Optical Fiber TFBG Glucose Biosensor via pH-Sensitive Polyelectrolyte Membrane
Abstract
1. Introduction
2. Experimental System and Method
2.1. Materials and Reagents
2.2. Experimental System
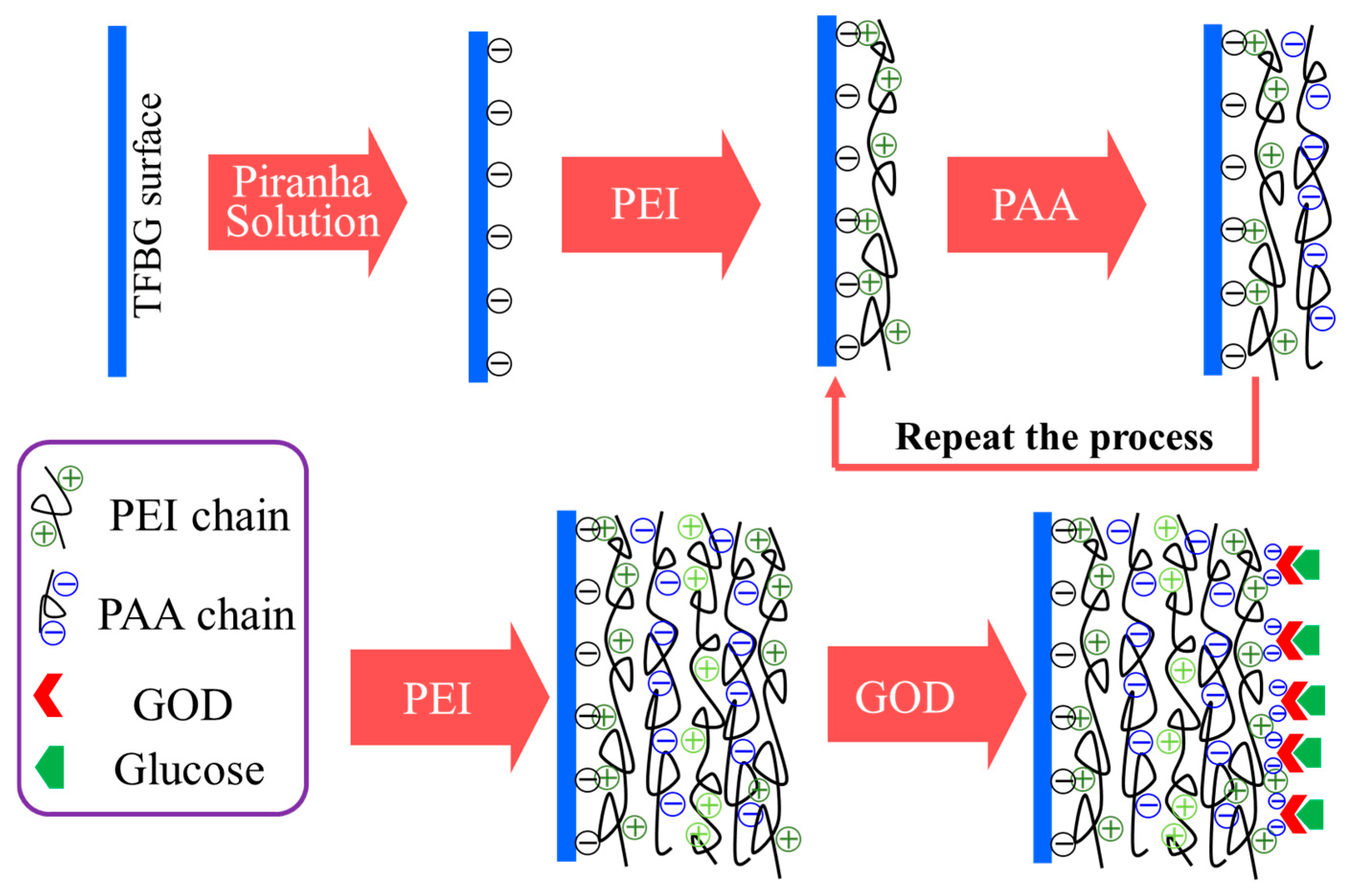
2.3. Fabrication of Optical Fiber TFBG Glucose Biosensor
3. Results and Discussion
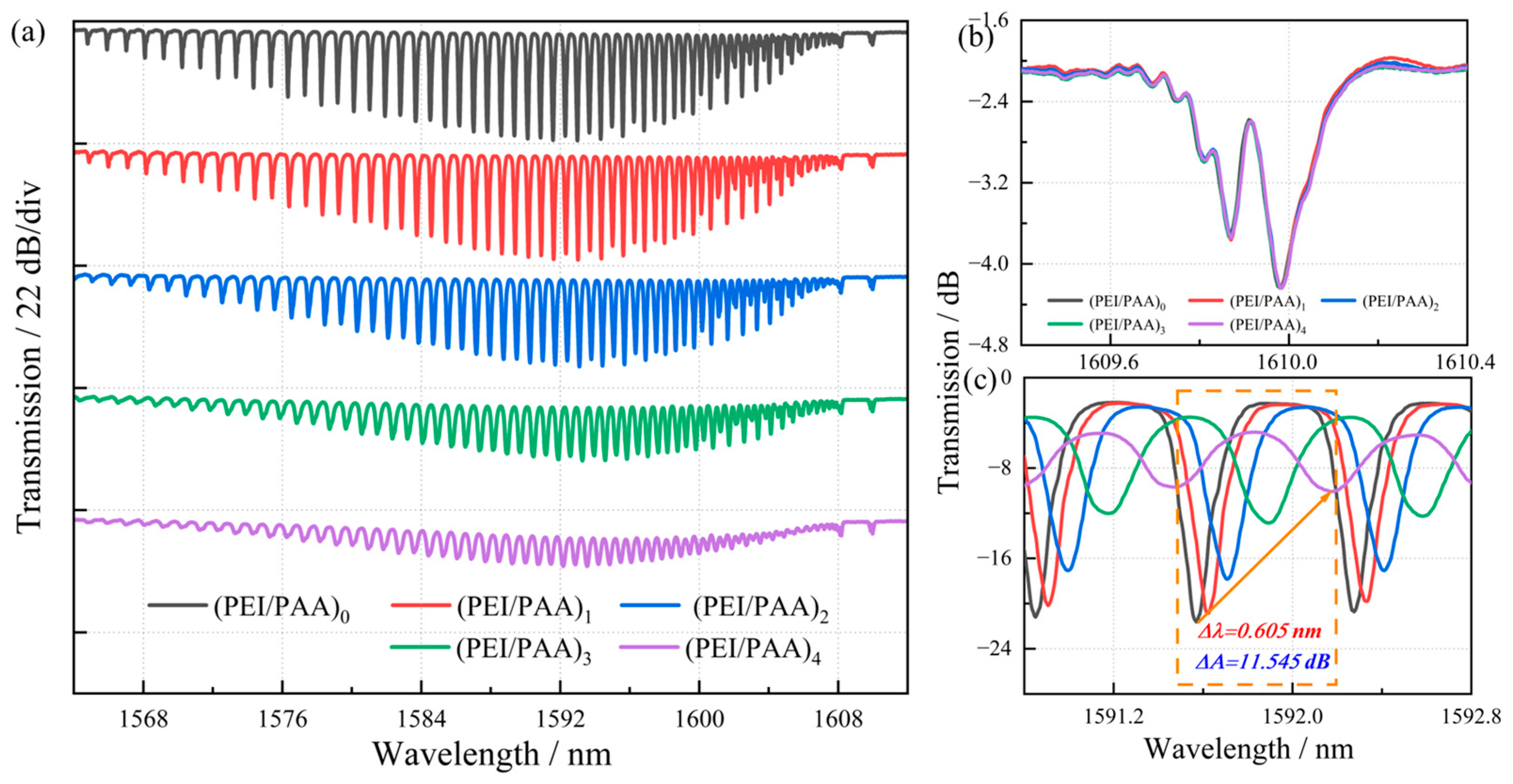
3.1. Spectral Evolution of TFBG with Polyelectrolyte Membranes
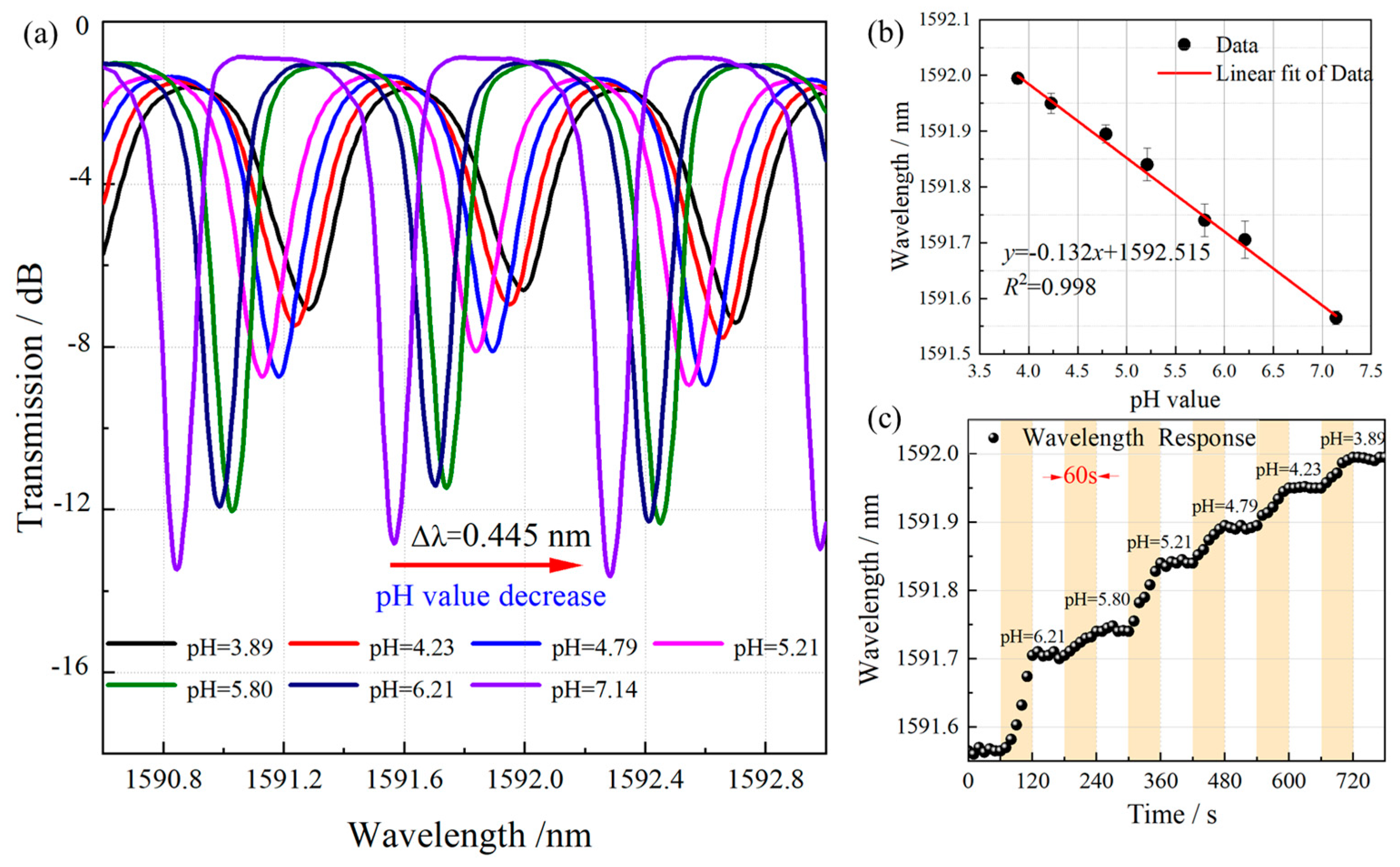
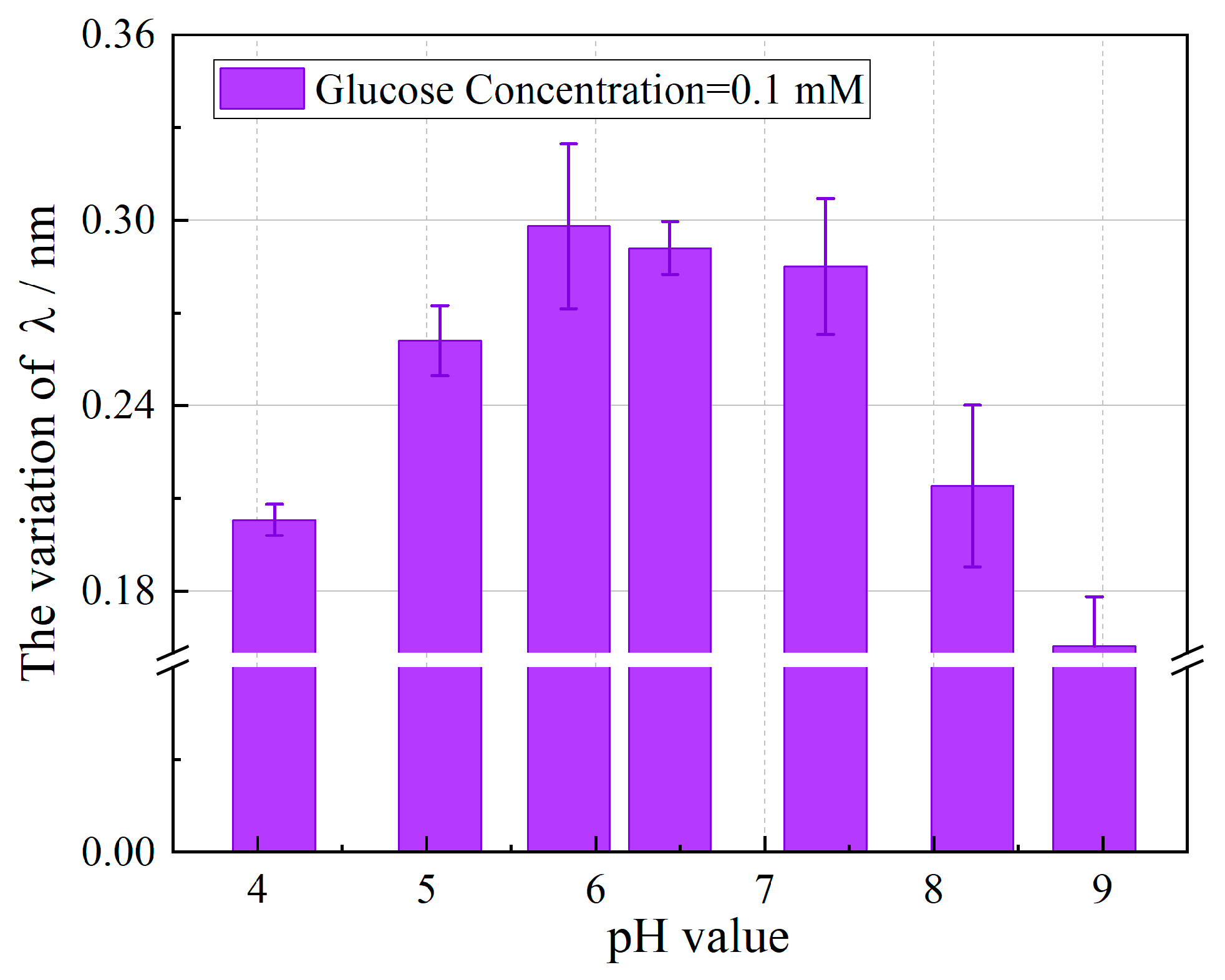
3.2. pH-Sensing Performance
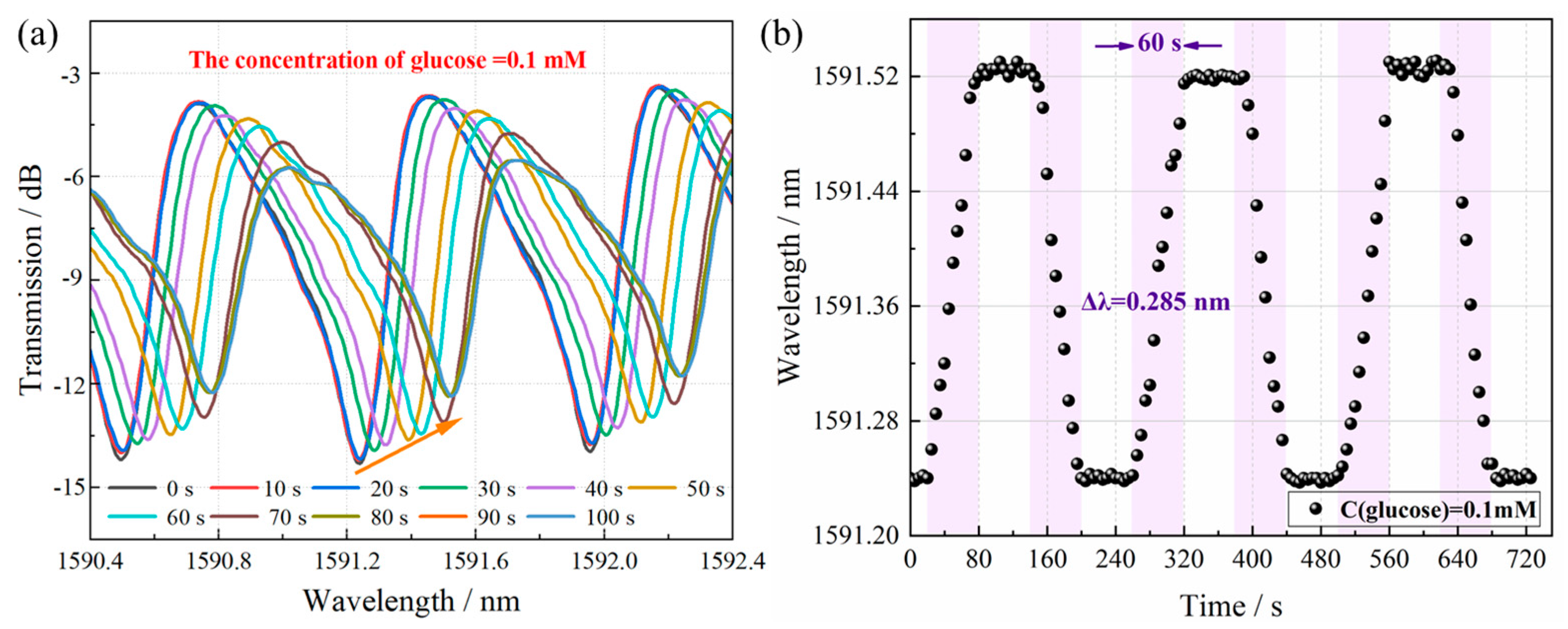
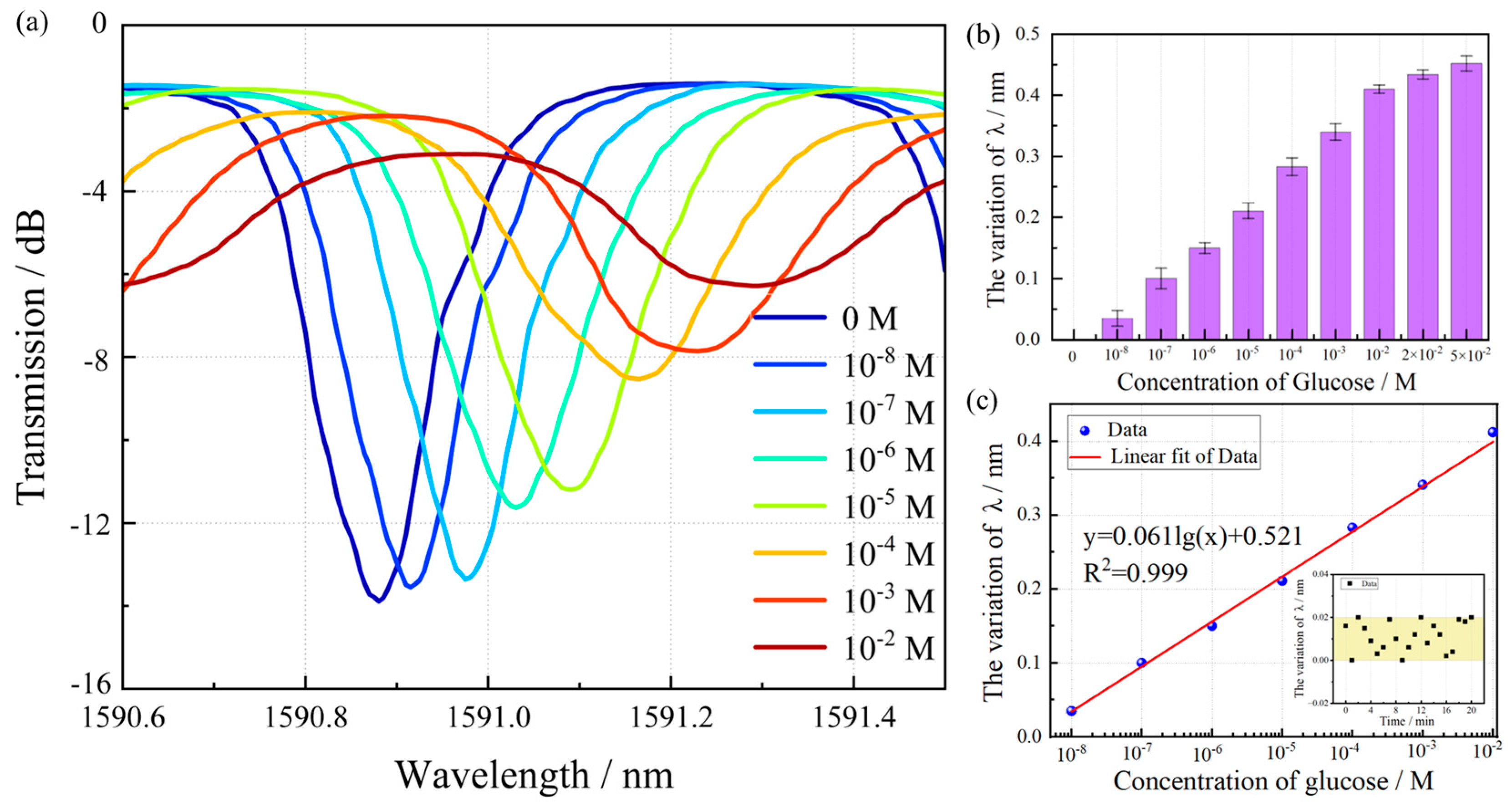
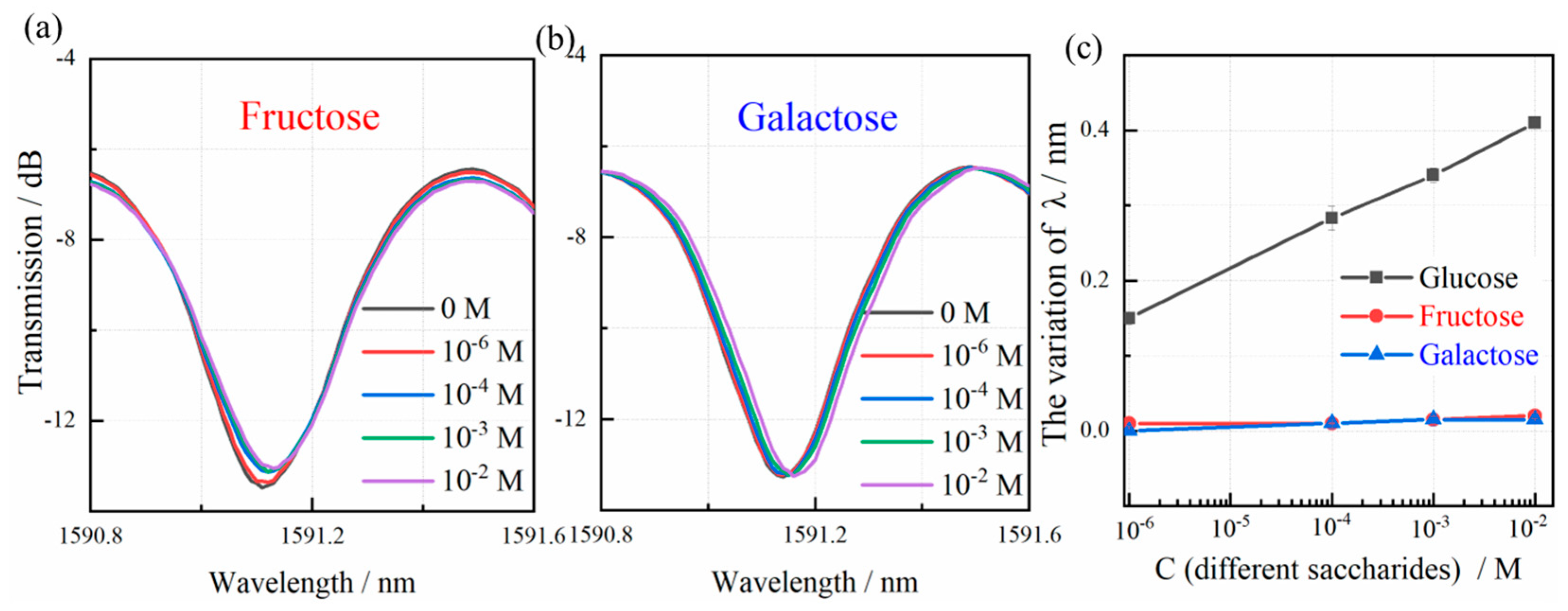
3.3. Glucose Detection and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lemos, S.G.; Nogueira, A.R.A.; Torre-Neto, A.; Parra, A.; Alonso, J. Soil calcium and pH monitoring sensor system. Agric. Food Chem. 2007, 55, 4658–4663. [Google Scholar] [CrossRef]
- Pathak, A.K.; Chaudhary, D.K.; Singh, V.K. Broad range and highly sensitive optical pH sensor based on hierarchical ZnO microflowers over tapered silica fiber. Sens. Actuators A Phys. 2018, 280, 399–405. [Google Scholar] [CrossRef]
- Escobedo, P.; Ramos-Lorente, C.E.; Martínez-Olmos, A.; Carvajal, M.A.; Ortega-Muñoz, M.; de Orbe-Payá, I.; Hernández-Mateo, F.; Santoyo-González, F.; Capitán-Vallvey, L.F.; Palma, A.J.; et al. Wireless wearable wristband for continuous sweat pH monitoring. Sens. Actuators B Chem. 2021, 327, 128948. [Google Scholar] [CrossRef]
- Orouji, A.; Abbasi-Moayed, S.; Ghasemi, F.; Hormozi-Nezhad, M.R. A wide-range pH indicator based on colorimetric patterns of gold@silver nanorods. Sens. Actuators B Chem. 2022, 358, 131479. [Google Scholar] [CrossRef]
- Wu, Y.; Bakker, E. Direct energy transfer from a pH glass electrode to a liquid crystal display. Anal. Chem. 2022, 94, 10408–10414. [Google Scholar] [CrossRef]
- Corres, J.M.; Matias, I.R.; del Villar, I.; Arregui, F.J. Design of pH sensors in long-period fiber gratings using polymeric nanocoatings. IEEE Sens. J. 2007, 7, 455–463. [Google Scholar] [CrossRef]
- Yulianti, I.; Supa’at, A.S.M.; Idrus, S.M.; Kurdi, O.; Anwar, M.R.S. Sensitivity improvement of a fiber Bragg grating pH sensor with elastomeric coating. Meas. Sci. Technol. 2012, 23, 015104. [Google Scholar] [CrossRef]
- Khanikar, T.; Singh, V.K. PANI-PVA composite film coated optical fiber probe as a stable and highly sensitive pH sensor. Opt. Mater. 2019, 88, 244–251. [Google Scholar] [CrossRef]
- Lei, Y.S.; Gong, Z.D.; Li, Y.Y.; Zhang, J.; Liu, Z.; Sun, Z.J.; Ouyang, X.; Tang, Y.Q.; Chan, C.C. Highly sensitive physiological sensor based on tapered fiber-optic interferometer for sweat pH detection. IEEE Sens. J. 2023, 23, 11627–11634. [Google Scholar] [CrossRef]
- Zhang, B.H.; Mumtaz, F.; Roman, M.; Alla, D.R.; Gerald, R.E.; Huang, J. Miniaturized fluorescence pH sensor with assembly free ball lens on a tapered multimode optical fiber. Opt. Express 2024, 32, 4228–4241. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.N. Fiber-Optic SPR pH Sensor Based on MMF-NCF-MMF structure and self-Assembled Nanofilm. IEEE T. Instrum. Meas. 2021, 70, 1–9. [Google Scholar] [CrossRef]
- Albert, J.; Shao, L.Y.; Caucheteur, C. Tilted fiber Bragg grating sensors. Laser Photonics Rev. 2013, 7, 83. [Google Scholar] [CrossRef]
- Chubchev, E.D.; Dolzhenko, E.I.; Tomyshev, K.A.; Nechepurenko, I.A.; Dorofeenko, A.V.; Butov, O.V. Analysis of Propagation/Leakage transition of fiber cladding modes for processing of data from dielectric tilted fiber Bragg grating sensors. Measurement 2025, 253, 117479. [Google Scholar] [CrossRef]
- Jean-Ruel, H.; Albert, J. Recent advances and current trends in optical fiber biosensors based on tilted fiber Bragg gratings. TrAC Trends Anal. Chem. 2024, 174, 117663. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.; Lu, M.D.; Du, Y.T.; Chen, M.; Meng, S.; Ji, W.; Sun, C.S.; Peng, W. Near-infrared band Gold nanoparticles-Au film “hot spot” model based label-free ultratrace lead (II) ions detection via fiber SPR DNAzyme biosensor. Sens. Actuators B Chem. 2021, 337, 129816. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, H.; Liu, Q.; Liu, F.; Han, X.; Liu, X.; Li, K.; Xiao, G.; Albert, J.; Lu, X.; et al. Operando monitoring of ion activities in aqueous batteries with plasmonic fiber-optic sensors. Nat. Commun. 2022, 13, 547. [Google Scholar] [CrossRef]
- Shen, C.; Huang, Z.; Chen, X.; Wang, Z.; Zhou, J.; Wang, Z.; Liu, D.; Li, C.; Zhao, T.; Zhang, Y.; et al. Rapid ultra-sensitive nucleic acid detection using plasmonic fiber-optic spectral combs and gold nanoparticle-tagged targets. Biosens. Bioelectron. 2023, 242, 115719. [Google Scholar] [CrossRef]
- Wang, F.; Ren, C.Y.; Lu, M.D.; Zhang, Y.; Peng, W. Ultrasensitive detection of platinum ion via a plasmonic fiber-optic aptasensor. Opt. Laser Technol. 2024, 179, 111277. [Google Scholar] [CrossRef]
- Han, X.; Liu, W.; Zu, L.; Wu, W.; Xie, J.; You, D.; Du, M.; Guo, T. In situ surface turbidity sensor based on localized light scattering from tilted fiber Bragg gratings. Opt. Lett. 2024, 49, 650–653. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Yan, X.Y.; Peng, B.; Li, Z.C.; Xia, X.D.; Lu, C.H.; You, D.T.; Li, K.W.; Guo, T. Ultrafast and reproducible fiber-optic hydrogen sensor via a tilted fiber grating with Pd/WO3 nanocoating. Photonic Sens. 2025, 15, 250229. [Google Scholar] [CrossRef]
- Caucheteur, C.; Guo, T.; Albert, J. Multiresonant fiber gratings. Opt. Photonics News 2022, 33, 42–49. [Google Scholar] [CrossRef]
- LopezAldaba, A.; González-Vila, Á.; Debliquy, M.; Lopez-Amo, M.; Caucheteur, C.; Lahem, D. Polyaniline-coated tilted fiber Bragg gratings for pH sensing. Sens. Actuators B Chem. 2018, 254, 1087–1093. [Google Scholar] [CrossRef]
- Li, P.; Huang, Y.; Wang, E.B.; Cao, J.W.; Pei, Y.H.; Lyu, G.H. Tilted fiber Bragg grating pH sensor based on polyaniline reaction deposition film layer. Opt. Quantum Electron. 2024, 56, 1353. [Google Scholar] [CrossRef]
- Xie, Y.S.; Li, Y.X.; Lin, H.F.; Wang, X.R.; Liao, W.J.; Liu, Z.Y.; Lin, L. Real-time pH Sensor in bacterial microenvironments using liquid crystal core-shell microspheres. Anal. Chem. 2024, 96, 11472–11478. [Google Scholar] [CrossRef]
- Yang, D.W.; Sun, R.R.; Sun, H.X.; Li, Q.; Zhang, H.; Zhang, X.F.; Shi, L.; Yao, L.; Tang, Y.L. A FRET biosensor constructed using pH sensitive G-quadruplex DNA for detecting mitochondrial autophagy. Talanta 2025, 281, 126885. [Google Scholar] [CrossRef]
- Ding, Z.W.; Ravikumar, R.; Zhao, C.L.; Chen, L.H.; Chan, C.C. Chitosan/poly (acrylic acid) based fiber-optic surface plasmon resonance sensor for Cu2+ ions detection. J. Light. Technol. 2019, 37, 2246–2252. [Google Scholar] [CrossRef]
- Yang, Y.-H.; Haile, M.; Park, Y.T.; Malek, F.A.; Grunlan, J.C. Super gas barrier of all-polymer multilayer thin films. Macromolecules. 2011, 44, 1450–1459. [Google Scholar] [CrossRef]
- Shao, L.Y.; Yin, M.J.; Tam, H.Y.; Albert, J. Fiber optic pH Sensor with self-assembled polymer multilayer nanocoatings. Sensors 2013, 13, 1425–1434. [Google Scholar] [CrossRef]
- Pereira, J.M.; Mendes, J.P.; Dias, B.; Almeida, J.M.M.M.d.; Coelho, L.C.C. Optical pH sensor based on a long-period fiber grating coated with a polymeric layer-by-layer electrostatic self-assembled nanofilm. Sensors 2024, 24, 1662. [Google Scholar] [CrossRef]
- Vashist, S.K.; Zheng, D.; Al-Rubeaan, K.; Luong, J.H.T.; Sheu, F.S. Technology behind commercial devices for blood glucose monitoring in diabetes management: A review. Anal. Chim. Acta. 2011, 703, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Lu, M.D.; Yuan, H.Z.; Zhang, Y.; Ji, W.; Sun, C.S.; Peng, W. pM level and large dynamic range glucose detection based on a sandwich type plasmonic fiber sensor. J. Lightwave Technol. 2021, 39, 3882–3889. [Google Scholar] [CrossRef]
- Deep, A.; Tiwari, U.; Kumar, P.; Mishra, V.; Jain, S.C.; Singh, N.; Kapur, P.; Bharadwaj, L.M. Immobilization of enzyme on long period grating fibers for sensitive glucose detection. Biosens. Bioelectron. 2012, 33, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Xiang, X.Y.; Ding, L.Y.; Luo, Z.H.; Zhao, J.; Huang, J.; Li, H.J.; Jiang, X.D. A novel large-diameter optical fiber glucose biosensor based on glucose oxidase-graphene oxide-gold nanoparticles. IEEE Sens. J. 2023, 23, 6832–6839. [Google Scholar] [CrossRef]
- Zhang, X.J.; Wu, Z.; Liu, F.; Fu, Q.Q.; Chen, X.Y.; Xu, J.; Zhang, Z.C.; Huang, Y.Y.; Tang, Y.; Guo, T.; et al. Hydrogen peroxide and glucose concentration measurement using optical fiber grating sensors with corrodible plasmonic nanocoatings. Biomed. Opt. Express. 2018, 9, 1735–1744. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Luo, S.T.; Gui, Y.Q.; Wang, X.; Tian, Z.Y.; Yu, H.H. Difunctional hydrogel optical fiber fluorescence sensor for continuous and simultaneous monitoring of glucose and pH. Biosensors. 2023, 13, 287. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, X.J.; Li, K.W.; Guo, T.; Ianoul, A.; Albert, J. Discrimination of bulk and surface refractive index change in plasmonic sensors with narrow bandwidth resonance combs. ACS Sens. 2021, 6, 3013–3023. [Google Scholar] [CrossRef]
- Bankar, S.B.; Bule, M.V.; Singhal, R.S.; Ananthanarayan, L. Glucose oxidase—An overview. Biotechnol. Adv. 2009, 27, 489–501. [Google Scholar] [CrossRef]
- Luo, B.; Yan, Z.; Sun, Z.; Liu, Y.; Zhao, M.; Zhang, L. Biosensor based on excessively tilted fiber grating in thin-cladding optical fiber for sensitive and selective detection of low glucose concentration. Opt. Express. 2015, 23, 32429–32440. [Google Scholar] [CrossRef]
- Yin, M.J.; Huang, B.B.; Gao, S.R.; Zhang, A.P.; Ye, X.S. Optical fiber LPG biosensor integrated microfluidic chip for ultrasensitive glucose detection. Biomed. Opt. Express. 2016, 7, 2067–2077. [Google Scholar] [CrossRef]
- Lin, H.T.; Li, M.S.; Ding, L.Y.; Huang, J. A fiber optic biosensor based on hydrogel-immobilized enzyme complex for continuous determination of cholesterol and glucose. Appl. Biochem. Biotechnol. 2019, 187, 1569–1580. [Google Scholar] [CrossRef]
- Yu, S.; Ding, L.Y.; Lin, H.T.; Wu, W.; Huang, J. A novel optical fiber glucose biosensor based on carbon quantum dots-glucose oxidase/cellulose acetate complex sensitive film. Biosens. Bioelectron. 2019, 146, 111760. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liang, T.; Wang, H.; Huang, Z.; Hu, Z.; Xie, K.; Zhang, W.; Wei, Y.; Wang, G.; Yan, J.; et al. Ultrasensitive glucose biosensor using micro-nano interface of tilted fiber grating coupled with biofunctionalized Au nanoparticles. IEEE Sens. J. 2022, 22, 4122–4134. [Google Scholar] [CrossRef]
- Xi, J.W.; Sun, H.; Li, J.Z.; Deng, L.; Yang, Y.X.; Zheng, H.R.; Feng, D.Y.; Huang, X.; Zhang, J.Q.; Li, X. Tilted fiber Bragg grating sensor based on surface plasmon resonance and electrospinning for glucose detection. Microchem. J. 2024, 204, 110978. [Google Scholar] [CrossRef]
- Dai, Z.H.; Ni, J.; Huang, X.H.; Lu, G.F.; Bao, J.C. Direct electrochemistry of glucose oxidase immobilized on a hexagonal mesoporous silica-MCM-41 matrix. Bioelectrochemistry. 2007, 70, 250–256. [Google Scholar] [CrossRef]
- Yan, Z.Q.; Xue, M.; He, Q.; Lu, W.; Meng, Z.H.; Yan, D.; Qiu, L.L.; Zhou, L.J.; Yu, Y.J. A non-enzymatic urine glucose sensor with 2-D photonic crystal hydrogel. Anal. Bioanal. Chem. 2016, 408, 8317–8323. [Google Scholar] [CrossRef]


| Methods | LOD | Detection Range | Response | Ref. |
|---|---|---|---|---|
| LPG-(PEI/PAA)4(PEI/GOD)1 | 1 nM | 1 nM–10 µM | 70 s | [39] |
| Fiber Optic -PNIPAAm-GOD | 11.4 mg/dL | 50–700 mg/dL | 200 s | [40] |
| Fiber-Fluorescent-CQDs-GOD/CA | 25.79 nM | 10 nM–200 μM | ~10 s | [41] |
| EX-TFG-AuNPs-GOD | 2.5 nM | 1 nM–5 mM | 55 s | [42] |
| TFBG-SPR- Electrospinning-GOD | 100 mg/dL | 100–500 mg/dL | 30 s | [43] |
| TFBG -(PEI/PAA)4(PEI/GOD)1 | 27.7 nM | 10 nM–10 mM | 60 s | This work |
| Sample | Glucose Added (mM) | Glucose Found (mM) | Recovery (%) | Accuracy (%) |
|---|---|---|---|---|
| Rabbit serum | 2.00 | 2.03 | 101.50 | 98.50 |
| 4.00 | 3.89 | 97.25 | 97.25 | |
| 6.00 | 6.11 | 101.83 | 98.17 | |
| Artificial urine | 2.00 | 1.87 | 93.50 | 93.50 |
| 4.00 | 4.13 | 103.25 | 96.75 | |
| 6.00 | 5.99 | 99.83 | 99.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Zhou, X.; Zhang, J.; Cheng, S. Optical Fiber TFBG Glucose Biosensor via pH-Sensitive Polyelectrolyte Membrane. Biosensors 2025, 15, 642. https://doi.org/10.3390/bios15100642
Wang F, Zhou X, Zhang J, Cheng S. Optical Fiber TFBG Glucose Biosensor via pH-Sensitive Polyelectrolyte Membrane. Biosensors. 2025; 15(10):642. https://doi.org/10.3390/bios15100642
Chicago/Turabian StyleWang, Fang, Xinyuan Zhou, Jianzhong Zhang, and Shenhang Cheng. 2025. "Optical Fiber TFBG Glucose Biosensor via pH-Sensitive Polyelectrolyte Membrane" Biosensors 15, no. 10: 642. https://doi.org/10.3390/bios15100642
APA StyleWang, F., Zhou, X., Zhang, J., & Cheng, S. (2025). Optical Fiber TFBG Glucose Biosensor via pH-Sensitive Polyelectrolyte Membrane. Biosensors, 15(10), 642. https://doi.org/10.3390/bios15100642




