Microspectrometer-Enabled Real-Time Concentration Monitoring in the Microfluidic Protein Enrichment Chip
Abstract
:1. Introduction
2. Design and Working Principle
3. Experimental Setup
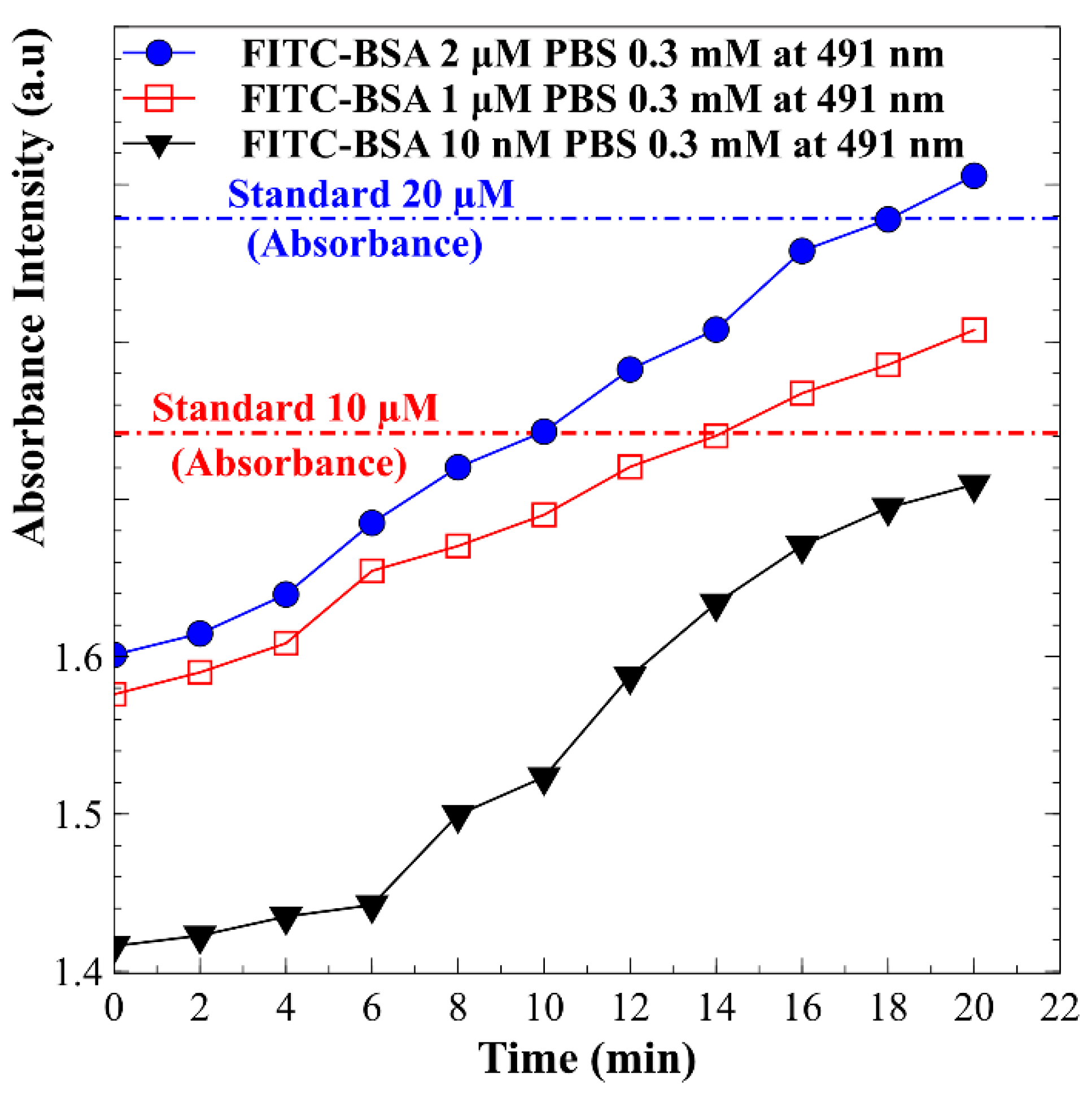
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, M.; Gupta, A.; Zhang, X.; Chattopadhyay, A.N.; Fedeli, S.; Huang, R.; Yang, J.; Rotello, V.M. Identification of Proteins Using Supramolecular Gold Nanoparticle-Dye Sensor Arrays. Anal. Sens. 2023, 3, e202200080. [Google Scholar] [CrossRef] [PubMed]
- Duffy, D.C. Digital detection of proteins. Lab. Chip 2023, 23, 818–847. [Google Scholar] [CrossRef]
- Momenbeitollahi, N.; Cloet, T.; Li, H. Pushing the detection limits: Strategies towards highly sensitive optical-based protein detection. Anal. Bioanal. Chem. 2021, 413, 5995–6011. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Lu, Y.; Zhang, Q.; Liu, L.; Li, S.; Yao, Y.; Jiang, J.; Liu, G.L.; Liu, Q. Protein detecting with smartphone-controlled electrochemical impedance spectroscopy for point-of-care applications. Sens. Actuators B Chem. 2016, 222, 994–1002. [Google Scholar] [CrossRef]
- de Los Santos-Ramirez, J.M.; Boyas-Chavez, P.G.; Cerrillos-Ordoñez, A.; Mata-Gomez, M.; Gallo-Villanueva, R.C.; Perez-Gonzalez, V.H. Trends and challenges in microfluidic methods for protein manipulation—A review. Electrophoresis 2024, 45, 69–100. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Wu, H.F.; Amstislavskaya, T.G.; Li, C.-Y.; Jen, C.-P. A simple electrokinetic protein preconcentrator utilizing nano-interstices. Biomicrofluidics 2016, 10, 024121. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Zhou, M.; Liu, X.; Li, Q.; Drummer, D.; Jiang, B. Investigation of parameters and porous plug enhanced enrichment with field-amplified sample stacking in microchip. Phys. Fluids 2023, 35, 012017. [Google Scholar] [CrossRef]
- Chen, D.; Timperman, A.T. Analyte Enrichment via Ion Concentration Polarization with Hydrogel Plugs Polymerized in PDMS Microchannels by a Facile and Comprehensive Method for Improved Polymerization. Anal. Chem. 2022, 94, 15586–15594. [Google Scholar] [CrossRef]
- Gholinejad, M.; Jabari Moghadam, A.; Mousavi Shaegh, S.A. Analysis of preconcentration patterns in microfluidic ion concentration polarization devices. Phys. Fluids 2022, 34, 012014. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiao, Y.; Wang, C.; Zhang, C.; Wang, H.; Feng, Z.; Gu, Y.; Wang, Z. Design and characterization of small-diameter tissue-engineered blood vessels constructed by electrospun polyurethane-core and gelatin-shell coaxial fiber. Bioengineered 2021, 12, 5769–5788. [Google Scholar] [CrossRef] [PubMed]
- Noble, J.E. Quantification of protein concentration using UV absorbance and Coomassie dyes. Methods Enzymol. 2014, 536, 17–26. [Google Scholar] [PubMed]
- Fang, X.; Zheng, Y.; Duan, Y.; Liu, Y.; Zhong, W. Recent Advances in Design of Fluorescence-Based Assays for High-Throughput Screening. Anal. Chem. 2019, 91, 482–504. [Google Scholar] [CrossRef]
- Birhanu, A.G. Mass spectrometry-based proteomics as an emerging tool in clinical laboratories. Clin. Proteom. 2023, 20, 32. [Google Scholar] [CrossRef] [PubMed]
- Verdaasdonk, J.S.; Lawrimore, J.; Bloom, K. Determining absolute protein numbers by quantitative fluorescence microscopy. Methods Cell Biol. 2014, 123, 347–365. [Google Scholar] [PubMed]
- Xia, Y.; Whitesides, G.M. Soft lithography. Annu. Rev. Mater. Res. 1998, 28, 153–184. [Google Scholar]
- Lee, J.H.; Song, Y.-A.; Han, J. Multiplexed proteomic sample preconcentration device using surface-patterned ion-selective membrane. Lab. Chip 2008, 8, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Yang, H.; Sivagnanam, V.; Gijs, M.A.M. Microfluidic protein preconcentrator using a microchannel-integrated nafion strip: Experiment and modeling. Anal. Chem. 2010, 82, 9989–9997. [Google Scholar] [CrossRef]
- Tran Nhu, C.; Do Quang, L.; Jen, C.-P.; Chu Duc, T.; Thanh, T.B. Development of a Protein Enrichment and Detection Microfluidic Platform Based on Ion Concentration Polarization (ICP) and Electrochemical Impedance Spectroscopy (EIS) Techniques. IEEE Sens. Lett. 2024, 8, 4502904. [Google Scholar] [CrossRef]
- Wu, H.F.; Amstislavskaya, T.G.; Chen, P.-H.; Wu, T.-F.; Chen, Y.-H.; Jen, C.-P. Preconcentration-enhanced immunosensing for whole human cancer cell lysate based on a nanofluidic preconcentrator. BioChip J. 2016, 10, 159–166. [Google Scholar] [CrossRef]
- Chiang, P.-J.; Kuo, C.-C.; Zamay, T.N.; Zamay, A.S.; Jen, C.-P. Quantitative evaluation of the depletion efficiency of nanofractures generated by nanoparticle-assisted junction gap breakdown for protein concentration. Microelectron. Eng. 2014, 115, 39–45. [Google Scholar] [CrossRef]
- Goldfarb, A.R.; Saidel, L.J. Ultraviolet absorption spectra of proteins. Science 1951, 114, 156–157. [Google Scholar] [CrossRef] [PubMed]
- Miranda, S.; Vilanova, A.; Lopes, T.; Mendes, A. TiO2-coated window for facilitated gas evolution in PEC solar water splitting. RSC Adv. 2017, 7, 29665–29671. [Google Scholar] [CrossRef]
- Gross, A.; Stangl, F.; Hoenes, K.; Sift, M.; Hessling, M. Improved Drinking Water Disinfection with UVC-LEDs for Escherichia Coli and Bacillus Subtilis Utilizing Quartz Tubes as Light Guide. Water 2015, 7, 4605–4621. [Google Scholar] [CrossRef]
- Sheng, J.; Wu, Y.; Yang, X.; Zhang, J. UV-laser irradiation on the soda-lime silicate glass. Int. J. Hydrogen Energy 2009, 34, 1123–1125. [Google Scholar] [CrossRef]
- Zhou, H.; Gao, Y.; Liu, Y.; Wu, Y.; Fang, Y.; Wang, B.; Xu, B. Targeted fluorescent imaging of a novel FITC-labeled PSMA ligand in prostate cancer. Amino Acids 2022, 54, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Fang, C.; Li, B.; Zhang, Z.; Cao, C.; Cai, M.; Su, S.; Sun, X.; Shi, X.; Li, C.; et al. First-in-human liver-tumour surgery guided by multispectral fluorescence imaging in the visible and near-infrared-I/II windows. Nat. Biomed. Eng. 2020, 4, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Hungerford, G.; Benesch, J.; Mano, J.F.; Reis, R.L. Effect of the labelling ratio on the photophysics of fluorescein isothiocyanate (FITC) conjugated to bovine serum albumin. Photochem. Photobiol. Sci. Off. J. Eur. Photochem. Assoc. Eur. Soc. Photobiol. 2007, 6, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H. A Simple Outline of Methods for Protein Isolation and Purification. Endocrinol. Metab. 2017, 32, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Bukusoglu, E.; Koku, H.; Çulfaz-Emecen, P.Z. Addressing challenges in the ultrafiltration of biomolecules from complex aqueous environments. Curr. Opin. Colloid Interface Sci. 2020, 46, 52–64. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.-L.; Huang, W.-S.; Wu, Y.H.; Jen, C.-P. Microspectrometer-Enabled Real-Time Concentration Monitoring in the Microfluidic Protein Enrichment Chip. Biosensors 2025, 15, 1. https://doi.org/10.3390/bios15010001
Li D-L, Huang W-S, Wu YH, Jen C-P. Microspectrometer-Enabled Real-Time Concentration Monitoring in the Microfluidic Protein Enrichment Chip. Biosensors. 2025; 15(1):1. https://doi.org/10.3390/bios15010001
Chicago/Turabian StyleLi, Dong-Li, Wen-Shu Huang, Yi Hung Wu, and Chun-Ping Jen. 2025. "Microspectrometer-Enabled Real-Time Concentration Monitoring in the Microfluidic Protein Enrichment Chip" Biosensors 15, no. 1: 1. https://doi.org/10.3390/bios15010001
APA StyleLi, D.-L., Huang, W.-S., Wu, Y. H., & Jen, C.-P. (2025). Microspectrometer-Enabled Real-Time Concentration Monitoring in the Microfluidic Protein Enrichment Chip. Biosensors, 15(1), 1. https://doi.org/10.3390/bios15010001





