Advances in Wearable Biosensors for Wound Healing and Infection Monitoring
Abstract
1. Introduction
2. Biological Basis of Wound Healing and Infection
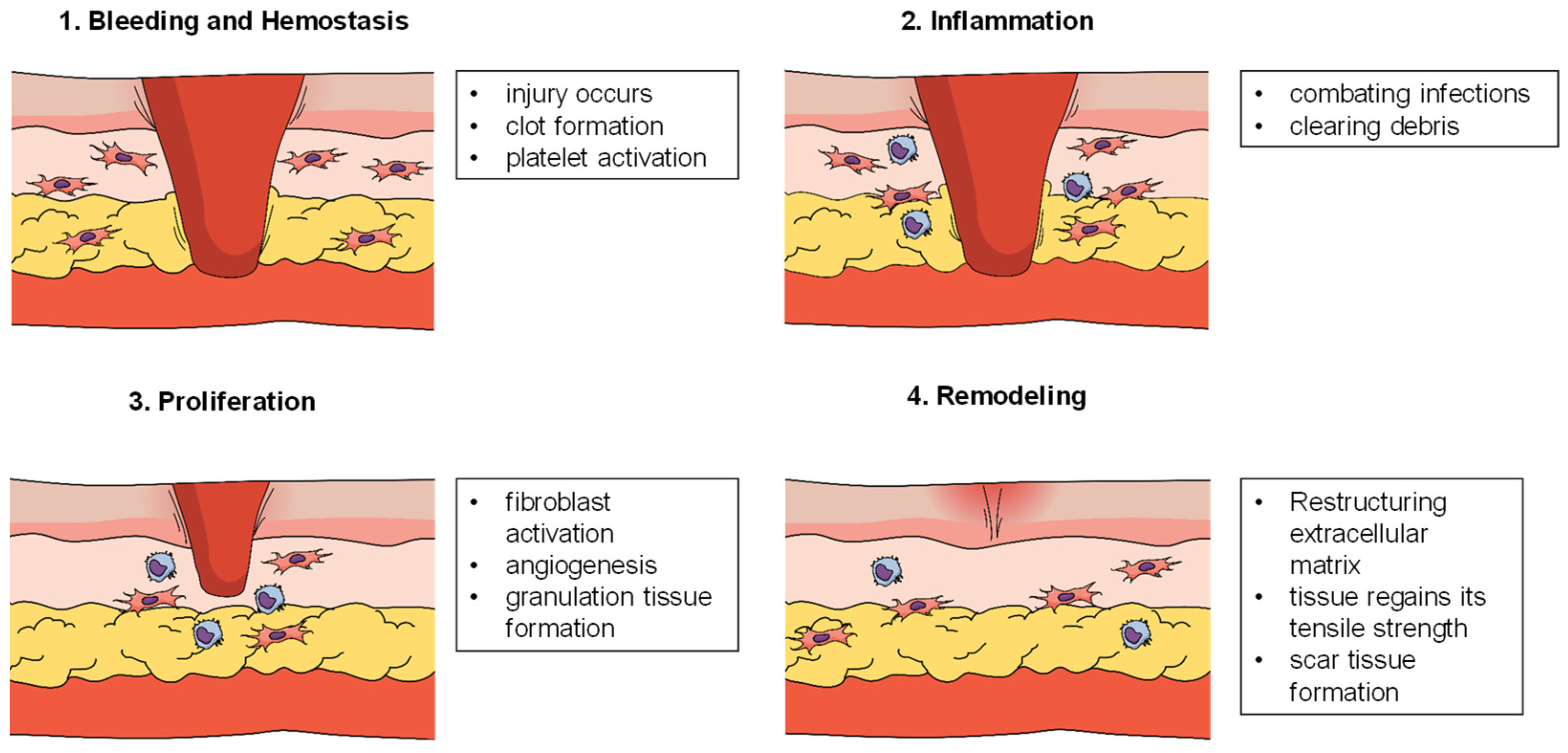
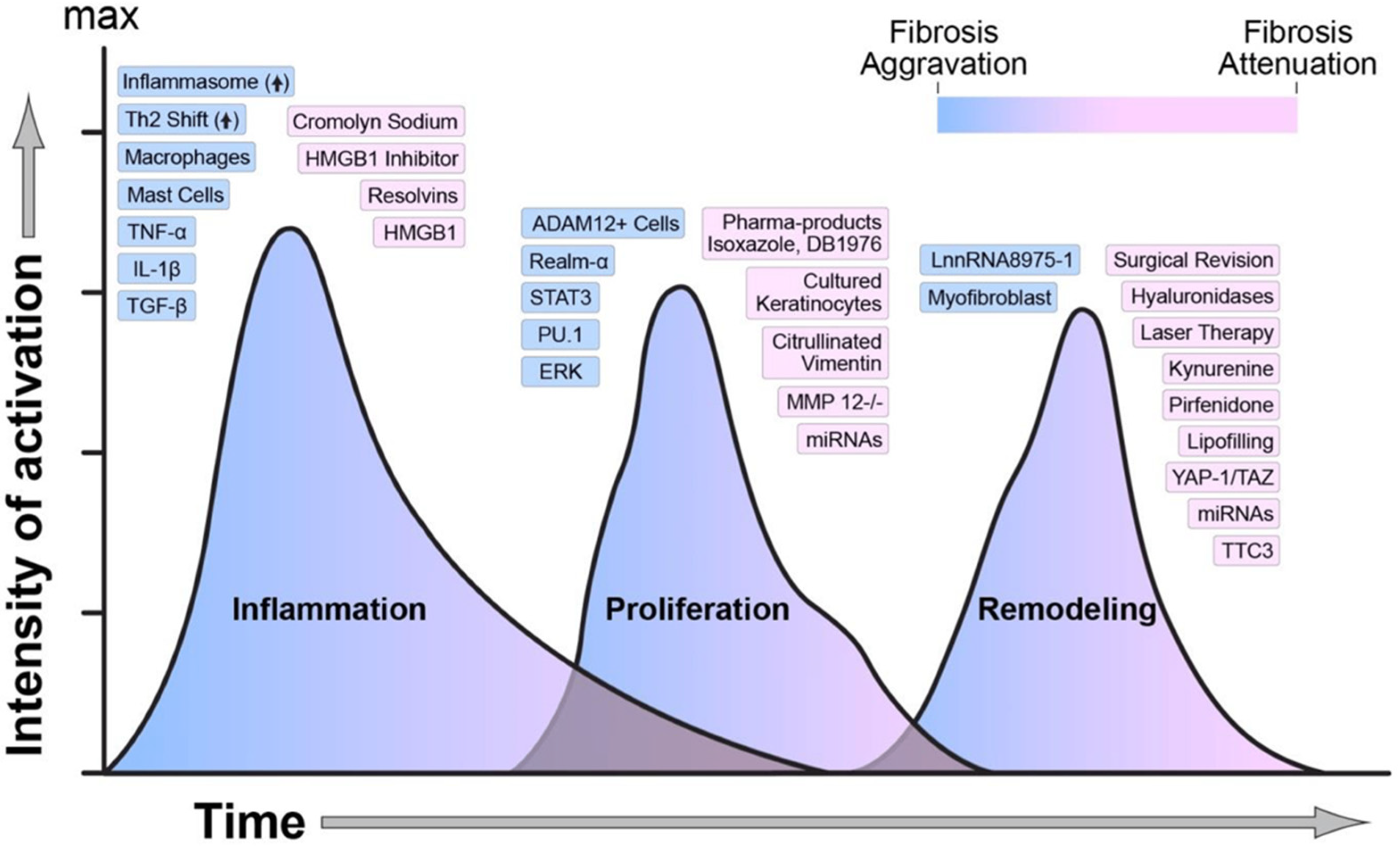
2.1. Stages of Wound Healing
2.2. Role of Biomarkers in Assessing Wound Status and Infections
3. Advancements in Wearable Biosensor Technologies
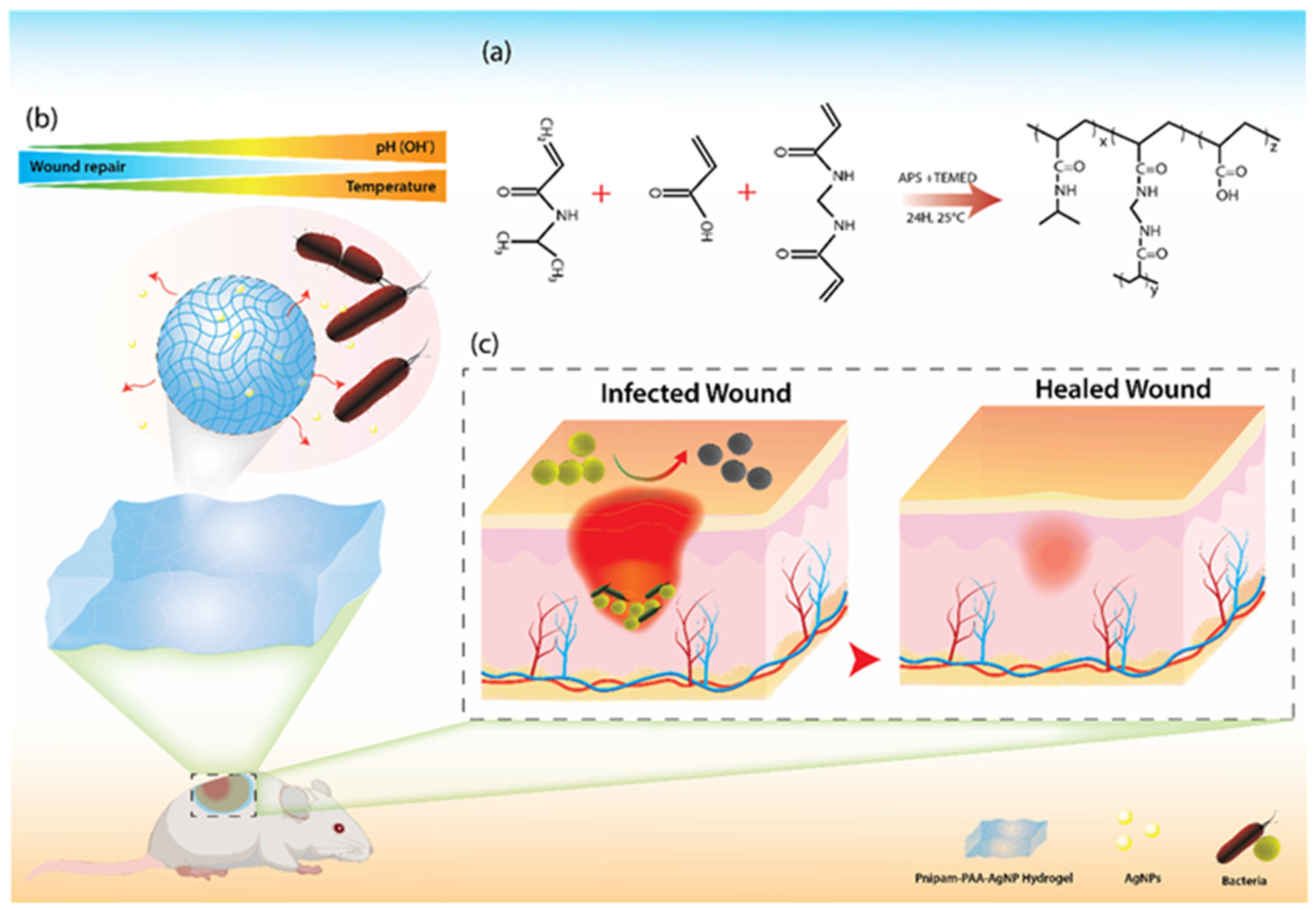
3.1. Types of Sensors
3.2. Materials and Designs
3.3. Enhancing Technical Summaries with Key Performance Parameters
3.4. Integration with AI and IoT
4. Key Applications in Wound Healing and Infection Monitoring
4.1. Real-Time Monitoring
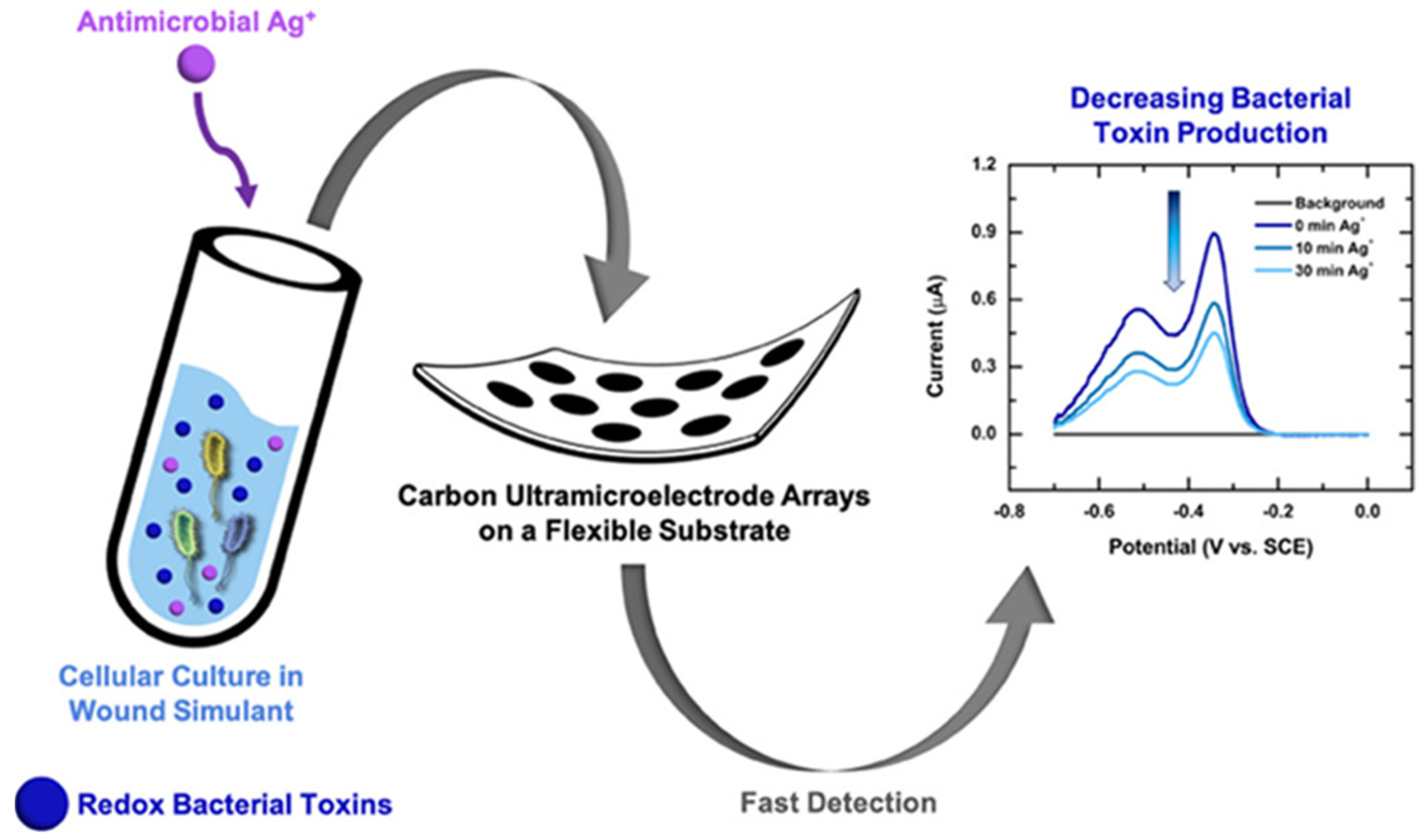
4.2. Early Infection Detection
4.3. Therapeutic Feedback Systems
5. Challenges and Limitations
5.1. Technical Issues
5.2. Clinical Translation
5.3. Power and Energy
6. Future Perspectives
6.1. Emerging Technologies Such as Bioresorbable Sensors and Multi-Biomarker Platforms
6.2. Validation and Application of Wound-Healing Biosensors in Clinical Settings
6.3. Potential of AI and Machine Learning for Predictive Analytics
6.4. Opportunities for Large-Scale Adoption in Telemedicine and Personalized Care
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dong, R.; Guo, B. Smart wound dressings for wound healing. Nano Today 2021, 41, 101290. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional hydrogels as wound dressing to enhance wound healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Las Heras, K.; Igartua, M.; Santos-Vizcaino, E.; Hernandez, R.M. Chronic wounds: Current status, available strategies and emerging therapeutic solutions. J. Control. Release 2020, 328, 532–550. [Google Scholar] [CrossRef] [PubMed]
- Falanga, V.; Isseroff, R.R.; Soulika, A.M.; Romanelli, M.; Margolis, D.; Kapp, S.; Granick, M.; Harding, K. Chronic wounds. Nat. Rev. Dis. Primers 2022, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, E.; Liu, P.Y.; Schultz, G.S.; Martins-Green, M.M.; Tanaka, R.; Weir, D.; Gould, L.J.; Armstrong, D.G.; Gibbons, G.W.; Wolcott, R. Chronic wounds: Treatment consensus. Wound Repair Regen. 2022, 30, 156–171. [Google Scholar] [CrossRef]
- Sen, C.K. Human wound and its burden: Updated 2020 compendium of estimates. Adv. Wound Care 2021, 10, 281–292. [Google Scholar] [CrossRef]
- Uberoi, A.; McCready-Vangi, A.; Grice, E.A. The wound microbiota: Microbial mechanisms of impaired wound healing and infection. Nat. Rev. Microbiol. 2024, 22, 507–521. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.; Rodrigues, P.M.; Pintado, M.; Tavaria, F.K. A systematic review of natural products for skin applications: Targeting inflammation, wound healing, and photo-aging. Phytomedicine 2023, 115, 154824. [Google Scholar] [CrossRef]
- Lipsky, B.A.; Dryden, M.; Gottrup, F.; Nathwani, D.; Seaton, R.A.; Stryja, J. Antimicrobial stewardship in wound care: A Position Paper from the British Society for Antimicrobial Chemotherapy and European Wound Management Association. J. Antimicrob. Chemother. 2016, 71, 3026–3035. [Google Scholar] [CrossRef]
- Zeng, Q.; Qi, X.; Shi, G.; Zhang, M.; Haick, H. Wound dressing: From nanomaterials to diagnostic dressings and healing evaluations. ACS Nano 2022, 16, 1708–1733. [Google Scholar] [CrossRef]
- Wang, C.; Shirzaei Sani, E.; Shih, C.-D.; Lim, C.T.; Wang, J.; Armstrong, D.G.; Gao, W. Wound management materials and technologies from bench to bedside and beyond. Nat. Rev. Mater. 2024, 9, 550–566. [Google Scholar] [CrossRef]
- Pusta, A.; Tertiș, M.; Cristea, C.; Mirel, S. Wearable sensors for the detection of biomarkers for wound infection. Biosensors 2021, 12, 1. [Google Scholar] [CrossRef]
- Kalasin, S.; Sangnuang, P.; Surareungchai, W. Intelligent wearable sensors interconnected with advanced wound dressing bandages for contactless chronic skin monitoring: Artificial intelligence for predicting tissue regeneration. Anal. Chem. 2022, 94, 6842–6852. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound healing: A cellular perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Li, W.; Zhang, T.; Ma, H.; Cao, Y.; Wang, K.; Zhou, Y.; Shamim, A.; Zheng, L.; Wang, X. Wireless Technologies in Flexible and Wearable Sensing: From Materials Design, System Integration to Applications. Adv. Mater. 2024, 36, 2400333. [Google Scholar] [CrossRef]
- Ahmed, Z.; Mohamed, K.; Zeeshan, S.; Dong, X. Artificial intelligence with multi-functional machine learning platform development for better healthcare and precision medicine. Database 2020, 2020, baaa010. [Google Scholar] [CrossRef]
- Ghosh, R.; Singh, P.; Pandit, A.H.; Tariq, U.; Bhunia, B.K.; Kumar, A. Emerging Technological Advancement for Chronic Wound Treatment and Their Role in Accelerating Wound Healing. ACS Appl. Bio Mater. 2024, 7, 7101–7132. [Google Scholar] [CrossRef] [PubMed]
- Peña, O.A.; Martin, P. Cellular and molecular mechanisms of skin wound healing. Nat. Rev. Mol. Cell Biol. 2024, 25, 599–616. [Google Scholar] [CrossRef]
- Guo, B.; Dong, R.; Liang, Y.; Li, M. Haemostatic materials for wound healing applications. Nat. Rev. Chem. 2021, 5, 773–791. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. The inflammatory response in myocardial injury, repair, and remodelling. Nat. Rev. Cardiol. 2014, 11, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Pellicoro, A.; Ramachandran, P.; Iredale, J.P.; Fallowfield, J.A. Liver fibrosis and repair: Immune regulation of wound healing in a solid organ. Nat. Rev. Immunol. 2014, 14, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Landén, N.X.; Li, D.; Ståhle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef]
- Wang, H.; Yang, L. Applications of injectable hemostatic materials in wound healing: Principles, strategies, performance requirements, and future perspectives. Theranostics 2023, 13, 4615–4635. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Maheshwari, A.; Chandra, A. Biomarkers for wound healing and their evaluation. J. Wound Care 2016, 25, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in chronic wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef]
- Tidball, J.G. Regulation of muscle growth and regeneration by the immune system. Nat. Rev. Immunol. 2017, 17, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Cañedo-Dorantes, L.; Cañedo-Ayala, M. Skin acute wound healing: A comprehensive review. Int. J. Inflamm. 2019, 2019, 3706315. [Google Scholar] [CrossRef]
- Spielman, A.F.; Griffin, M.F.; Parker, J.; Cotterell, A.C.; Wan, D.C.; Longaker, M.T. Beyond the scar: A basic science review of wound remodeling. Adv. Wound Care 2023, 12, 57–67. [Google Scholar] [CrossRef]
- El Ayadi, A.; Jay, J.W.; Prasai, A. Current Approaches Targeting the Wound Healing Phases to Attenuate Fibrosis and Scarring. Int. J. Mol. Sci. 2020, 21, 1105. [Google Scholar] [CrossRef] [PubMed]
- Lindley, L.E.; Stojadinovic, O.; Pastar, I.; Tomic-Canic, M. Biology and biomarkers for wound healing. Plast. Reconstr. Surg. 2016, 138, 18S–28S. [Google Scholar] [CrossRef] [PubMed]
- Mota, F.A.; Pereira, S.A.; Araujo, A.R.; Passos, M.L.; Saraiva, M.L.M. Biomarkers in the diagnosis of wounds infection: An analytical perspective. TrAC Trends Anal. Chem. 2021, 143, 116405. [Google Scholar] [CrossRef]
- Jankowska, D.; Bannwarth, M.; Schulenburg, C.; Faccio, G.; Maniura-Weber, K.; Rossi, R.; Scherer, L.; Richter, M.; Boesel, L. Simultaneous detection of pH value and glucose concentrations for wound monitoring applications. Biosens. Bioelectron. 2017, 87, 312–319. [Google Scholar] [CrossRef]
- Proksch, E. pH in nature, humans and skin. J. Dermatol. 2018, 45, 1044–1052. [Google Scholar] [CrossRef]
- Ono, S.; Imai, R.; Ida, Y.; Shibata, D.; Komiya, T.; Matsumura, H. Increased wound pH as an indicator of local wound infection in second degree burns. Burns 2015, 41, 820–824. [Google Scholar] [CrossRef] [PubMed]
- Basu, P.; Banerjee, A.; Okoro, P.D.; Masoumi, A.; Kanjilal, B.; Akbari, M.; Martins-Green, M.; Armstrong, D.G.; Noshadi, I. Integration of Functional Polymers and Biosensors to Enhance Wound Healing. Adv. Healthc. Mater. 2024, 13, 2401461. [Google Scholar] [CrossRef]
- Mahmoud, N.N.; Hamad, K.; Al Shibitini, A.; Juma, S.; Sharifi, S.; Gould, L.; Mahmoudi, M. Investigating inflammatory markers in wound healing: Understanding implications and identifying artifacts. ACS Pharmacol. Transl. Sci. 2024, 7, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Zhang, Y.; Ma, N.; Shi, J.; Hou, Y. NF-κB role on tumor proliferation, migration, invasion and immune escape. Cancer Gene Ther. 2024, 31, 1599–1610. [Google Scholar] [CrossRef] [PubMed]
- Audu, C.O.; Melvin, W.J.; Joshi, A.D.; Wolf, S.J.; Moon, J.Y.; Davis, F.M.; Barrett, E.C.; Mangum, K.D.; Deng, H.; Xing, X. Macrophage-specific inhibition of the histone demethylase JMJD3 decreases STING and pathologic inflammation in diabetic wound repair. Cell. Mol. Immunol. 2022, 19, 1251–1262. [Google Scholar] [CrossRef]
- Lou, D.; Pang, Q.; Pei, X.; Dong, S.; Li, S.; Tan, W.-Q.; Ma, L. Flexible wound healing system for pro-regeneration, temperature monitoring and infection early warning. Biosens. Bioelectron. 2020, 162, 112275. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, B.; Huang, R.; Lin, Z.; Li, Y.; Li, J.; Li, X. Flexible integrated sensing platform for monitoring wound temperature and predicting infection. Microb. Biotechnol. 2021, 14, 1566–1579. [Google Scholar] [CrossRef]
- Chen, W.; He, C.; Qiao, N.; Guo, Z.; Hu, S.; Song, Y.; Wang, H.; Zhang, Z.; Ke, B.; Sun, X. Dual drugs decorated bacteria irradiate deep hypoxic tumor and arouse strong immune responses. Biomaterials 2022, 286, 121582. [Google Scholar] [CrossRef]
- Tan, C.; Dong, Z.; Li, Y.; Zhao, H.; Huang, X.; Zhou, Z.; Jiang, J.-W.; Long, Y.-Z.; Jiang, P.; Zhang, T.-Y. A high performance wearable strain sensor with advanced thermal management for motion monitoring. Nat. Commun. 2020, 11, 3530. [Google Scholar] [CrossRef]
- Yang, B.; Li, X.; Hou, Y.; Meier, A.; Cheng, X.; Choi, J.-H.; Wang, F.; Wang, H.; Wagner, A.; Yan, D. Non-invasive (non-contact) measurements of human thermal physiology signals and thermal comfort/discomfort poses-a review. Energy Build. 2020, 224, 110261. [Google Scholar] [CrossRef]
- Wang, G.; Yang, F.; Zhou, W.; Xiao, N.; Luo, M.; Tang, Z. The initiation of oxidative stress and therapeutic strategies in wound healing. Biomed. Pharmacother. 2023, 157, 114004. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Huang, J.; Li, Y.; Lv, X.; Zhou, H.; Wang, H.; Xu, Y.; Wang, C.; Wang, J.; Liu, Z. ROS-scavenging hydrogel to promote healing of bacteria infected diabetic wounds. Biomaterials 2020, 258, 120286. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar] [CrossRef]
- Demirci-Cekic, S.; Özkan, G.; Avan, A.N.; Uzunboy, S.; Çapanoğlu, E.; Apak, R. Biomarkers of oxidative stress and antioxidant defense. J. Pharm. Biomed. Anal. 2022, 209, 114477. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhong, B.; Lou, Z.; Han, W.; Wang, L. The advancement of intelligent dressings for monitoring chronic wound infections. Chem. Eng. J. 2024, 484, 149643. [Google Scholar] [CrossRef]
- Zheng, X.T.; Yang, Z.; Sutarlie, L.; Thangaveloo, M.; Yu, Y.; Salleh, N.A.B.M.; Chin, J.S.; Xiong, Z.; Becker, D.L.; Loh, X.J. Battery-free and AI-enabled multiplexed sensor patches for wound monitoring. Sci. Adv. 2023, 9, eadg6670. [Google Scholar] [CrossRef] [PubMed]
- Daulton, E.; Wicaksono, A.; Bechar, J.; Covington, J.A.; Hardwicke, J. The detection of wound infection by ion mobility chemical analysis. Biosensors 2020, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Nakhjavani, S.A.; Mirzajani, H.; Cararra, S.; Onbaşlı, M.C. Advances in Biosensor Technologies for Infectious Diseases Detection. TrAC Trends Anal. Chem. 2024, 180, 117979. [Google Scholar] [CrossRef]
- Fernandez, M.L.; Upton, Z.; Edwards, H.; Finlayson, K.; Shooter, G.K. Elevated uric acid correlates with wound severity. Int. Wound J. 2012, 9, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhou, H.; Gerhard, E.M.; Zhang, S.; Rodríguez, F.I.P.; Pan, T.; Yang, H.; Lin, Y.; Yang, J.; Cheng, H. Smart bioadhesives for wound healing and closure. Bioact. Mater. 2023, 19, 360–375. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Zhang, B.; Lin, X.; Fu, X.; An, Y.; Zou, Y.; Wang, J.-X.; Wang, Z.; Yu, T. Lactate metabolism in human health and disease. Signal Transduct. Target. Ther. 2022, 7, 305. [Google Scholar] [CrossRef]
- Burgess, J.L.; Wyant, W.A.; Abdo Abujamra, B.; Kirsner, R.S.; Jozic, I. Diabetic wound-healing science. Medicina 2021, 57, 1072. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.I.; Sculean, A.; Graves, D.T. Diabetic wound healing in soft and hard oral tissues. Transl. Res. 2021, 236, 72–86. [Google Scholar] [CrossRef]
- Olive, A.J.; Sassetti, C.M. Metabolic crosstalk between host and pathogen: Sensing, adapting and competing. Nat. Rev. Microbiol. 2016, 14, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Vo, D.-K.; Trinh, K.T.L. Advances in Wearable Biosensors for Healthcare: Current Trends, Applications, and Future Perspectives. Biosensors 2024, 14, 560. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Qiao, Z.; Niu, Y.; Yeo, J.C.; Liu, Y.; Qi, J.; Fan, S.; Liu, X.; Lee, J.Y.; Lim, C.T. Wearable flexible microfluidic sensing technologies. Nat. Rev. Bioeng. 2023, 1, 950–971. [Google Scholar] [CrossRef]
- Mota, F.A.; Passos, M.L.; Santos, J.L.; Saraiva, M.L.M. Comparative analysis of electrochemical and optical sensors for detection of chronic wounds biomarkers: A review. Biosens. Bioelectron. 2024, 251, 116095. [Google Scholar] [CrossRef] [PubMed]
- Barhoum, A.; Altintas, Z.; Devi, K.S.; Forster, R.J. Electrochemiluminescence biosensors for detection of cancer biomarkers in biofluids: Principles, opportunities, and challenges. Nano Today 2023, 50, 101874. [Google Scholar] [CrossRef]
- Sakthivel, K.; Lin, W.-C.; Lee, Y.-Y.; Huang, B.-W.; Chen, Y.-L.; Chang-Chien, G.-P.; Sheu, J.-K. Advancements in Electrochemical Biosensing of Cardiovascular Disease Biomarkers. J. Mater. Chem. B 2024, 12, 6305–6327. [Google Scholar]
- Li, S.; Zhang, H.; Zhu, M.; Kuang, Z.; Li, X.; Xu, F.; Miao, S.; Zhang, Z.; Lou, X.; Li, H. Electrochemical biosensors for whole blood analysis: Recent progress, challenges, and future perspectives. Chem. Rev. 2023, 123, 7953–8039. [Google Scholar] [CrossRef]
- Youssef, K.; Ullah, A.; Rezai, P.; Hasan, A.; Amirfazli, A. Recent advances in biosensors for real time monitoring of pH, temperature, and oxygen in chronic wounds. Mater. Today Bio 2023, 22, 100764. [Google Scholar] [CrossRef] [PubMed]
- Kalita, N.; Gogoi, S.; Minteer, S.D.; Goswami, P. Advances in Bioelectrode Design for Developing Electrochemical Biosensors. ACS Meas. Sci. Au 2023, 3, 404–433. [Google Scholar] [CrossRef] [PubMed]
- Fritea, L.; Banica, F.; Costea, T.O.; Moldovan, L.; Dobjanschi, L.; Muresan, M.; Cavalu, S. Metal Nanoparticles and Carbon-Based Nanomaterials for Improved Performances of Electrochemical (Bio)Sensors with Biomedical Applications. Materials 2021, 14, 6319. [Google Scholar] [CrossRef] [PubMed]
- Luong, J.H.T.; Narayan, T.; Solanki, S.; Malhotra, B.D. Recent Advances of Conducting Polymers and Their Composites for Electrochemical Biosensing Applications. J. Funct. Biomater. 2020, 11, 71. [Google Scholar] [CrossRef]
- Welch, E.C.; Powell, J.M.; Clevinger, T.B.; Fairman, A.E.; Shukla, A. Advances in Biosensors and Diagnostic Technologies Using Nanostructures and Nanomaterials. Adv. Funct. Mater. 2021, 31, 2104126. [Google Scholar] [CrossRef]
- Wang, C.-F.; Sun, X.-Y.; Su, M.; Wang, Y.-P.; Lv, Y.-K. Electrochemical biosensors based on antibody, nucleic acid and enzyme functionalized graphene for the detection of disease-related biomolecules. Analyst 2020, 145, 1550–1562. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, C.; Zhao, H.; An, H.; Cao, H.; Zhang, Y.; Fan, Z. Tuning sulfur doping in graphene for highly sensitive dopamine biosensors. Carbon 2015, 86, 197–206. [Google Scholar] [CrossRef]
- Macovei, D.-G.; Irimes, M.-B.; Hosu, O.; Cristea, C.; Tertis, M. Point-of-care electrochemical testing of biomarkers involved in inflammatory and inflammatory-associated medical conditions. Anal. Bioanal. Chem. 2023, 415, 1033–1063. [Google Scholar] [CrossRef]
- Ahmad, A.; Imran, M.; Ahsan, H. Biomarkers as biomedical bioindicators: Approaches and techniques for the detection, analysis, and validation of novel Biomarkers of diseases. Pharmaceutics 2023, 15, 1630. [Google Scholar] [CrossRef] [PubMed]
- Shrikrishna, N.S.; Sharma, R.; Sahoo, J.; Kaushik, A.; Gandhi, S. Navigating the landscape of optical biosensors. Chem. Eng. J. 2024, 490, 151661. [Google Scholar] [CrossRef]
- Yang, L.; Hou, H.; Li, J. Frontiers in Fluorescence imaging: Tools for the In-Situ Sensing of Disease Biomarkers. J. Mater. Chem. B 2025, 13, 1133–1158. [Google Scholar] [CrossRef] [PubMed]
- Kazanskiy, N.L.; Khonina, S.N.; Butt, M.A. A review on flexible wearables-Recent developments in non-invasive continuous health monitoring. Sens. Actuators A Phys. 2024, 366, 114993. [Google Scholar] [CrossRef]
- Wong, S.H.D.; Deen, G.R.; Bates, J.S.; Maiti, C.; Lam, C.Y.K.; Pachauri, A.; AlAnsari, R.; Bělský, P.; Yoon, J.; Dodda, J.M. Smart skin-adhesive patches: From design to biomedical applications. Adv. Funct. Mater. 2023, 33, 2213560. [Google Scholar] [CrossRef]
- Krishnan, S. Colorimetric visual sensors for point-of-needs testing. Sens. Actuators Rep. 2022, 4, 100078. [Google Scholar] [CrossRef]
- Shende, P.; Prabhakar, B.; Patil, A. Color changing sensors: A multimodal system for integrated screening. TrAC Trends Anal. Chem. 2019, 121, 115687. [Google Scholar] [CrossRef]
- Liu, L.; Li, X.; Nagao, M.; Elias, A.L.; Narain, R.; Chung, H.-J. A pH-Indicating colorimetric tough hydrogel patch towards applications in a substrate for smart wound dressings. Polymers 2017, 9, 558. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Zheng, Y.; Jiang, X.; Zhou, C.; Jin, H.; Jin, K.; Wu, W.; Haick, H. Wearable sensors and systems for wound healing-related pH and temperature detection. Micromachines 2021, 12, 430. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ochoa, M.; Waimin, J.F.; Rahimi, R.; Ziaie, B. A pH-regulated drug delivery dermal patch for targeting infected regions in chronic wounds. Lab A Chip 2019, 19, 2265–2274. [Google Scholar] [CrossRef]
- Clemente, F.; Antonacci, A.; Giardi, M.T.; Frisulli, V.; Tambaro, F.P.; Scognamiglio, V. Last trends in Point-of-Care (POC) diagnostics for the management of hematological indices in home care patients. Biosensors 2023, 13, 345. [Google Scholar] [CrossRef]
- Liu, J.; Geng, Z.; Fan, Z.; Liu, J.; Chen, H. Point-of-care testing based on smartphone: The current state-of-the-art (2017–2018). Biosens. Bioelectron. 2019, 132, 17–37. [Google Scholar] [CrossRef]
- Mahato, K.; Saha, T.; Ding, S.; Sandhu, S.S.; Chang, A.-Y.; Wang, J. Hybrid multimodal wearable sensors for comprehensive health monitoring. Nat. Electron. 2024, 7, 735–750. [Google Scholar] [CrossRef]
- Shrivastava, S.; Trung, T.Q.; Lee, N.-E. Recent progress, challenges, and prospects of fully integrated mobile and wearable point-of-care testing systems for self-testing. Chem. Soc. Rev. 2020, 49, 1812–1866. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Hu, C.; Yttri, E.A.; Panat, R. Recent advances in 3D printing of biomedical sensing devices. Adv. Funct. Mater. 2022, 32, 2107671. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yoo, S.; Liu, C.; Kwak, S.S.; Walter, J.R.; Xu, S.; Rogers, J.A. Skin-interfaced wireless biosensors for perinatal and paediatric health. Nat. Rev. Bioeng. 2023, 1, 631–647. [Google Scholar] [CrossRef]
- Zhao, C.; Park, J.; Root, S.E.; Bao, Z. Skin-inspired soft bioelectronic materials, devices and systems. Nat. Rev. Bioeng. 2024, 2, 671–690. [Google Scholar] [CrossRef]
- Xiong, Z.; Achavananthadith, S.; Lian, S.; Madden, L.E.; Ong, Z.X.; Chua, W.; Kalidasan, V.; Li, Z.; Liu, Z.; Singh, P. A wireless and battery-free wound infection sensor based on DNA hydrogel. Sci. Adv. 2021, 7, eabj1617. [Google Scholar] [CrossRef] [PubMed]
- Kalkal, A.; Kumar, S.; Kumar, P.; Pradhan, R.; Willander, M.; Packirisamy, G.; Kumar, S.; Malhotra, B.D. Recent advances in 3D printing technologies for wearable (bio) sensors. Addit. Manuf. 2021, 46, 102088. [Google Scholar] [CrossRef]
- Thirumalai, D.; Santhamoorthy, M.; Kim, S.-C.; Lim, H.-R. Conductive polymer-based hydrogels for wearable electrochemical biosensors. Gels 2024, 10, 459. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xie, S.; Wang, Z.; Liu, F.; Yang, Y.; Tang, C.; Wu, X.; Liu, P.; Li, Y.; Saiyin, H. Functionalized helical fibre bundles of carbon nanotubes as electrochemical sensors for long-term in vivo monitoring of multiple disease biomarkers. Nat. Biomed. Eng. 2020, 4, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Mackin, C.; Weng, W.-H.; Zhu, J.; Luo, Y.; Luo, S.-X.L.; Lu, A.-Y.; Hempel, M.; McVay, E.; Kong, J. Integrated biosensor platform based on graphene transistor arrays for real-time high-accuracy ion sensing. Nat. Commun. 2022, 13, 5064. [Google Scholar] [CrossRef]
- Huang, H.; Su, S.; Wu, N.; Wan, H.; Wan, S.; Bi, H.; Sun, L. Graphene-based sensors for human health monitoring. Front. Chem. 2019, 7, 399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Y.; He, X.; Yang, Y.; Chen, X.; Li, J. Advances in microneedle technology for biomedical detection. Biomater. Sci. 2024, 12, 5134–5149. [Google Scholar] [CrossRef]
- Erdem, Ö.; Eş, I.; Akceoglu, G.A.; Saylan, Y.; Inci, F. Recent advances in microneedle-based sensors for sampling, diagnosis and monitoring of chronic diseases. Biosensors 2021, 11, 296. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Z.; Xiao, M.; Li, Z.; Zhu, Z. Advances in biomedical systems based on microneedles: Design, fabrication, and application. Biomater. Sci. 2024, 12, 530–563. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Zhu, R.; Peng, I.; Xu, Z.; Jiang, Y. Wearable and Implantable Biosensors: Mechanisms and Applications for Closed-Loop Therapeutic Systems. J. Mater. Chem. B 2024, 12, 8577–8604. [Google Scholar] [CrossRef]
- Jiang, Y.; Islam, M.N.; He, R.; Huang, X.; Cao, P.F.; Advincula, R.C.; Dahotre, N.; Dong, P.; Wu, H.F.; Choi, W. Recent advances in 3D printed sensors: Materials, design, and manufacturing. Adv. Mater. Technol. 2023, 8, 2200492. [Google Scholar] [CrossRef]
- Muñoz, J.; Pumera, M. 3D-printed biosensors for electrochemical and optical applications. TrAC Trends Anal. Chem. 2020, 128, 115933. [Google Scholar] [CrossRef]
- Tsegay, F.; Elsherif, M.; Alam, F.; Butt, H. Smart 3D printed auxetic hydrogel skin wound dressings. ACS Appl. Bio Mater. 2022, 5, 5545–5553. [Google Scholar] [CrossRef] [PubMed]
- Astaneh, M.E.; Fereydouni, N. Silver Nanoparticles in 3D Printing: A New Frontier in Wound Healing. ACS Omega 2024, 9, 41107–41129. [Google Scholar] [CrossRef] [PubMed]
- Distler, T.; Boccaccini, A.R. 3D printing of electrically conductive hydrogels for tissue engineering and biosensors—A review. Acta Biomater 2020, 101, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Sharma, A.; Ahmed, A.; Sundramoorthy, A.K.; Furukawa, H.; Arya, S.; Khosla, A. Recent Advances in Electrochemical Biosensors: Applications, Challenges, and Future Scope. Biosensors 2021, 11, 336. [Google Scholar] [CrossRef] [PubMed]
- Salvo, P.; Dini, V.; Kirchhain, A.; Janowska, A.; Oranges, T.; Chiricozzi, A.; Lomonaco, T.; Di Francesco, F.; Romanelli, M. Sensors and Biosensors for C-Reactive Protein, Temperature and pH, and Their Applications for Monitoring Wound Healing: A Review. Sensors 2017, 17, 2952. [Google Scholar] [CrossRef]
- Koklu, A.; Ohayon, D.; Wustoni, S.; Druet, V.; Saleh, A.; Inal, S. Organic Bioelectronic Devices for Metabolite Sensing. Chem. Rev. 2022, 122, 4581–4635. [Google Scholar] [CrossRef] [PubMed]
- Diacci, C.; Burtscher, B.; Berto, M.; Ruoko, T.-P.; Lienemann, S.; Greco, P.; Berggren, M.; Borsari, M.; Simon, D.T.; Bortolotti, C.A.; et al. Organic Electrochemical Transistor Aptasensor for Interleukin-6 Detection. ACS Appl. Mater. Interfaces 2024, 16, 61467–61474. [Google Scholar] [CrossRef] [PubMed]
- Gomes, N.O.; Paschoalin, R.T.; Bilatto, S.; Sorigotti, A.R.; Farinas, C.S.; Mattoso, L.H.C.; Machado, S.A.S.; Oliveira, O.N., Jr.; Raymundo-Pereira, P.A. Flexible, Bifunctional Sensing Platform Made with Biodegradable Mats for Detecting Glucose in Urine. ACS Sustain. Chem. Eng. 2023, 11, 2209–2218. [Google Scholar] [CrossRef]
- Chanmugam, A.; Langemo, D.; Thomason, K.; Haan, J.; Altenburger, E.A.; Tippett, A.; Henderson, L.; Zortman, T.A. Relative Temperature Maximum in Wound Infection and Inflammation as Compared with a Control Subject Using Long-Wave Infrared Thermography. Adv. Ski. Wound Care 2017, 30, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.-H.; Samandari, M.; Li, C.; Li, H.; Song, D.; Zhang, Y.; Tamayol, A.; Wang, X. Multimodal sensing and therapeutic systems for wound healing and management: A review. Sens. Actuators Rep. 2022, 4, 100075. [Google Scholar] [CrossRef]
- Prakashan, D.; Kaushik, A.; Gandhi, S. Smart sensors and wound dressings: Artificial intelligence-supported chronic skin monitoring—A review. Chem. Eng. J. 2024, 497, 154371. [Google Scholar] [CrossRef]
- Fernandez, M.L.; Upton, Z.; Shooter, G.K. Uric Acid and Xanthine Oxidoreductase in Wound Healing. Curr. Rheumatol. Rep. 2013, 16, 396. [Google Scholar] [CrossRef]
- Brown, M.S.; Ashley, B.; Koh, A. Wearable Technology for Chronic Wound Monitoring: Current Dressings, Advancements, and Future Prospects. Front. Bioeng. Biotechnol. 2018, 6, 47. [Google Scholar] [CrossRef]
- Barbosa, A.I.; Reis, N.M. A critical insight into the development pipeline of microfluidic immunoassay devices for the sensitive quantitation of protein biomarkers at the point of care. Analyst 2017, 142, 858–882. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Li, Y.; Qiao, X.; Zeng, X.; Luo, X. An antifouling electrochemical biosensor based on oxidized bacterial cellulose and quaternized chitosan for reliable detection of involucrin in wound exudate. Anal. Chim. Acta 2024, 1316, 342821. [Google Scholar] [CrossRef]
- Reichel, T.; Held, S.; Schwarz, A.; Hacker, S.; Wesemann, F.; Donath, L.; Krüger, K. Acute response of biomarkers in plasma from capillary blood after a strenuous endurance exercise bout. Eur. J. Appl. Physiol. 2023, 123, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Samant, P.P.; Niedzwiecki, M.M.; Raviele, N.; Tran, V.; Mena-Lapaix, J.; Walker, D.I.; Felner, E.I.; Jones, D.P.; Miller, G.W.; Prausnitz, M.R. Sampling interstitial fluid from human skin using a microneedle patch. Sci. Transl. Med. 2020, 12, eaaw0285. [Google Scholar] [CrossRef] [PubMed]
- Haller, H.L.; Sander, F.; Popp, D.; Rapp, M.; Hartmann, B.; Demircan, M.; Nischwitz, S.P.; Kamolz, L.P. Oxygen, pH, Lactate, and Metabolism—How Old Knowledge and New Insights Might Be Combined for New Wound Treatment. Medicina 2021, 57, 1190. [Google Scholar] [CrossRef]
- Teymourian, H.; Tehrani, F.; Mahato, K.; Wang, J. Lab under the Skin: Microneedle Based Wearable Devices. Adv. Healthc. Mater. 2021, 10, 2002255. [Google Scholar] [CrossRef] [PubMed]
- Manickam, P.; Mariappan, S.A.; Murugesan, S.M.; Hansda, S.; Kaushik, A.; Shinde, R.; Thipperudraswamy, S.P. Artificial Intelligence (AI) and Internet of Medical Things (IoMT) Assisted Biomedical Systems for Intelligent Healthcare. Biosensors 2022, 12, 562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, Y.; Jiang, N.; Yetisen, A.K. Wearable artificial intelligence biosensor networks. Biosens. Bioelectron. 2023, 219, 114825. [Google Scholar] [CrossRef]
- Jin, X.; Liu, C.; Xu, T.; Su, L.; Zhang, X. Artificial intelligence biosensors: Challenges and prospects. Biosens. Bioelectron. 2020, 165, 112412. [Google Scholar] [CrossRef] [PubMed]
- Ariyaluran Habeeb, R.A.; Nasaruddin, F.; Gani, A.; Targio Hashem, I.A.; Ahmed, E.; Imran, M. Real-time big data processing for anomaly detection: A Survey. Int. J. Inf. Manag. 2019, 45, 289–307. [Google Scholar] [CrossRef]
- Shaw, I.; Ali, Y.S.; Nie, C.; Zhang, K.; Chen, C.; Xiao, Y. Integrating Artificial Intelligence and Microfluidics Technology for Psoriasis Therapy: A Comprehensive Review for Research and Clinical Applications. Adv. Intell. Syst. 2024, 2400558. [Google Scholar] [CrossRef]
- Rim, T.H.; Lee, G.; Kim, Y.; Tham, Y.-C.; Lee, C.J.; Baik, S.J.; Kim, Y.A.; Yu, M.; Deshmukh, M.; Lee, B.K.; et al. Prediction of systemic biomarkers from retinal photographs: Development and validation of deep-learning algorithms. Lancet Digit. Health 2020, 2, e526–e536. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.; Singh, K.R.B.; Yadav, A.K.; Nayak, V.; Singh, J.; Solanki, P.R.; Singh, R.P. Internet of things (IoT) in nano-integrated wearable biosensor devices for healthcare applications. Biosens. Bioelectron. X 2022, 11, 100153. [Google Scholar] [CrossRef]
- Mamdiwar, S.D.; R, A.; Shakruwala, Z.; Chadha, U.; Srinivasan, K.; Chang, C.-Y. Recent Advances on IoT-Assisted Wearable Sensor Systems for Healthcare Monitoring. Biosensors 2021, 11, 372. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.; Yang, F.; Jiang, Z.; Wu, K.; Hou, R.; Zhu, Y. Smart wound dressing for advanced wound management: Real-time monitoring and on-demand treatment. Mater. Des. 2023, 229, 111917. [Google Scholar] [CrossRef]
- Basatneh, R.; Najafi, B.; Armstrong, D.G. Health Sensors, Smart Home Devices, and the Internet of Medical Things: An Opportunity for Dramatic Improvement in Care for the Lower Extremity Complications of Diabetes. J. Diabetes Sci. Technol. 2018, 12, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, J.T.; Fernandes-Taylor, S.; Barnes, M.L.; Tomsejova, A.; Saunders, R.S.; Kent, K.C. Conceptualizing smartphone use in outpatient wound assessment: Patients’ and caregivers’ willingness to use technology. J. Surg. Res. 2015, 198, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Albahri, A.S.; Alwan, J.K.; Taha, Z.K.; Ismail, S.F.; Hamid, R.A.; Zaidan, A.A.; Albahri, O.S.; Zaidan, B.B.; Alamoodi, A.H.; Alsalem, M.A. IoT-based telemedicine for disease prevention and health promotion: State-of-the-Art. J. Netw. Comput. Appl. 2021, 173, 102873. [Google Scholar] [CrossRef]
- Manikkath, J.; Subramony, J.A. Toward closed-loop drug delivery: Integrating wearable technologies with transdermal drug delivery systems. Adv. Drug Deliv. Rev. 2021, 179, 113997. [Google Scholar] [CrossRef] [PubMed]
- Derakhshandeh, H.; Kashaf, S.S.; Aghabaglou, F.; Ghanavati, I.O.; Tamayol, A. Smart Bandages: The Future of Wound Care. Trends Biotechnol. 2018, 36, 1259–1274. [Google Scholar] [CrossRef]
- Bao, S.; Wang, Y.; Yao, L.; Chen, S.; Wang, X.; Luo, Y.; Lyu, H.; Yu, Y.; Zhou, P.; Zhou, Y. Research trends and hot topics of wearable sensors in wound care over past 18 years: A bibliometric analysis. Heliyon 2024, 10, e38762. [Google Scholar] [CrossRef]
- Mishra, A.; Kushare, A.; Gupta, M.N.; Ambre, P. Advanced Dressings for Chronic Wound Management. ACS Appl. Bio Mater. 2024, 7, 2660–2676. [Google Scholar] [CrossRef] [PubMed]
- Hassan Akhtar, M.; Azhar Hayat Nawaz, M.; Abbas, M.; Liu, N.; Han, W.; Lv, Y.; Yu, C. Advances in pH Sensing: From Traditional Approaches to Next-Generation Sensors in Biological Contexts. Chem. Rec. 2024, 24, e202300369. [Google Scholar] [CrossRef]
- Mariani, F.; Serafini, M.; Gualandi, I.; Arcangeli, D.; Decataldo, F.; Possanzini, L.; Tessarolo, M.; Tonelli, D.; Fraboni, B.; Scavetta, E. Advanced Wound Dressing for Real-Time pH Monitoring. ACS Sens. 2021, 6, 2366–2377. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; McCarty, S.; Hunt, J.A.; Woods, E.J. The effects of pH on wound healing, biofilms, and antimicrobial efficacy. Wound Repair Regen. 2014, 22, 174–186. [Google Scholar] [CrossRef]
- Das, I.J.; Bal, T. pH factors in chronic wound and pH-responsive polysaccharide-based hydrogel dressings. Int. J. Biol. Macromol. 2024, 279, 135118. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Trotsyuk, A.A.; Niu, S.; Henn, D.; Chen, K.; Shih, C.-C.; Larson, M.R.; Mermin-Bunnell, A.M.; Mittal, S.; Lai, J.-C.; et al. Wireless, closed-loop, smart bandage with integrated sensors and stimulators for advanced wound care and accelerated healing. Nat. Biotechnol. 2023, 41, 652–662. [Google Scholar] [CrossRef]
- Wang, C.; Shirzaei Sani, E.; Gao, W. Wearable Bioelectronics for Chronic Wound Management. Adv. Funct. Mater. 2022, 32, 2111022. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, C.; Tittelbach, J.; Hipler, U.-C.; Elsner, P. Clinical efficacy of dressings for treatment of heavily exuding chronic wounds. Chronic Wound Care Manag. Res. 2015, 2, 101–111. [Google Scholar] [CrossRef]
- Nuutila, K.; Eriksson, E. Moist Wound Healing with Commonly Available Dressings. Adv. Wound Care 2020, 10, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ji, H.; Gao, L.; Hao, R.; Shi, Y.; Yang, J.; Hao, Y.; Chen, J. Wearable hydrogel-based health monitoring systems: A new paradigm for health monitoring? Chem. Eng. J. 2024, 495, 153382. [Google Scholar] [CrossRef]
- Lee, H.K.; Yang, Y.J.; Koirala, G.R.; Oh, S.; Kim, T.-I. From lab to wearables: Innovations in multifunctional hydrogel chemistry for next-generation bioelectronic devices. Biomaterials 2024, 310, 122632. [Google Scholar] [CrossRef] [PubMed]
- Malone, M.; Schultz, G. Challenges in the diagnosis and management of wound infection. Br. J. Dermatol. 2022, 187, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Badea, M.; Tiwari, S.; Marty, J.L. Wearable Biosensors: An Alternative and Practical Approach in Healthcare and Disease Monitoring. Molecules 2021, 26, 748. [Google Scholar] [CrossRef]
- Yavarinasab, A.; Flibotte, S.; Liu, S.; Tropini, C. An impedance-based chemiresistor for the real-time, simultaneous detection of gut microbiota-generated short-chain fatty acids. Sens. Actuators B Chem. 2023, 393, 134182. [Google Scholar] [CrossRef]
- Basic, A.; Dahlén, G. Microbial metabolites in the pathogenesis of periodontal diseases: A narrative review. Front. Oral. Health 2023, 4, 1210200. [Google Scholar] [CrossRef] [PubMed]
- Tegl, G.; Schiffer, D.; Sigl, E.; Heinzle, A.; Guebitz, G.M. Biomarkers for infection: Enzymes, microbes, and metabolites. Appl. Microbiol. Biotechnol. 2015, 99, 4595–4614. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Xu, N.; Wu, X.; Liu, M.; Liu, Y.; Zhao, J.; Zhang, T.; Zhao, J.; Zhou, Y.; Xie, Q.; et al. Enhanced heterogeneous interface to construct intelligent conductive hydrogel gas sensor for individualized treatment of infected wounds. Int. J. Biol. Macromol. 2024, 258, 128520. [Google Scholar] [CrossRef] [PubMed]
- Salinas Alvarez, C.; Sierra-Sosa, D.; Garcia-Zapirain, B.; Yoder-Himes, D.; Elmaghraby, A. Detection of Volatile Compounds Emitted by Bacteria in Wounds Using Gas Sensors. Sensors 2019, 19, 1523. [Google Scholar] [CrossRef]
- Li, H.; Li, B.; Lv, D.; Li, W.; Lu, Y.; Luo, G. Biomaterials releasing drug responsively to promote wound healing via regulation of pathological microenvironment. Adv. Drug Deliv. Rev. 2023, 196, 114778. [Google Scholar] [CrossRef]
- Joorabloo, A.; Liu, T. Smart theranostics for wound monitoring and therapy. Adv. Colloid Interface Sci. 2024, 330, 103207. [Google Scholar] [CrossRef] [PubMed]
- Maierhofer, M.; Rieger, V.; Mayr, T. Optical ammonia sensors based on fluorescent aza-BODIPY dyes—A flexible toolbox. Anal. Bioanal. Chem. 2020, 412, 7559–7567. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Selvaganapathy, P.R. Porous biocompatible colorimetric nanofiber-based sensor for selective ammonia detection on personal wearable protective equipment. Sens. Actuators B Chem. 2023, 393, 134270. [Google Scholar] [CrossRef]
- Wang, X.; Chen, C.; Hu, J.; Liu, C.; Ning, Y.; Lu, F. Current strategies for monitoring and controlling bacterial biofilm formation on medical surfaces. Ecotoxicol. Environ. Saf. 2024, 282, 116709. [Google Scholar] [CrossRef]
- Ahmed, A.; Rushworth Jo, V.; Hirst Natalie, A.; Millner Paul, A. Biosensors for Whole-Cell Bacterial Detection. Clin. Microbiol. Rev. 2014, 27, 631–646. [Google Scholar] [CrossRef]
- Zhou, Q.; Si, Z.; Wang, K.; Li, K.; Hong, W.; Zhang, Y.; Li, P. Enzyme-triggered smart antimicrobial drug release systems against bacterial infections. J. Control. Release 2022, 352, 507–526. [Google Scholar] [CrossRef] [PubMed]
- Kirchhain, A.; Poma, N.; Salvo, P.; Tedeschi, L.; Melai, B.; Vivaldi, F.; Bonini, A.; Franzini, M.; Caponi, L.; Tavanti, A.; et al. Biosensors for measuring matrix metalloproteinases: An emerging research field. TrAC Trends Anal. Chem. 2019, 110, 35–50. [Google Scholar] [CrossRef]
- Lei, Z.; Jian, M.; Li, X.; Wei, J.; Meng, X.; Wang, Z. Biosensors and bioassays for determination of matrix metalloproteinases: State of the art and recent advances. J. Mater. Chem. B 2020, 8, 3261–3291. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Ding, C.; Qin, M.; Li, J. Hydrogel-Based Biosensors for Bacterial Infections. Small 2024, 20, 2306960. [Google Scholar] [CrossRef]
- Zhang, J.; Kim, M.-H.; Lee, S.; Park, S. Integration of nanobiosensors into organ-on-chip systems for monitoring viral infections. Nano Converg. 2024, 11, 47. [Google Scholar] [CrossRef] [PubMed]
- Simoska, O.; Duay, J.; Stevenson, K.J. Electrochemical Detection of Multianalyte Biomarkers in Wound Healing Efficacy. ACS Sens. 2020, 5, 3547–3557. [Google Scholar] [CrossRef] [PubMed]
- Cicha, I.; Priefer, R.; Severino, P.; Souto, E.B.; Jain, S. Biosensor-Integrated Drug Delivery Systems as New Materials for Biomedical Applications. Biomolecules 2022, 12, 1198. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Singh, D.; Gaur, P.; Joshi, D. Intelligent automated drug administration and therapy: Future of healthcare. Drug Deliv. Transl. Res. 2021, 11, 1878–1902. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tong, S.; Niu, J.; Cao, C.; Gao, A.; Jiao, Y.; Fu, Y.; Li, D.; Pan, X.; Cui, D.; et al. Microneedles: Multifunctional devices for drug delivery, body fluid extraction, and bio-sensing. Nanoscale 2024, 17, 740–773. [Google Scholar] [CrossRef]
- Wu, C.; Yu, Q.; Huang, C.; Li, F.; Zhang, L.; Zhu, D. Microneedles as transdermal drug delivery system for enhancing skin disease treatment. Acta Pharm. Sin. B 2024, 14, 5161–5180. [Google Scholar] [CrossRef] [PubMed]
- Nordström, R.; Malmsten, M. Delivery systems for antimicrobial peptides. Adv. Colloid Interface Sci. 2017, 242, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Haidari, H.; Vasilev, K.; Cowin, A.J.; Kopecki, Z. Bacteria-Activated Dual pH- and Temperature-Responsive Hydrogel for Targeted Elimination of Infection and Improved Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 51744–51762. [Google Scholar] [CrossRef] [PubMed]
- Horta-Velázquez, A.; Mota-Morales, J.D.; Morales-Narváez, E. Next-generation of smart dressings: Integrating multiplexed sensors and theranostic functions. Int. J. Biol. Macromol. 2024, 254, 127737. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lorenzo, C.; Concheiro, A. Smart drug delivery systems: From fundamentals to the clinic. Chem. Commun. 2014, 50, 7743–7765. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, M.; Rahimi, R.; Zhou, J.; Jiang, H.; Yoon, C.K.; Maddipatla, D.; Narakathu, B.B.; Jain, V.; Oscai, M.M.; Morken, T.J.; et al. Integrated sensing and delivery of oxygen for next-generation smart wound dressings. Microsyst. Nanoeng. 2020, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Gu, Z.; Zhou, L.; Hao, M.; An, H.; Song, K.; Wu, X.; Zhang, K.; Zhao, Z.; Dong, Y.; et al. Recent Progress in Intelligent Wearable Sensors for Health Monitoring and Wound Healing Based on Biofluids. Front. Bioeng. Biotechnol. 2021, 9, 765987. [Google Scholar] [CrossRef] [PubMed]
- Janghorban, M.; Aradanas, I.; Kazemi, S.; Ngaju, P.; Pandey, R. Recent Advances, Opportunities, and Challenges in Developing Nucleic Acid Integrated Wearable Biosensors for Expanding the Capabilities of Wearable Technologies in Health Monitoring. Biosensors 2022, 12, 986. [Google Scholar] [CrossRef] [PubMed]
- Chenani, H.; Saeidi, M.; Rastkhiz, M.A.; Bolghanabadi, N.; Aghaii, A.H.; Orouji, M.; Hatamie, A.; Simchi, A. Challenges and Advances of Hydrogel-Based Wearable Electrochemical Biosensors for Real-Time Monitoring of Biofluids: From Lab to Market. A Review. Anal. Chem. 2024, 96, 8160–8183. [Google Scholar] [CrossRef] [PubMed]
- Friedel, M.; Thompson, I.A.P.; Kasting, G.; Polsky, R.; Cunningham, D.; Soh, H.T.; Heikenfeld, J. Opportunities and challenges in the diagnostic utility of dermal interstitial fluid. Nat. Biomed. Eng. 2023, 7, 1541–1555. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-W.; Wu, Y.-F.; Ahmed, T.; Pan, S.-C.; Cheng, C.-M. Point-of-care detection devices for wound care and monitoring. Trends in Biotechnology 2024, 42, 74–90. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical Sensors and Biosensors Based on Nanomaterials and Nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef]
- Xu, M.; Obodo, D.; Yadavalli, V.K. The design, fabrication, and applications of flexible biosensing devices. Biosens. Bioelectron. 2019, 124–125, 96–114. [Google Scholar] [CrossRef]
- Li, Z.; Askim, J.R.; Suslick, K.S. The Optoelectronic Nose: Colorimetric and Fluorometric Sensor Arrays. Chem. Rev. 2019, 119, 231–292. [Google Scholar] [CrossRef]
- Yin, S.; Yu, Z.; Song, N.; Guo, Z.; Li, W.; Ma, J.; Wang, X.; Liu, J.; Liang, M. A long lifetime and highly sensitive wearable microneedle sensor for the continuous real-time monitoring of glucose in interstitial fluid. Biosens. Bioelectron. 2024, 244, 115822. [Google Scholar] [CrossRef] [PubMed]
- ElSaboni, Y.; Hunt, J.A.; Stanley, J.; Moffatt, C.; Wei, Y. Development of a textile based protein sensor for monitoring the healing progress of a wound. Sci. Rep. 2022, 12, 7972. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Kim, J.; Walter, J.R.; Ghaffari, R.; Rogers, J.A. Translational gaps and opportunities for medical wearables in digital health. Sci. Transl. Med. 2022, 14, eabn6036. [Google Scholar] [CrossRef]
- Oksel Karakus, C.; Bilgi, E.; Winkler, D.A. Biomedical nanomaterials: Applications, toxicological concerns, and regulatory needs. Nanotoxicology 2021, 15, 331–351. [Google Scholar] [CrossRef] [PubMed]
- Amaral, C.; Paiva, M.; Rodrigues, A.R.; Veiga, F.; Bell, V. Global Regulatory Challenges for Medical Devices: Impact on Innovation and Market Access. Appl. Sci. 2024, 14, 9304. [Google Scholar] [CrossRef]
- Abbas, J.J.; Smith, B.; Poluta, M.; Velazquez-Berumen, A. Improving health-care delivery in low-resource settings with nanotechnology: Challenges in multiple dimensions. Nanobiomedicine 2017, 4, 1849543517701158. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Wang, D.; Zhao, S.; Lou, Z.; Shen, G. Wearable Sensors-Enabled Human–Machine Interaction Systems: From Design to Application. Adv. Funct. Mater. 2021, 31, 2008936. [Google Scholar] [CrossRef]
- Dunn, L.; Prosser, H.C.; Tan, J.T.; Vanags, L.Z.; Ng, M.K.; Bursill, C.A. Murine model of wound healing. J. Vis. Exp. 2013, 75, e50265. [Google Scholar] [CrossRef]
- Chen, L.; Mirza, R.; Kwon, Y.; DiPietro, L.A.; Koh, T.J. The murine excisional wound model: Contraction revisited. Wound Repair Regen. 2015, 23, 874–877. [Google Scholar] [CrossRef]
- Thirabowonkitphithan, P.; Phuengmaung, P.; Leelahavanichkul, A.; Laiwattanapaisal, W. MWCNTs/PVA Hydrogel-Modified Electrochemical Sensors for Ex Vivo and In Vivo Detection of Pyocyanin Biomarker for Pseudomonas aeruginosa Wound Infection. ACS Appl. Electron. Mater. 2023, 5, 821–831. [Google Scholar] [CrossRef]
- Sun, T.; He, J.; Qian, S.; Zheng, Y.; Zhang, K.; Luo, J.; Tian, F. Collaborative detection for wound infections using electronic nose and FAIMS technology based on a rat wound model. Sens. Actuators B Chem. 2020, 320, 128595. [Google Scholar] [CrossRef]
- Seaton, M.; Hocking, A.; Gibran, N.S. Porcine Models of Cutaneous Wound Healing. ILAR J. 2015, 56, 127–138. [Google Scholar] [CrossRef]
- Chen, C.-M.; Kwasnicki, R.M.; Curto, V.F.; Yang, G.-Z.; Lo, B.P.L. Tissue oxygenation sensor and an active in vitro phantom for sensor validation. IEEE Sens. J. 2019, 19, 8233–8240. [Google Scholar] [CrossRef]
- Zeng, X.; Peng, R.; Fan, Z.; Lin, Y. Self-powered and wearable biosensors for healthcare. Mater. Today Energy 2022, 23, 100900. [Google Scholar] [CrossRef]
- Song, Y.; Mukasa, D.; Zhang, H.; Gao, W. Self-Powered Wearable Biosensors. Acc. Mater. Res. 2021, 2, 184–197. [Google Scholar] [CrossRef]
- Parrilla, M.; De Wael, K. Wearable Self-Powered Electrochemical Devices for Continuous Health Management. Adv. Funct. Mater. 2021, 31, 2107042. [Google Scholar] [CrossRef]
- Shen, J.; Li, Z.; Yu, J.; Ding, B. Humidity-resisting triboelectric nanogenerator for high performance biomechanical energy harvesting. Nano Energy 2017, 40, 282–288. [Google Scholar] [CrossRef]
- Moloudian, G.; Hosseinifard, M.; Kumar, S.; Simorangkir, R.B.V.B.; Buckley, J.L.; Song, C.; Fantoni, G.; O’Flynn, B. RF Energy Harvesting Techniques for Battery-Less Wireless Sensing, Industry 4.0, and Internet of Things: A Review. IEEE Sens. J. 2024, 24, 5732–5745. [Google Scholar] [CrossRef]
- Chamanian, S.; Uluşan, H.; Zorlu, Ö.; Baghaee, S.; Uysal-Biyikoglu, E.; Külah, H. Wearable battery-less wireless sensor network with electromagnetic energy harvesting system. Sens. Actuators A Phys. 2016, 249, 77–84. [Google Scholar] [CrossRef]
- Gao, Z.; Zhou, Y.; Zhang, J.; Foroughi, J.; Peng, S.; Baughman, R.H.; Wang, Z.L.; Wang, C.H. Advanced Energy Harvesters and Energy Storage for Powering Wearable and Implantable Medical Devices. Adv. Mater. 2024, 36, 2404492. [Google Scholar] [CrossRef] [PubMed]
- Engmann, F.; Katsriku, F.A.; Abdulai, J.-D.; Adu-Manu, K.S.; Banaseka, F.K. Prolonging the Lifetime of Wireless Sensor Networks: A Review of Current Techniques. Wirel. Commun. Mob. Comput. 2018, 2018, 8035065. [Google Scholar] [CrossRef]
- Li, S.; Lu, D.; Li, S.; Liu, J.; Xu, Y.; Yan, Y.; Rodriguez, J.Z.; Bai, H.; Avila, R.; Kang, S.; et al. Bioresorbable, wireless, passive sensors for continuous pH measurements and early detection of gastric leakage. Sci. Adv. 2024, 10, eadj0268. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-K.; Murphy, R.K.J.; Hwang, S.-W.; Lee, S.M.; Harburg, D.V.; Krueger, N.A.; Shin, J.; Gamble, P.; Cheng, H.; Yu, S.; et al. Bioresorbable silicon electronic sensors for the brain. Nature 2016, 530, 71–76. [Google Scholar] [CrossRef]
- Liu, H.; Song, J.; Zhao, Z.; Zhao, S.; Tian, Z.; Yan, F. Organic Electrochemical Transistors for Biomarker Detections. Adv. Sci. 2024, 11, 2305347. [Google Scholar] [CrossRef]
- Chatterjee, S.; Saxena, M.; Padmanabhan, D.; Jayachandra, M.; Pandya, H.J. Futuristic medical implants using bioresorbable materials and devices. Biosens. Bioelectron. 2019, 142, 111489. [Google Scholar] [CrossRef]
- La Mattina, A.A.; Mariani, S.; Barillaro, G. Bioresorbable materials on the rise: From electronic components and physical sensors to in vivo monitoring systems. Adv. Sci. 2020, 7, 1902872. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, E.S.; Dervin, S.; Ganguly, P.; Dahiya, R. Biodegradable materials for sustainable health monitoring devices. ACS Appl. Bio Mater. 2020, 4, 163–194. [Google Scholar] [CrossRef]
- Deng, X.; Gould, M.; Ali, M.A. A review of current advancements for wound healing: Biomaterial applications and medical devices. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 2542–2573. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lee, G.; Li, S.; Hu, Z.; Zhao, K.; Rogers, J.A. Advances in bioresorbable materials and electronics. Chem. Rev. 2023, 123, 11722–11773. [Google Scholar] [CrossRef] [PubMed]
- Darvishi, S.; Tavakoli, S.; Kharaziha, M.; Girault, H.H.; Kaminski, C.F.; Mela, I. Advances in the sensing and treatment of wound biofilms. Angew. Chem. 2022, 134, e202112218. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.S.; Dias, S.B.; Jelinek, H.F.; Hadjileontiadis, L.J.; Pappa, A.-M. The convergence of traditional and digital biomarkers through AI-assisted biosensing: A new era in translational diagnostics? Biosens. Bioelectron. 2023, 235, 115387. [Google Scholar] [CrossRef]
- Noushin, T.; Hossain, N.I.; Tabassum, S. IoT-enabled integrated smart wound sensor for multiplexed monitoring of inflammatory biomarkers at the wound site. Front. Nanotechnol. 2022, 4, 851041. [Google Scholar] [CrossRef]
- Bhide, A.; Ganguly, A.; Parupudi, T.; Ramasamy, M.; Muthukumar, S.; Prasad, S. Next-generation continuous metabolite sensing toward emerging sensor needs. ACS Omega 2021, 6, 6031–6040. [Google Scholar] [CrossRef]
- Zhou, J.; Yao, D.; Qian, Z.; Hou, S.; Li, L.; Jenkins, A.T.A.; Fan, Y. Bacteria-responsive intelligent wound dressing: Simultaneous In situ detection and inhibition of bacterial infection for accelerated wound healing. Biomaterials 2018, 161, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Hochlenert, D.; Bogoclu, C.; Cremanns, K.; Gierschner, L.; Ludmann, D.; Mertens, M.; Tromp, T.; Weggen, A.; Otten, H. Sensor-Assisted Wound Therapy in Plantar Diabetic Foot Ulcer Treatment: A Randomized Clinical Trial. J. Diabetes Sci. Technol. 2023, 19322968231213095. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, S.; Kanwal, T.; Ahmad, N.; Fatima, B.; Najam-ul-Haq, M.; Hussain, D. Advances and challenges in portable optical biosensors for onsite detection and point-of-care diagnostics. TrAC Trends Anal. Chem. 2024, 173, 117640. [Google Scholar] [CrossRef]
- Weigelt, M.A.; Lev-Tov, H.A.; Tomic-Canic, M.; Lee, W.D.; Williams, R.; Strasfeld, D.; Kirsner, R.S.; Herman, I.M. Advanced wound diagnostics: Toward transforming wound care into precision medicine. Adv. Wound Care 2022, 11, 330–359. [Google Scholar] [CrossRef]
- Baker, S.; Xiang, W. Artificial intelligence of things for smarter healthcare: A survey of advancements, challenges, and opportunities. IEEE Commun. Surv. Tutor. 2023, 25, 1261–1293. [Google Scholar] [CrossRef]
- Hosseini, E.S.; Bhattacharjee, M.; Manjakkal, L.; Dahiya, R. Healing and monitoring of chronic wounds: Advances in wearable technologies. In Digital Health; Elsevier: Amsterdam, The Netherlands, 2021; pp. 85–99. [Google Scholar]
- Yang, C.; Yang, C.; Chen, Y.; Liu, J.; Liu, Z.; Chen, H.-J. The trends in wound management: Sensing, therapeutic treatment, and “theranostics”. J. Sci. Adv. Mater. Devices 2023, 8, 100619. [Google Scholar] [CrossRef]
- Ngiam, K.Y.; Khor, W. Big data and machine learning algorithms for health-care delivery. Lancet Oncol. 2019, 20, e262–e273. [Google Scholar] [CrossRef]
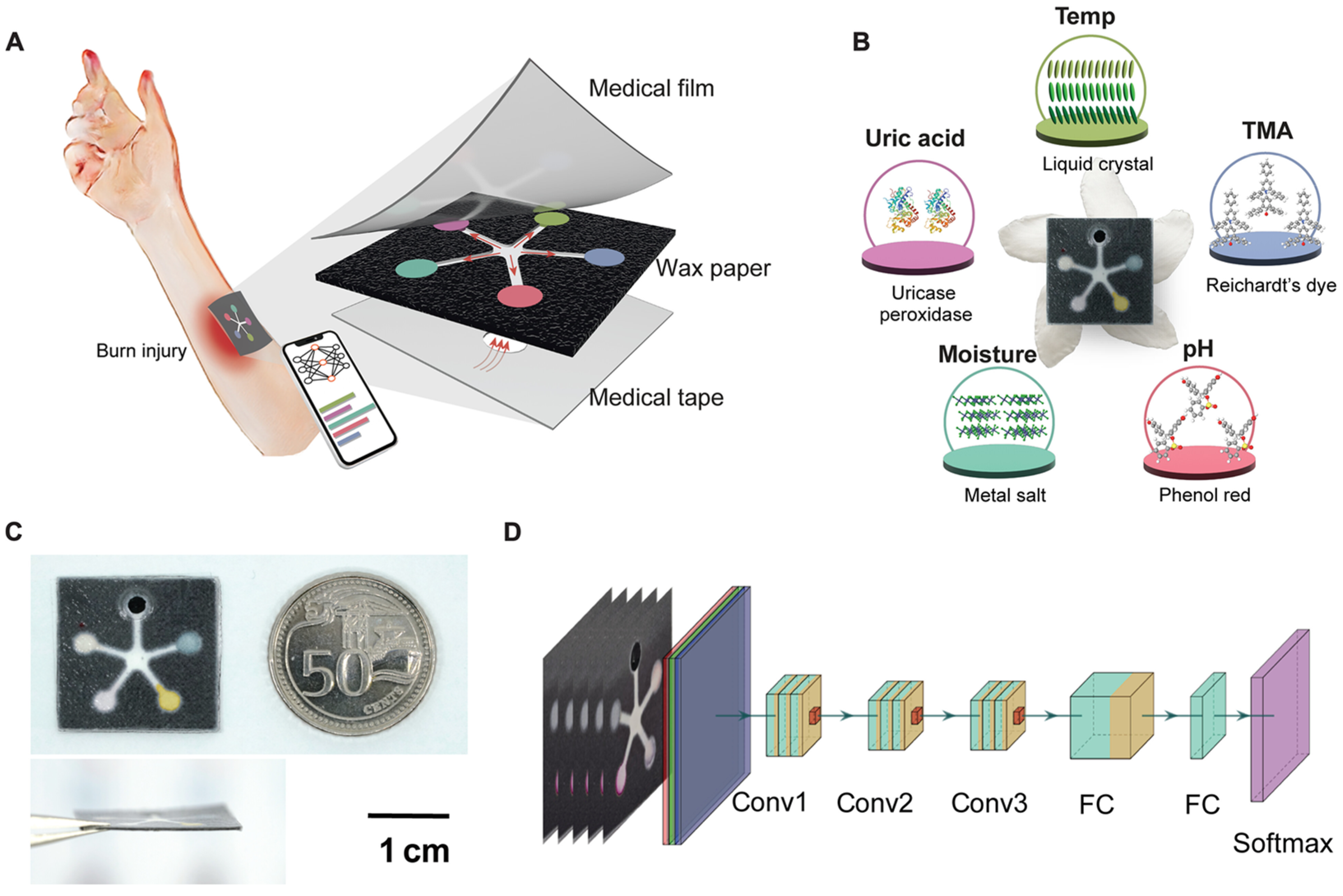

| Biomarker | Normal Range | Clinically Relevant Range | Detection Sensitivity | Relevance in Wound Healing | Measurement Location | Reference |
|---|---|---|---|---|---|---|
| Temperature | 31.1–36.5 °C | ≥3 °C above surrounding skin | ±0.5 °C | Indicates localized infection and inflammation | Wound Fluid, Capillary Blood | [110,111,112] |
| pH | 4.2–5.6 | ≥7 (7.15–8.9 in chronic wounds) | ±0.1 pH units | Helps differentiate chronic wounds from healing wounds, indicating infection or delayed healing | Wound Fluid, Interstitial Fluid | [34,111,112] |
| Uric acid | 220–750 µM | >1 mM (chronic wounds leg ulcers) | µM range | Elevated levels suggest oxidative stress, which can delay wound healing. | Wound Fluid, Interstitial Fluid | [112,113] |
| Interleukin-1 (IL-1) | <5 pg/mL | ≥5 pg/mL | pg/mL range | A key inflammatory biomarker, elevated in infected wounds | Capillary Blood, Wound Fluid | [114,115] |
| Interleukin-6 (IL-6) | <2.4 pg/mL | ≥2.4 pg/mL | pg/mL range | A key inflammatory biomarker, elevated in infected wounds | Capillary Blood, Wound Fluid | [114,115] |
| Tumor Necrosis Factor-alpha (TNF-α) | <14 pg/mL | ≥14 pg/mL | pg/mL range | Elevated levels are associated with chronic inflammation, indicating infection or delayed healing. | Capillary Blood | [115] |
| Trimethylamine (TMA) | Not typically present | >30 (ppm) Elevated in infected wounds | ppm | Marker for bacterial activity, particularly in anaerobic infections | Wound Fluid | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vo, D.-K.; Trinh, K.T.L. Advances in Wearable Biosensors for Wound Healing and Infection Monitoring. Biosensors 2025, 15, 139. https://doi.org/10.3390/bios15030139
Vo D-K, Trinh KTL. Advances in Wearable Biosensors for Wound Healing and Infection Monitoring. Biosensors. 2025; 15(3):139. https://doi.org/10.3390/bios15030139
Chicago/Turabian StyleVo, Dang-Khoa, and Kieu The Loan Trinh. 2025. "Advances in Wearable Biosensors for Wound Healing and Infection Monitoring" Biosensors 15, no. 3: 139. https://doi.org/10.3390/bios15030139
APA StyleVo, D.-K., & Trinh, K. T. L. (2025). Advances in Wearable Biosensors for Wound Healing and Infection Monitoring. Biosensors, 15(3), 139. https://doi.org/10.3390/bios15030139






