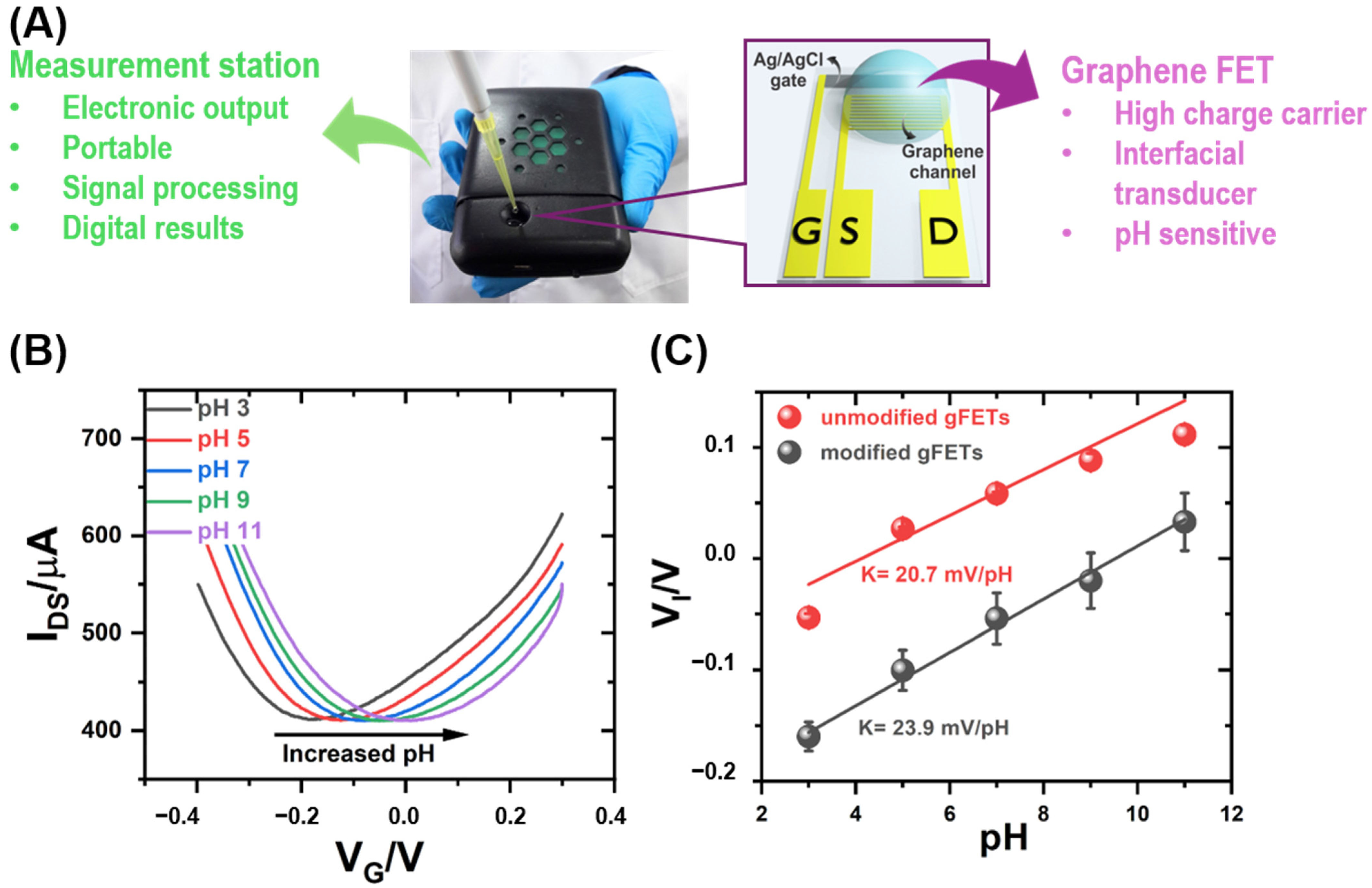
2.4. Measurement Set-Up
2.5. Measurement of Sensitivity to pH Changes
2.6. Modification of the gFETs with Streptavidin
2.7. Urea Hydrolysis Measurements on b-Urease-Streptavidin gFETs
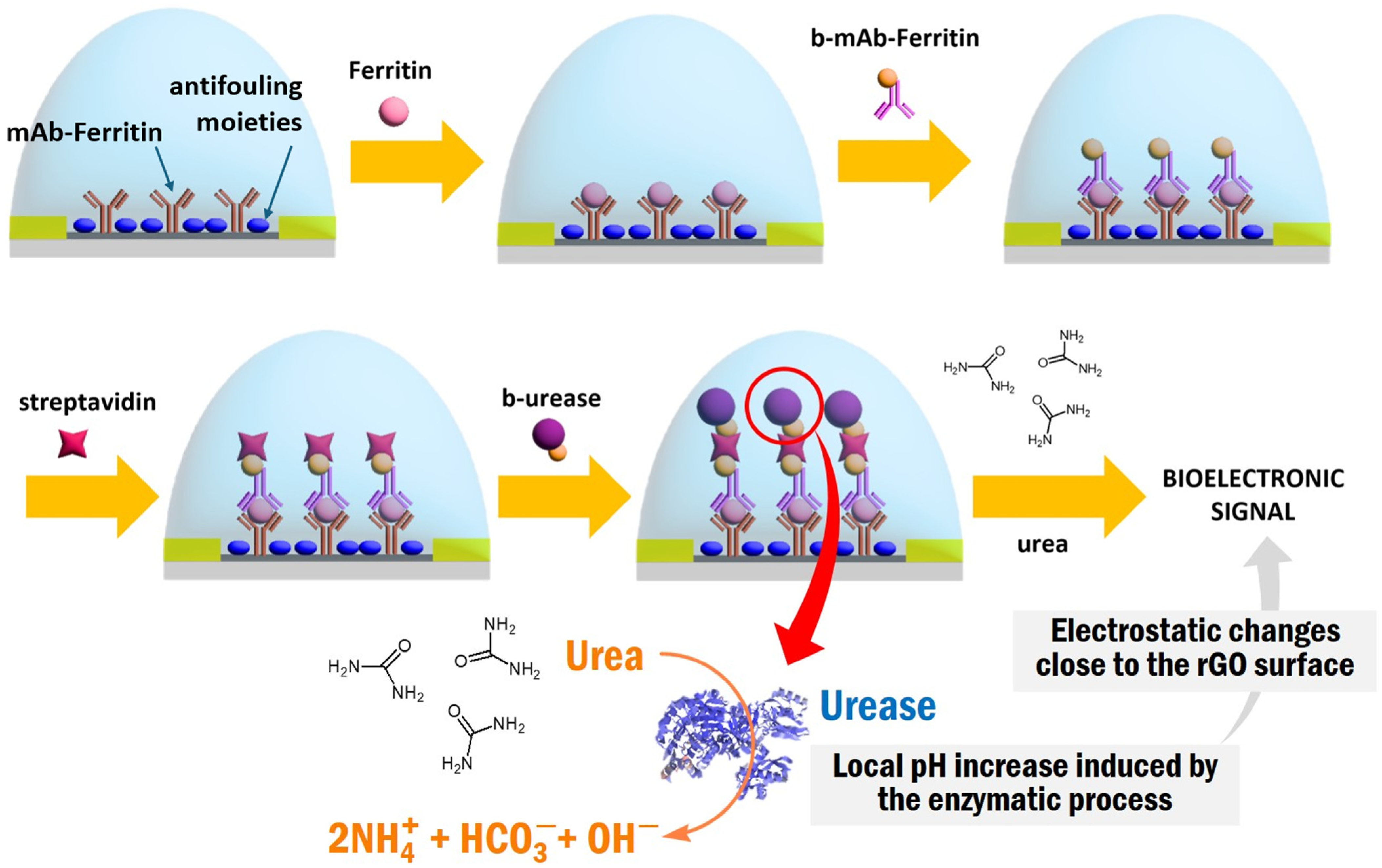
2.8. Modification of the gFETs with mAb-Ferritin
3. Results and Discussion
3.1. pH Monitoring by gFET Readout
3.2. Study and Optimization of the Enzymatic and Indirect Readout
3.3. ELISA-gFET for Ferritin Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Worwood, M. Ferritin. Blood Rev. 1990, 4, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Granick, S. Structure and Physiological Functions of Ferritin. Physiol. Rev. 1951, 31, 489–511. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Knovich, M.A.; Coffman, L.G.; Torti, F.M.; Torti, S.V. Serum Ferritin: Past, Present and Future. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2010, 1800, 760–769. [Google Scholar] [CrossRef]
- Worwood, M.; Cook, J.D. Serum Ferritin. CRC Crit. Rev. Clin. Lab. Sci. 1979, 10, 171–204. [Google Scholar] [CrossRef] [PubMed]
- Arosio, P.; Adelman, T.G.; Drysdale, J.W. On Ferritin Heterogeneity. Further Evidence for Heteropolymers. J. Biol. Chem. 1978, 253, 4451–4458. [Google Scholar] [CrossRef]
- Granick, S. Ferritin: Its properties and significance for iron metabolism. Chem. Rev. 1946, 38, 379–403. [Google Scholar] [CrossRef]
- Jacobs, A.; Worwood, M. Ferritin in Serum Clinical y Bioquimica. N. Engl. J. Med. 1975, 292, 18. [Google Scholar] [CrossRef]
- Jacobs, A.; Miller, F.; Worwood, M.; Beamish, M.R.; Wardrop, C.A.J. Ferritin in the Serum of Normal Subjects and Patients with Iron Deficiency and Iron Overload. Br. Med. J. 1972, 4, 206. [Google Scholar] [CrossRef]
- Knovich, M.A.; Storey, J.A.; Coffman, L.G.; Torti, S.V.; Torti, F.M. Ferritin for the Clinician. Blood Rev. 2009, 23, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.E.; Yu, X.; Kozinetz, C.A.; McClain, K.L. Highly Elevated Ferritin Levels and the Diagnosis of Hemophagocytic Lymphohistiocytosis. Pediatr. Blood Cancer 2008, 50, 1227–1235. [Google Scholar] [CrossRef]
- Martı’n, F.M.; Santolaria, F.; Batista, N.; Milena, A.; Gonzaíez-Reimers, E.; Brito, M.J.; Oramas, J. Cytokine levels (il-6 and ifn-g), acute phase response and nutritional status as prognostic factors in lung cancer. Cytokine 1999, 11, 80–86. [Google Scholar] [CrossRef]
- Nielsen, P.; Günther, U.; Dürken, M.; Fischer, R.; Düllmann, J. Serum Ferritin Iron in Iron Overload and Liver Damage: Correlation to Body Iron Stores and Diagnostic Relevance. J. Lab. Clin. Med. 2000, 135, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Vreugdenhil, G.; Baltust, C.A.M.; Van, H.G.; Swaakf, A.J.G. Anaemia of chronic disease: Diagnostic significance of erythrocyte and serological parameters in iron deficient rheumatoid arthritis patients. Rheumatology 1990, 29, 105–110. [Google Scholar] [CrossRef]
- Mahroum, N.; Alghory, A.; Kiyak, Z.; Alwani, A.; Seida, R.; Alrais, M.; Shoenfeld, Y. Ferritin–from Iron, through Inflammation and Autoimmunity, to COVID-19. J. Autoimmun. 2022, 126, 102778. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, K.; Kaur, H.; Sarma, P.; Bhattacharyya, A.; Sharma, D.J.; Prajapat, M.; Pathak, M.; Kothari, A.; Kumar, S.; Rana, S.; et al. Serum Ferritin as a Predictive Biomarker in COVID-19. A Systematic Review, Meta-Analysis and Meta-Regression Analysis. J. Crit. Care 2022, 67, 172–181. [Google Scholar] [CrossRef]
- Addison, G.M.; Beamish, M.R.; Hales, C.N.; Hodgkins, M.; Jacobs, A.; Llewellin, P. An Immunoradiometric Assay for Ferritin in the Serum of Normal Subjects and Patients with Iron Deficiency and Iron Overload. J. Clin. Pathol. 1972, 25, 326–329. [Google Scholar] [CrossRef]
- Linpisarn, S.; Kricka, L.J.; Kennedy, J.H.; Whitehead, T.P. Sensitive Sandwich Enzyme Immunoassay for Serum Ferritin on Microtitre Plates. Ann. Clin. Biochem. 1981, 18, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Barnett, M.D.; Gordon1, Y.B.; Amess, J.A.L.; Mollin, D.L. Measurement of Ferritin in Serum by Radioimmunoassay. J. Clin. Pathol. 1978, 31, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Yang, F.; Sha, Y.; Yang, X. Real-Time Immunoassay of Ferritin Using Surface Plasmon Resonance Biosensor. Talanta 2003, 60, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.F.; Hsu, W.L.; Hwang, J.M.; Chen, C.Y. Development of an Immunosensor for Human Ferritin, a Nonspecific Tumor Marker, Based on Surface Plasmon Resonance. Biosens. Bioelectron. 2004, 19, 999–1005. [Google Scholar] [CrossRef]
- Morgan, C.L.; Newman, D.J.; Price, C.P. Immunosensors: Technology and Opportunities in Laboratory Medicine. Clin. Chem. 1996, 42, 193–209. [Google Scholar] [CrossRef]
- Cao, C.; Kim, J.P.; Kim, B.W.; Chae, H.; Yoon, H.C.; Yang, S.S.; Sim, S.J. A Strategy for Sensitivity and Specificity Enhancements in Prostate Specific Antigen-A1-Antichymotrypsin Detection Based on Surface Plasmon Resonance. Biosens. Bioelectron. 2006, 21, 2106–2113. [Google Scholar] [CrossRef]
- Seo, G.; Lee, G.; Kim, M.J.; Baek, S.H.; Choi, M.; Ku, K.B.; Lee, C.S.; Jun, S.; Park, D.; Kim, H.G.; et al. Rapid Detection of COVID-19 Causative Virus (SARS-CoV-2) in Human Nasopharyngeal Swab Specimens Using Field-Effect Transistor-Based Biosensor. ACS Nano 2020, 14, 5135–5142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Lieber, C.M. Nano-Bioelectronics. Chem. Rev. 2016, 116, 215–257. [Google Scholar] [CrossRef] [PubMed]
- Fenoy, G.E.; von Bilderling, C.; Knoll, W.; Azzaroni, O.; Marmisollé, W.A. PEDOT:Tosylate-Polyamine-Based Organic Electrochemical Transistors for High-Performance Bioelectronics. Adv. Electron. Mater. 2021, 7, 202100059. [Google Scholar] [CrossRef]
- Christodouleas, D.C.; Kaur, B.; Chorti, P. From Point-of-Care Testing to EHealth Diagnostic Devices (EDiagnostics). ACS Cent. Sci. 2018, 4, 1600–1616. [Google Scholar] [CrossRef]
- Nisar, S.; Dastgeer, G.; Shazad, Z.M.; Zulfiqar, M.W.; Rasheed, A.; Iqbal, M.Z.; Hussain, K.; Rabani, I.; Kim, D.; Irfan, A.; et al. 2D Materials in Advanced Electronic Biosensors for Point-of-Care Devices. Adv. Sci. 2024, 2401386. [Google Scholar] [CrossRef]
- Lee, W.H.; Park, J.; Kim, Y.; Kim, K.S.; Hong, B.H.; Cho, K. Control of Graphene Field-Effect Transistors by Interfacial Hydrophobic Self-Assembled Monolayers. Adv. Mater. 2011, 23, 3460–3464. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and Prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Jiang, L.; van Geest, E.P.; Lima, L.M.C.; Schneider, G.F. Sensing at the Surface of Graphene Field-Effect Transistors. Adv. Mater. 2017, 29, 1603610. [Google Scholar] [CrossRef]
- Her, J.L.; Pan, T.M.; Lin, W.Y.; Wang, K.S.; Li, L.J. Label-Free Detection of Alanine Aminotransferase Using a Graphene Field-Effect Biosensor. Sens. Actuators B Chem. 2013, 182, 396–400. [Google Scholar] [CrossRef]
- Kim, D.J.; Sohn, I.Y.; Jung, J.H.; Yoon, O.J.; Lee, N.E.; Park, J.S. Reduced Graphene Oxide Field-Effect Transistor for Label-Free Femtomolar Protein Detection. Biosens. Bioelectron. 2013, 41, 621–626. [Google Scholar] [CrossRef]
- Ohno, Y.; Maehashi, K.; Matsumoto, K. Chemical and Biological Sensing Applications Based on Graphene Field-Effect Transistors. Biosens. Bioelectron. 2010, 26, 1727–1730. [Google Scholar] [CrossRef]
- Heller, I.; Chatoor, S.; Männik, J.; Zevenbergen, M.A.G.; Dekker, C.; Lemay, S.G. Influence of Electrolyte Composition on Liquid-Gated Carbon Nanotube and Graphene Transistors. J. Am. Chem. Soc. 2010, 132, 17149–17156. [Google Scholar] [CrossRef]
- Salvo, P.; Melai, B.; Calisi, N.; Paoletti, C.; Bellagambi, F.; Kirchhain, A.; Trivella, M.G.; Fuoco, R.; Di Francesco, F. Graphene-Based Devices for Measuring PH. Sens. Actuators B Chem. 2018, 256, 976–991. [Google Scholar] [CrossRef]
- Azzaroni, O.; Knoll, W. Graphene Field-Effect Transistors: Advanced Bioelectronic Devices for Sensing Applications; Wiley: Hoboken, NJ, USA, 2023; ISBN 978-3-527-84338-1. [Google Scholar]
- Sohn, I.Y.; Kim, D.J.; Jung, J.H.; Yoon, O.J.; Nguyen Thanh, T.; Tran Quang, T.; Lee, N.E. PH Sensing Characteristics and Biosensing Application of Solution-Gated Reduced Graphene Oxide Field-Effect Transistors. Biosens. Bioelectron. 2013, 45, 70–76. [Google Scholar] [CrossRef]
- Piccinini, E.; Fenoy, G.E.; Knoll, W.; Marmisollé, W.A.; Azzaroni, O. Polyelectrolyte-Enzyme Assemblies Integrated into Graphene Field-Effect Transistors for Biosensing Applications. In Graphene Field-Effect Transistors; Wiley: Hoboken, NJ, USA, 2023; pp. 285–299. ISBN 9783527843374. [Google Scholar]
- Fu, W.; Nef, C.; Knopfmacher, O.; Tarasov, A.; Weiss, M.; Calame, M.; Schönenberger, C. Graphene Transistors Are Insensitive to PH Changes in Solution. Nano Lett. 2011, 11, 3597–3600. [Google Scholar] [CrossRef]
- Guo, S.; Dong, S. Graphene Nanosheet: Synthesis, Molecular Engineering, Thin Film, Hybrids, and Energy and Analytical Applications. Chem. Soc. Rev. 2011, 40, 2644–2672. [Google Scholar] [CrossRef]
- Zhang, X.; Jing, Q.; Ao, S.; Schneider, G.F.; Kireev, D.; Zhang, Z.; Fu, W. Ultrasensitive Field-Effect Biosensors Enabled by the Unique Electronic Properties of Graphene. Small 2020, 16, 1902820. [Google Scholar] [CrossRef] [PubMed]
- Piccinini, E.; Bliem, C.; Reiner-Rozman, C.; Battaglini, F.; Azzaroni, O.; Knoll, W. Enzyme-Polyelectrolyte Multilayer Assemblies on Reduced Graphene Oxide Field-Effect Transistors for Biosensing Applications. Biosens. Bioelectron. 2017, 92, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Berninger, T.; Bliem, C.; Piccinini, E.; Azzaroni, O.; Knoll, W. Cascading Reaction of Arginase and Urease on a Graphene-Based FET for Ultrasensitive, Real-Time Detection of Arginine. Biosens. Bioelectron. 2018, 115, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Aroonyadet, N.; Wang, X.; Song, Y.; Chen, H.; Cote, R.J.; Thompson, M.E.; Datar, R.H.; Zhou, C. Highly Scalable, Uniform, and Sensitive Biosensors Based on Top-down Indium Oxide Nanoribbons and Electronic Enzyme-Linked Immunosorbent Assay. Nano Lett. 2015, 15, 1943–1951. [Google Scholar] [CrossRef]
- Stem, E.; Vacic, A.; Li, C.; Ishikawa, F.N.; Zhou, C.; Reed, M.A.; Fahmy, T.M. A Nanoelectronic Enzyme-Linked Immunosorbent Assay for Detection of Proteins in Physiological Solutions. Small 2010, 6, 232–238. [Google Scholar] [CrossRef]
- Liu, Q.; Aroonyadet, N.; Song, Y.; Wang, X.; Cao, X.; Liu, Y.; Cong, S.; Wu, F.; Thompson, M.E.; Zhou, C. Highly Sensitive and Quick Detection of Acute Myocardial Infarction Biomarkers Using In2O3 Nanoribbon Biosensors Fabricated Using Shadow Masks. ACS Nano 2016, 10, 10117–10125. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, D.; He, W.; Chen, N.; Zhou, L.; Yu, L.; Yang, Y.; Yuan, Q. Interface-Engineered Field-Effect Transistor Electronic Devices for Biosensing. Adv. Mater. 2023, 2306252. [Google Scholar] [CrossRef]
- Niyogi, S.; Bekyarova, E.; Itkis, M.E.; Zhang, H.; Shepperd, K.; Hicks, J.; Sprinkle, M.; Berger, C.; Lau, C.N.; Deheer, W.A.; et al. Spectroscopy of Covalently Functionalized Graphene. Nano Lett. 2010, 10, 4061–4066. [Google Scholar] [CrossRef]
- Ramasubramaniam, A.; Selhorst, R.; Alon, H.; Barnes, M.D.; Emrick, T.; Naveh, D. Combining 2D Inorganic Semiconductors and Organic Polymers at the Frontier of the Hard-Soft Materials Interface. J. Mater. Chem. C Mater. 2017, 5, 11158–11164. [Google Scholar] [CrossRef]
- Nandanapalli, K.R.; Mudusu, D.; Lee, S. Functionalization of Graphene Layers and Advancements in Device Applications. Carbon 2019, 152, 954–985. [Google Scholar] [CrossRef]
- Piccinini, E.; Allegretto, J.A.; Scotto, J.; Cantillo, A.L.; Fenoy, G.E.; Marmisollé, W.A.; Azzaroni, O. Surface Engineering of Graphene through Heterobifunctional Supramolecular-Covalent Scaffolds for Rapid COVID-19 Biomarker Detection. ACS Appl. Mater. Interfaces 2021, 13, 43696–43707. [Google Scholar] [CrossRef] [PubMed]
- Piccinini, E.; Fenoy, G.E.; Cantillo, A.L.; Allegretto, J.A.; Scotto, J.; Piccinini, J.M.; Marmisollé, W.A.; Azzaroni, O. Biofunctionalization of Graphene-Based FET Sensors through Heterobifunctional Nanoscaffolds: Technology Validation toward Rapid COVID-19 Diagnostics and Monitoring. Adv. Mater. Interfaces 2022, 9, 2102526. [Google Scholar] [CrossRef]
- Wang, J.; Gu, Z.; Liu, X.; Zhao, L.; Peng, H.; Li, J. An Electronic Enzyme-Linked Immunosorbent Assays Platform for Protein Analysis Based on Magnetic Beads and AlGaN/GaN High Electron Mobility Transistors. Analyst 2020, 145, 2725–2730. [Google Scholar] [CrossRef]
- Piccinini, E.; Azzaroni, O.; Marmisollé, W.A.; Piccinini, J.M.; Scotto, J.; Allegretto, J.A.; Cantillo, A.L.; Fenoy, G.E. Sensors and Systems Based on Field-Effect Transistors, Methods of Preparation and Devices for Their Operation. U.S. Patent Application 17/927,841, 27 July 2023. [Google Scholar]
- Goldsmith, B.R.; Locascio, L.; Gao, Y.; Lerner, M.; Walker, A.; Lerner, J.; Kyaw, J.; Shue, A.; Afsahi, S.; Pan, D.; et al. Digital Biosensing by Foundry-Fabricated Graphene Sensors. Sci. Rep. 2019, 9, 434. [Google Scholar] [CrossRef]
- Diforti, J.F.; Cunningham, T.; Piccinini, E.; Marmisollé, W.A.; Piccinini, J.M.; Azzaroni, O. Noninvasive and Multiplex Self-Test of Kidney Disease Biomarkers with Graphene-Based Lab-on-a-Chip (G-LOC): Toward Digital Diagnostics in the Hands of Patients. Anal. Chem. 2024, 96, 5832–5842. [Google Scholar] [CrossRef] [PubMed]
- Diforti, J.F.; Cunningham, T.; Zegalo, Z.; Piccinini, E.; Marmisollé, W.A.; Piccinini, J.M.; Azzaroni, O. Transforming Renal Diagnosis: Graphene-Enhanced Lab-On-a-Chip for Multiplexed Kidney Biomarker Detection in Capillary Blood. Adv. Sens. Res. 2024, 2400061. [Google Scholar] [CrossRef]
- Fenoy, G.E.; Marmisollé, W.A.; Azzaroni, O.; Knoll, W. Acetylcholine Biosensor Based on the Electrochemical Functionalization of Graphene Field-Effect Transistors. Biosens. Bioelectron. 2020, 148, 111796. [Google Scholar] [CrossRef] [PubMed]
- Zhan, B.; Li, C.; Yang, J.; Jenkins, G.; Huang, W.; Dong, X. Graphene Field-Effect Transistor and Its Application for Electronic Sensing. Small 2014, 10, 4042–4065. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Burke, P.J. Polyelectrolyte Multilayer Electrostatic Gating of Graphene Field-Effect Transistors. Nano Res. 2014, 7, 1650–1658. [Google Scholar] [CrossRef]
- Hess, L.H.; Lyuleeva, A.; Blaschke, B.M.; Sachsenhauser, M.; Seifert, M.; Garrido, J.A.; Deubel, F. Graphene Transistors with Multifunctional Polymer Brushes for Biosensing Applications. ACS Appl. Mater. Interfaces 2014, 6, 9705–9710. [Google Scholar] [CrossRef] [PubMed]
- Weber, P.C.; Ohlendorf, D.H.; Wendoloski, J.J.; Salemme, F.R. Structural Origins of High-Affinity Biotin Binding. Science 1987, 391, 85–88. [Google Scholar] [CrossRef]
- Michael Green, N. Avidin and Streptavidin. Methods Enzym. 1990, 184, 51–67. [Google Scholar] [CrossRef]
- Grubmüller, H.; Heymann, B.; Tavan, P. Ligand Binding: Molecular Mechanics Calculation of the Streptavidin-Biotin Rupture Force. Science 1996, 271, 997–999. [Google Scholar] [CrossRef] [PubMed]
- Peterson, R.D.; Cunningham, B.T.; Andrade, J.E. A Photonic Crystal Biosensor Assay for Ferritin Utilizing Iron-Oxide Nanoparticles. Biosens. Bioelectron. 2014, 56, 320–327. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Yang, H.; Pan, J.; Cui, X.; Shen, D.; Eremin, S.A.; Fang, Y.; Zhao, S. Lateral Flow Immunosensor for Ferritin Based on Dual Signal-Amplified Strategy by Rhodium Nanoparticles. ACS Appl. Bio Mater. 2020, 3, 8849–8856. [Google Scholar] [CrossRef] [PubMed]
- Boonkaew, S.; Teengam, P.; Jampasa, S.; Rengpipat, S.; Siangproh, W.; Chailapakul, O. Cost-Effective Paper-Based Electrochemical Immunosensor Using a Label-Free Assay for Sensitive Detection of Ferritin. Analyst 2020, 145, 5019–5026. [Google Scholar] [CrossRef] [PubMed]
- Yen, L.C.; Pan, T.M.; Lee, C.H.; Chao, T.S. Label-Free and Real-Time Detection of Ferritin Using a Horn-like Polycrystalline-Silicon Nanowire Field-Effect Transistor Biosensor. Sens. Actuators B Chem. 2016, 230, 398–404. [Google Scholar] [CrossRef]
- Senjo, H.; Higuchi, T.; Okada, S.; Takahashi, O. Hyperferritinemia: Causes and Significance in a General Hospital. Hematology 2018, 23, 817–822. [Google Scholar] [CrossRef]
- Hajian, R.; Balderston, S.; Tran, T.; deBoer, T.; Etienne, J.; Sandhu, M.; Wauford, N.A.; Chung, J.Y.; Nokes, J.; Athaiya, M.; et al. Detection of Unamplified Target Genes via CRISPR–Cas9 Immobilized on a Graphene Field-Effect Transistor. Nat. Biomed. Eng. 2019, 3, 427–437. [Google Scholar] [CrossRef]


| FET Material | IDS Range | IDS Dependence with pH | Urea for Readout | Analyte | Portable | Reference |
|---|---|---|---|---|---|---|
| In2O3 | 0–1 µA | Log (IDS) α pH | 100 mM | HIV-1 p24 | No | [44] |
| In2O3 | 0–2 µA | Log (IDS) α pH | 105:1 (urea to urease) | IL-2 | No | [45] |
| In2O3 | 0–2 µA | Log (IDS) α pH | 10 mM | Troponin I | No | [46] |
| rGO | 100–500 µA | IDS α pH | 1 mM | Ferritin | Yes | this work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Candia, M.L.; Piccinini, E.; Azzaroni, O.; Marmisollé, W.A. Digitalization of Enzyme-Linked Immunosorbent Assay with Graphene Field-Effect Transistors (G-ELISA) for Portable Ferritin Determination. Biosensors 2024, 14, 394. https://doi.org/10.3390/bios14080394
Candia ML, Piccinini E, Azzaroni O, Marmisollé WA. Digitalization of Enzyme-Linked Immunosorbent Assay with Graphene Field-Effect Transistors (G-ELISA) for Portable Ferritin Determination. Biosensors. 2024; 14(8):394. https://doi.org/10.3390/bios14080394
Chicago/Turabian StyleCandia, Melody L., Esteban Piccinini, Omar Azzaroni, and Waldemar A. Marmisollé. 2024. "Digitalization of Enzyme-Linked Immunosorbent Assay with Graphene Field-Effect Transistors (G-ELISA) for Portable Ferritin Determination" Biosensors 14, no. 8: 394. https://doi.org/10.3390/bios14080394
APA StyleCandia, M. L., Piccinini, E., Azzaroni, O., & Marmisollé, W. A. (2024). Digitalization of Enzyme-Linked Immunosorbent Assay with Graphene Field-Effect Transistors (G-ELISA) for Portable Ferritin Determination. Biosensors, 14(8), 394. https://doi.org/10.3390/bios14080394







