Fully Inkjet-Printed Flexible Graphene–Prussian Blue Platform for Electrochemical Biosensing
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Inkjet Printing, Post-Print Treatment, and Characterization of Graphene Electrodes
2.3. Synthesis and Characterization of Prussian Blue
2.3.1. Chemical Synthesis
2.3.2. Prussian Blue Nanoparticle Suspension
2.4. Electrochemical Measurements
2.5. Lactate Oxidase Immobilization
3. Results
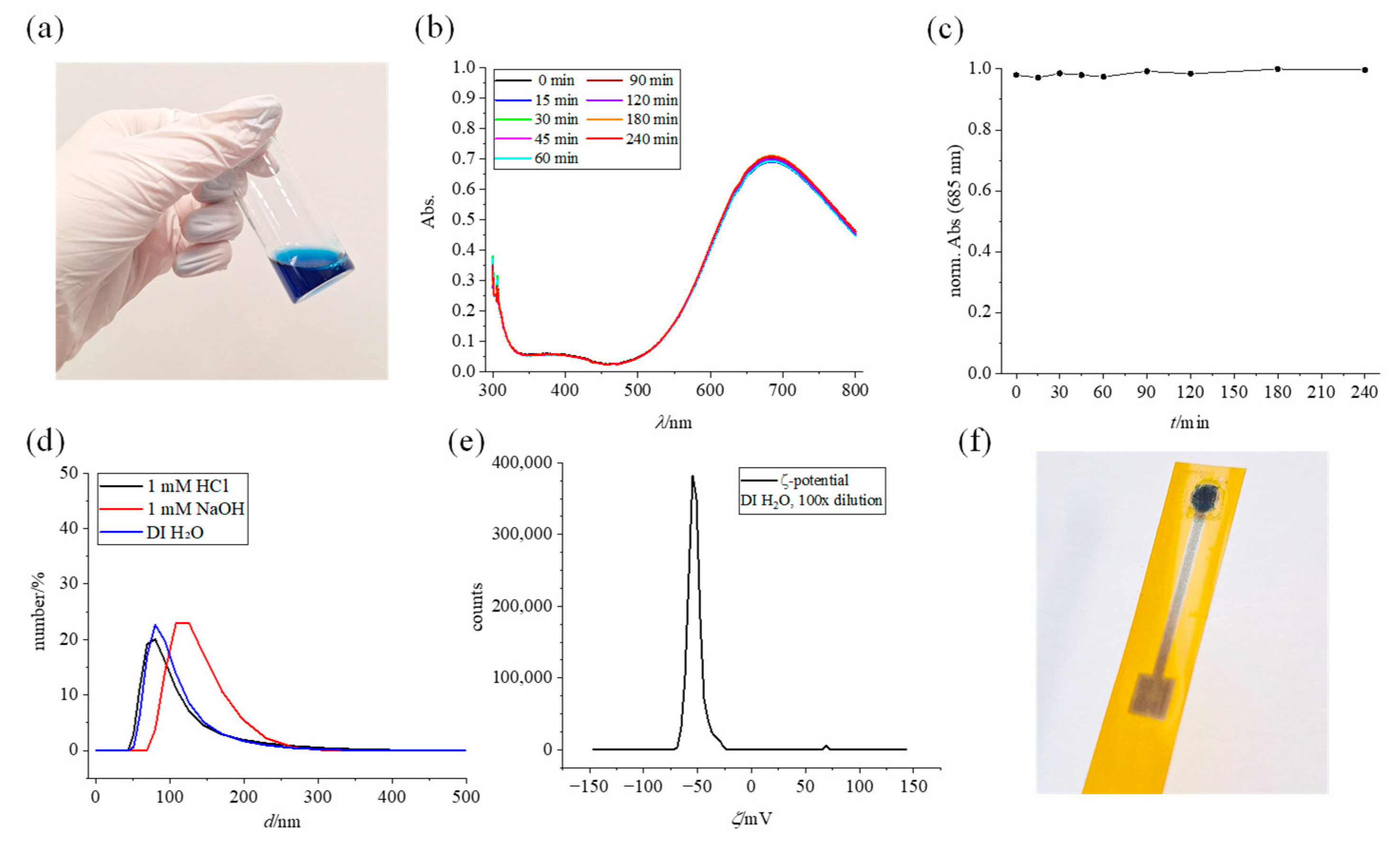
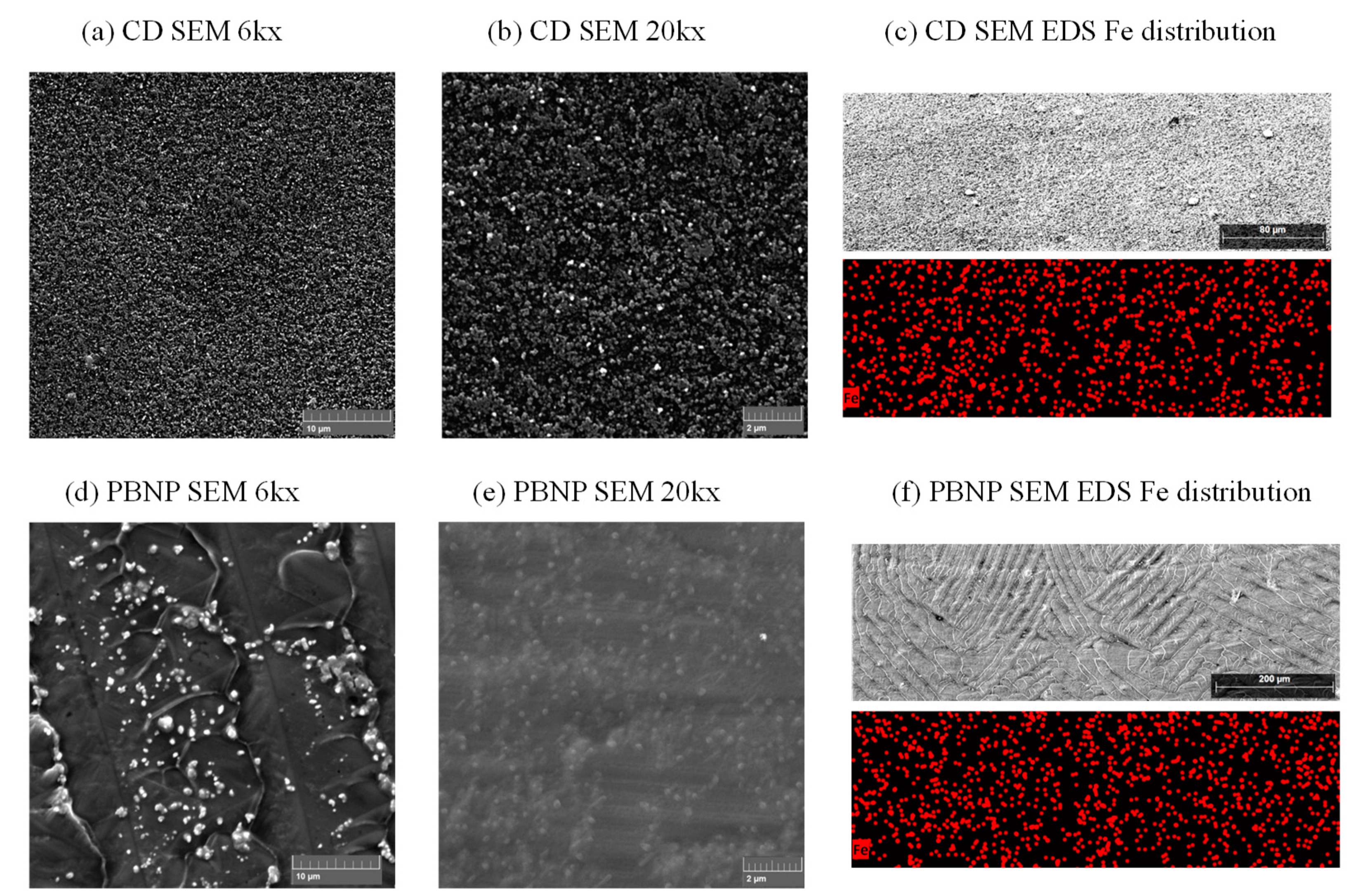
3.1. Prussian Blue Ink Characterization
3.2. Evaluation of PB Deposition Methods
3.2.1. Glassy Carbon Electrodes
3.2.2. Screen-Printed Carbon Electrodes
3.2.3. Inkjet-Printed Electrodes
3.3. Chronoamperometric Detections
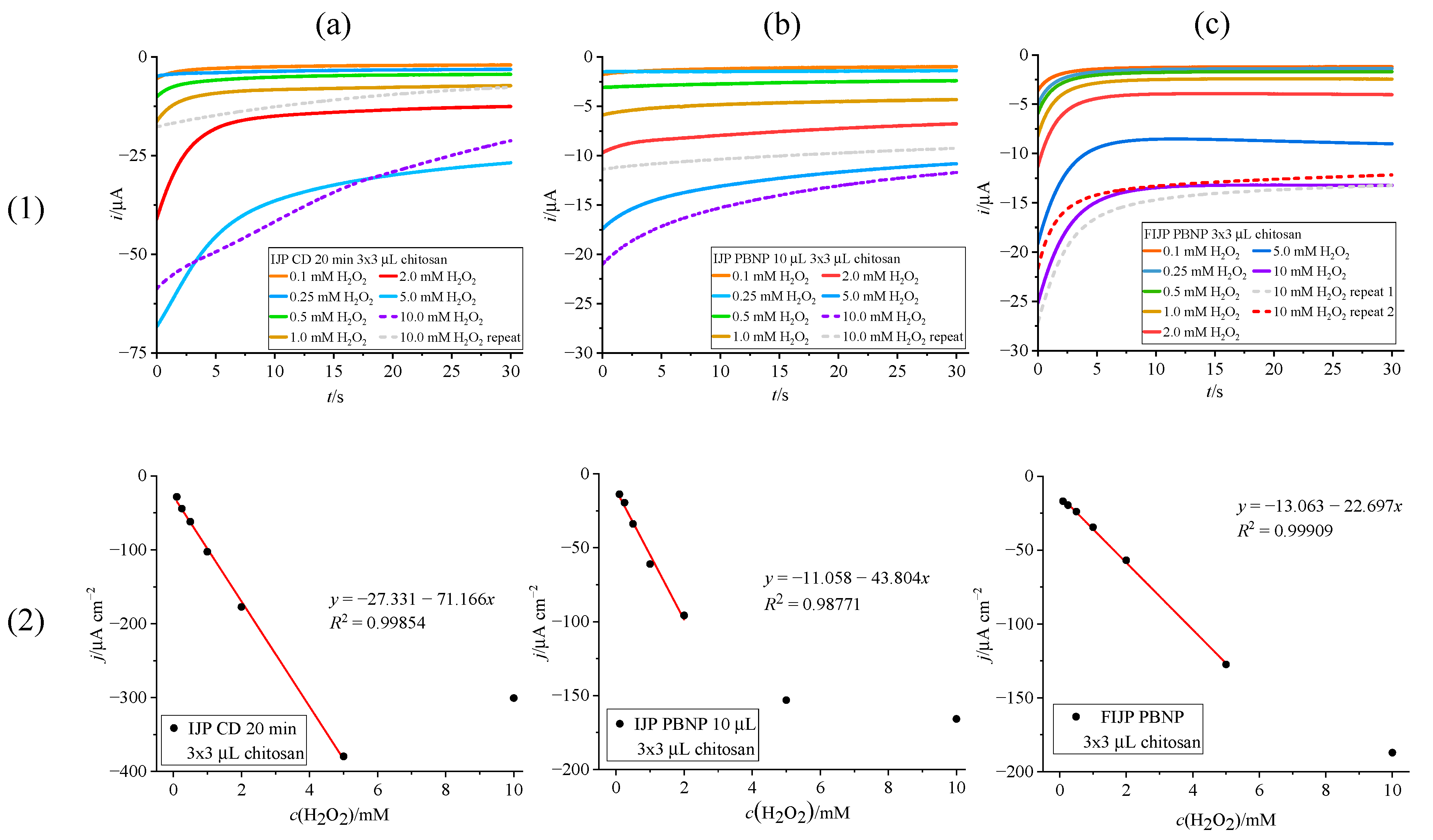
3.3.1. H2O2 Detection on Prussian Blue Modified Inkjet-Printed Electrodes with Chitosan Dummy Layer
3.3.2. Lactate Biosensor Assembly
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karyakin, A.A.; Karyakina, E.E. Prussian Blue-based ‘artificial peroxidase’ as a transducer for hydrogen peroxide detection. Application to biosensors. Sens. Actuators B Chem. 1999, 57, 268–273. [Google Scholar] [CrossRef]
- Ricci, F.; Amine, A.; Tuta, C.S.; Ciucu, A.A.; Lucarelli, F.; Palleschi, G.; Moscone, D. Prussian Blue and enzyme bulk-modified screen-printed electrodes for hydrogen peroxide and glucose determination with improved storage and operational stability. Anal. Chim. Acta 2003, 485, 111–120. [Google Scholar] [CrossRef]
- Vokhmyanina, D.; Daboss, E.; Sharapova, O.; Mogilnikova, M.; Karyakin, A. Single Printing Step Prussian Blue Bulk-Modified Transducers for Oxidase-Based Biosensors. Biosensors 2023, 13, 250. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S.; Arduini, F.; Moscone, D.; Palleschi, G.; Gonzalez-Macia, L.; Killard, A.J. Cholesterol biosensor based on inkjet-printed Prussian blue nanoparticle-modified screen-printed electrodes. Sens. Actuators B Chem. 2015, 221, 187–190. [Google Scholar] [CrossRef]
- Ricci, F.; Amine, A.; Palleschi, G.; Moscone, D. Prussian Blue based screen printed biosensors with improved characteristics of long-term lifetime and pH stability. Biosens. Bioelectron. 2003, 18, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Kassal, P.; Kim, J.; Kumar, R.; de Araujo, W.R.; Steinberg, I.M.; Steinberg, M.D.; Wang, J. Smart bandage with wireless connectivity for uric acid biosensing as an indicator of wound status. Electrochem. Commun. 2015, 56, 6–10. [Google Scholar] [CrossRef]
- Xuan, X.; Pérez-Ràfols, C.; Chen, C.; Cuartero, M.; Crespo, G.A. Lactate Biosensing for Reliable On-Body Sweat Analysis. ACS Sens. 2021, 6, 2763–2771. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef]
- Vinoth, R.; Nakagawa, T.; Mathiyarasu, J.; Mohan, A.M.V. Fully Printed Wearable Microfluidic Devices for High-Throughput Sweat Sampling and Multiplexed Electrochemical Analysis. ACS Sens. 2021, 6, 1174–1186. [Google Scholar] [CrossRef]
- Imani, S.; Bandodkar, A.J.; Mohan, A.M.V.; Kumar, R.; Yu, S.; Wang, J.; Mercier, P.P. A wearable chemical–electrophysiological hybrid biosensing system for real-time health and fitness monitoring. Nat. Commun. 2016, 7, 11650. [Google Scholar] [CrossRef]
- Wang, R.; Zhai, Q.; An, T.; Gong, S.; Cheng, W. Stretchable gold fiber-based wearable textile electrochemical biosensor for lactate monitoring in sweat. Talanta 2021, 222, 121484. [Google Scholar] [CrossRef] [PubMed]
- Karyakin, A.A.; Karyakina, E.E.; Gorton, L. On the mechanism of H2O2 reduction at Prussian Blue modified electrodes. Electrochem. Commun. 1999, 1, 78–82. [Google Scholar] [CrossRef]
- Haghighi, B.; Varma, S.; Alizadeh, F.M.; Yigzaw, Y.; Gorton, L. Prussian blue modified glassy carbon electrodes—Study on operational stability and its application as a sucrose biosensor. Talanta 2004, 64, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, R.; Blondeau, P.; Andrade, F.J. Distributed electrochemical sensors: Recent advances and barriers to market adoption. Anal. Bioanal. Chem. 2018, 410, 4077–4089. [Google Scholar] [CrossRef] [PubMed]
- O’Halloran, M.P.; Pravda, M.; Guilbault, G.G. Prussian Blue bulk modified screen-printed electrodes for H2O2 detection and for biosensors. Talanta 2001, 55, 605–611. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Lin, M.; Yin, L.; De la paz, E.; Pei, K.; Sonsa-ard, T.; de Loyola Silva, A.N.; Khorshed, A.A.; Zhang, F.; Tostado, N.; et al. An epidermal patch for the simultaneous monitoring of haemodynamic and metabolic biomarkers. Nat. Biomed. Eng. 2021, 5, 737–748. [Google Scholar] [CrossRef]
- Xu, Y.; De la Paz, E.; Paul, A.; Mahato, K.; Sempionatto, J.R.; Tostado, N.; Lee, M.; Hota, G.; Lin, M.; Uppal, A.; et al. In-ear integrated sensor array for the continuous monitoring of brain activity and of lactate in sweat. Nat. Biomed. Eng. 2023, 7, 1307–1320. [Google Scholar] [CrossRef] [PubMed]
- Ambaye, A.D.; Kefeni, K.K.; Mishra, S.B.; Nxumalo, E.N.; Ntsendwana, B. Recent developments in nanotechnology-based printing electrode systems for electrochemical sensors. Talanta 2021, 225, 121951. [Google Scholar] [CrossRef]
- Moya, A.; Gabriel, G.; Villa, R.; Javier del Campo, F. Inkjet-printed electrochemical sensors. Curr. Opin. Electrochem. 2017, 3, 29–39. [Google Scholar] [CrossRef]
- Rossetti, M.; Srisomwat, C.; Urban, M.; Rosati, G.; Maroli, G.; Yaman Akbay, H.G.; Chailapakul, O.; Merkoçi, A. Unleashing inkjet-printed nanostructured electrodes and battery-free potentiostat for the DNA-based multiplexed detection of SARS-CoV-2 genes. Biosens. Bioelectron. 2024, 250, 116079. [Google Scholar] [CrossRef]
- Setti, L.; Fraleoni-Morgera, A.; Mencarelli, I.; Filippini, A.; Ballarin, B.; Di Biase, M. An HRP-based amperometric biosensor fabricated by thermal inkjet printing. Sens. Actuators B Chem. 2007, 126, 252–257. [Google Scholar] [CrossRef]
- Das, S.R.; Nian, Q.; Cargill, A.A.; Hondred, J.A.; Ding, S.; Saei, M.; Cheng, G.J.; Claussen, J.C. 3D nanostructured inkjet printed graphene via UV-pulsed laser irradiation enables paper-based electronics and electrochemical devices. Nanoscale 2016, 8, 15870–15879. [Google Scholar] [CrossRef]
- Silvestri, A.; Vázquez-Díaz, S.; Misia, G.; Poletti, F.; López-Domene, R.; Pavlov, V.; Zanardi, C.; Cortajarena, A.L.; Prato, M. An Electroactive and Self-Assembling Bio-Ink, based on Protein-Stabilized Nanoclusters and Graphene, for the Manufacture of Fully Inkjet-Printed Paper-Based Analytical Devices. Small 2023, 19, 2300163. [Google Scholar] [CrossRef] [PubMed]
- Mass, M.; Veiga, L.S.; Garate, O.; Longinotti, G.; Moya, A.; Ramón, E.; Villa, R.; Ybarra, G.; Gabriel, G. Fully Inkjet-Printed Biosensors Fabricated with a Highly Stable Ink Based on Carbon Nanotubes and Enzyme-Functionalized Nanoparticles. Nanomaterials 2021, 11, 1645. [Google Scholar] [CrossRef]
- Shi, L.; Layani, M.; Cai, X.; Zhao, H.; Magdassi, S.; Lan, M. An inkjet printed Ag electrode fabricated on plastic substrate with a chemical sintering approach for the electrochemical sensing of hydrogen peroxide. Sens. Actuators B Chem. 2018, 256, 938–945. [Google Scholar] [CrossRef]
- Shamkhalichenar, H.; Choi, J.-W. An Inkjet-Printed Non-Enzymatic Hydrogen Peroxide Sensor on Paper. J. Electrochem. Soc. 2017, 164, B3101. [Google Scholar] [CrossRef]
- Cinti, S.; Arduini, F.; Moscone, D.; Palleschi, G.; Killard, A.J. Development of a Hydrogen Peroxide Sensor Based on Screen-Printed Electrodes Modified with Inkjet-Printed Prussian Blue Nanoparticles. Sensors 2014, 14, 14222–14234. [Google Scholar] [CrossRef] [PubMed]
- Ivanišević, I.; Kovačić, M.; Zubak, M.; Ressler, A.; Krivačić, S.; Katančić, Z.; Gudan Pavlović, I.; Kassal, P. Amphiphilic Silver Nanoparticles for Inkjet-Printable Conductive Inks. Nanomaterials 2022, 12, 4252. [Google Scholar] [CrossRef]
- Kralj, M.; Krivačić, S.; Ivanišević, I.; Zubak, M.; Supina, A.; Marciuš, M.; Halasz, I.; Kassal, P. Conductive Inks Based on Melamine Intercalated Graphene Nanosheets for Inkjet Printed Flexible Electronics. Nanomaterials 2022, 12, 2936. [Google Scholar] [CrossRef]
- Secor, E.B.; Ahn, B.Y.; Gao, T.Z.; Lewis, J.A.; Hersam, M.C. Rapid and Versatile Photonic Annealing of Graphene Inks for Flexible Printed Electronics. Adv. Mater. 2015, 27, 6683–6688. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Miao, Y.; Wu, X. Immobilization of Prussian blue nanoparticles onto thiol SAM modified Au electrodes for analysis of DL-homocysteine. Colloid J. 2007, 69, 660–665. [Google Scholar] [CrossRef]
- Hu, J.Y.; Lin, Y.P.; Liao, Y.C. Inkjet Printed Prussian Blue Films for Hydrogen Peroxide Detection. Anal. Sci. 2012, 28, 135–140. [Google Scholar] [CrossRef]
- Pajor-Świerzy, A.; Kruk, T.; Warszyński, P. Enhancement of the Electrocatalytic Properties of Prussian Blue Containing Multilayer Films Formed by Reduced Graphene Oxide. Colloids Interface Sci. Commun. 2014, 1, 6–9. [Google Scholar] [CrossRef]
- Wang, Z.W.; Yang, H.K.; Gao, B.W.; Tong, Y.; Zhang, X.J.; Su, L. Stability improvement of Prussian blue in nonacidic solutions via an electrochemical post-treatment method and the shape evolution of Prussian blue from nanospheres to nanocubes. Analyst 2014, 139, 1127–1133. [Google Scholar] [CrossRef]
- Roig, A.; Navarro, J.; Garcia, J.J.; Vicente, F. Voltammetric study on the stability of deposited prussian blue films against successive potential cycling. Electrochim. Acta 1994, 39, 437–442. [Google Scholar] [CrossRef]
- Dadić, M. Electrical Conductivity of the Complex Cyanides of the Prussian Blue Type. Croat. Chem. Acta 1960, 31, 101–106. [Google Scholar]
- de Mattos, I.L.; Gorton, L.; Ruzgas, T.; Karyakin, A.A. Sensor for hydrogen peroxide based on Prussian Blue modified electrode: Improvement of the operational stability. Anal. Sci. 2000, 16, 795–798. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Gunjikar, V.G.; Choudhary, V.R. Thermal analysis of ethyl cellulose: Influence of atmosphere and addition of diethyl phthalate as plasticizer. Thermochim. Acta 1989, 145, 173–178. [Google Scholar] [CrossRef]
- Lillis, B.; Grogan, C.; Berney, H.; Lane, W.A. Investigation into immobilisation of lactate oxidase to improve stability. Sens. Actuators B Chem. 2000, 68, 109–114. [Google Scholar] [CrossRef]
- Lyu, X.; Gonzalez, R.; Horton, A.; Li, T. Immobilization of Enzymes by Polymeric Materials. Catalysts 2021, 11, 1211. [Google Scholar] [CrossRef]
- Shukla, S.K.; Mishra, A.K.; Arotiba, O.A.; Mamba, B.B. Chitosan-based nanomaterials: A state-of-the-art review. Int. J. Biol. Macromol. 2013, 59, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Zamarayeva, A.M.; Yamamoto, N.A.D.; Toor, A.; Payne, M.E.; Woods, C.; Pister, V.I.; Khan, Y.; Evans, J.W.; Arias, A.C. Optimization of printed sensors to monitor sodium, ammonium, and lactate in sweat. APL Mater. 2020, 8, 100905. [Google Scholar] [CrossRef]
- Boeva, Z.; Mousavi, Z.; Sokalski, T.; Bobacka, J. Recent trends in non-invasive on-body chemical sensing. TrAC Trends Anal. Chem. 2024, 172, 117542. [Google Scholar] [CrossRef]
- Vokhmyanina, D.V.; Sharapova, O.E.; Buryanovataya, K.E.; Karyakin, A.A. Novel Siloxane Derivatives as Membrane Precursors for Lactate Oxidase Immobilization. Sensors 2023, 23, 4014. [Google Scholar] [CrossRef]




| Substrate | Electroactive Material | Type | Operating Potential/V | Linear Range/mM | Sensitivity /µA mM−1 cm−2 | Ref. |
|---|---|---|---|---|---|---|
| Graphite SPE | PBNP | SPE with IJP PB | 0 | 0.001–4.5 | 762 | [27] |
| Carbon SPE | PBNP | SPE with IJP PB | +0.15 | 0.02–0.7 | 0.164 | [32] |
| ITO coated glass | PEDOT:PSS/HRP | FIJP | −0.1 | 0.25–1 | 0.544 | [21] |
| Polyimide, paper | graphene | FIJP | +0.5 | 0.2–1.1 | 3.32 | [22] |
| Paper | graphene PtAuNC@ C6His16 | FIJP | +0.4 | 0.05–1 | 32.3 | [23] |
| PET | SWCNT, SiO2 NPs, HRP | FIJP | −0.23 | 1–3 | 57 | [24] |
| PVC | AgNP | FIJP | −0.4 | 0.1–6.8 | 287 | [25] |
| Paper | MWCNTs, AgNPs | FIJP | −0.3 | 0.001–0.7 | - | [26] |
| PI/graphene | Prussian Blue (covered with chitosan) | IJP with CD PB | −0.1 | 0.1–5 | 71.17 | This work |
| PI/graphene | Prussian Blue (covered with chitosan) | IJP with drop-cast PBNP | −0.1 | 0.1–2 | 43.80 | This work |
| PI/graphene | Prussian Blue (covered with chitosan) | FIJP (printed PBNP) | −0.1 | 0.1–5 | 22.70 | This work |
| Substrate | PB Deposition Method | Operating Potential/V | Linear Range/mM | Sensitivity 1 /µA mM−1 cm−2 | Ref. |
|---|---|---|---|---|---|
| SPE carbon | chemical | −0.05 | 1–50 | 0.30 | [7] |
| Au | electrodeposition | 0 | 2–30 | 3.11 | [8] |
| SPE carbon | drop-cast PBNPs | −0.17 | 1–25 | 0.031 | [9] |
| SPE carbon/PB | screen printing | −0.1 | 0–28 | 1.28 | [10] |
| SPE carbon/PB | screen printing | −0.2 | 0–30 | 0.3 | [16] |
| SPE carbon/PB | screen printing | −0.2 | 0–20 | 0.35 | [17] |
| IJP graphene | chemical | −0.3 | 3–25 | 0.5113 | This work |
| IJP graphene | drop-cast PBNPs | −0.3 | 5–25 | 0.2547 | This work |
| IJP graphene | inkjet-printed PBNPs | −0.3 | 3–50 | 0.1885 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boček, Ž.; Zubak, M.; Kassal, P. Fully Inkjet-Printed Flexible Graphene–Prussian Blue Platform for Electrochemical Biosensing. Biosensors 2025, 15, 28. https://doi.org/10.3390/bios15010028
Boček Ž, Zubak M, Kassal P. Fully Inkjet-Printed Flexible Graphene–Prussian Blue Platform for Electrochemical Biosensing. Biosensors. 2025; 15(1):28. https://doi.org/10.3390/bios15010028
Chicago/Turabian StyleBoček, Željka, Marko Zubak, and Petar Kassal. 2025. "Fully Inkjet-Printed Flexible Graphene–Prussian Blue Platform for Electrochemical Biosensing" Biosensors 15, no. 1: 28. https://doi.org/10.3390/bios15010028
APA StyleBoček, Ž., Zubak, M., & Kassal, P. (2025). Fully Inkjet-Printed Flexible Graphene–Prussian Blue Platform for Electrochemical Biosensing. Biosensors, 15(1), 28. https://doi.org/10.3390/bios15010028





