Intermolecular Structure Conversion-Based G4-TDF Nanostructures Functionalized μPADs for Fluorescent Determination of Potassium Ion in Serum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Instrumentation
2.3. Synthesis of G-Quadruplex/Tetrahedral DNA Framework (G4-TDF)
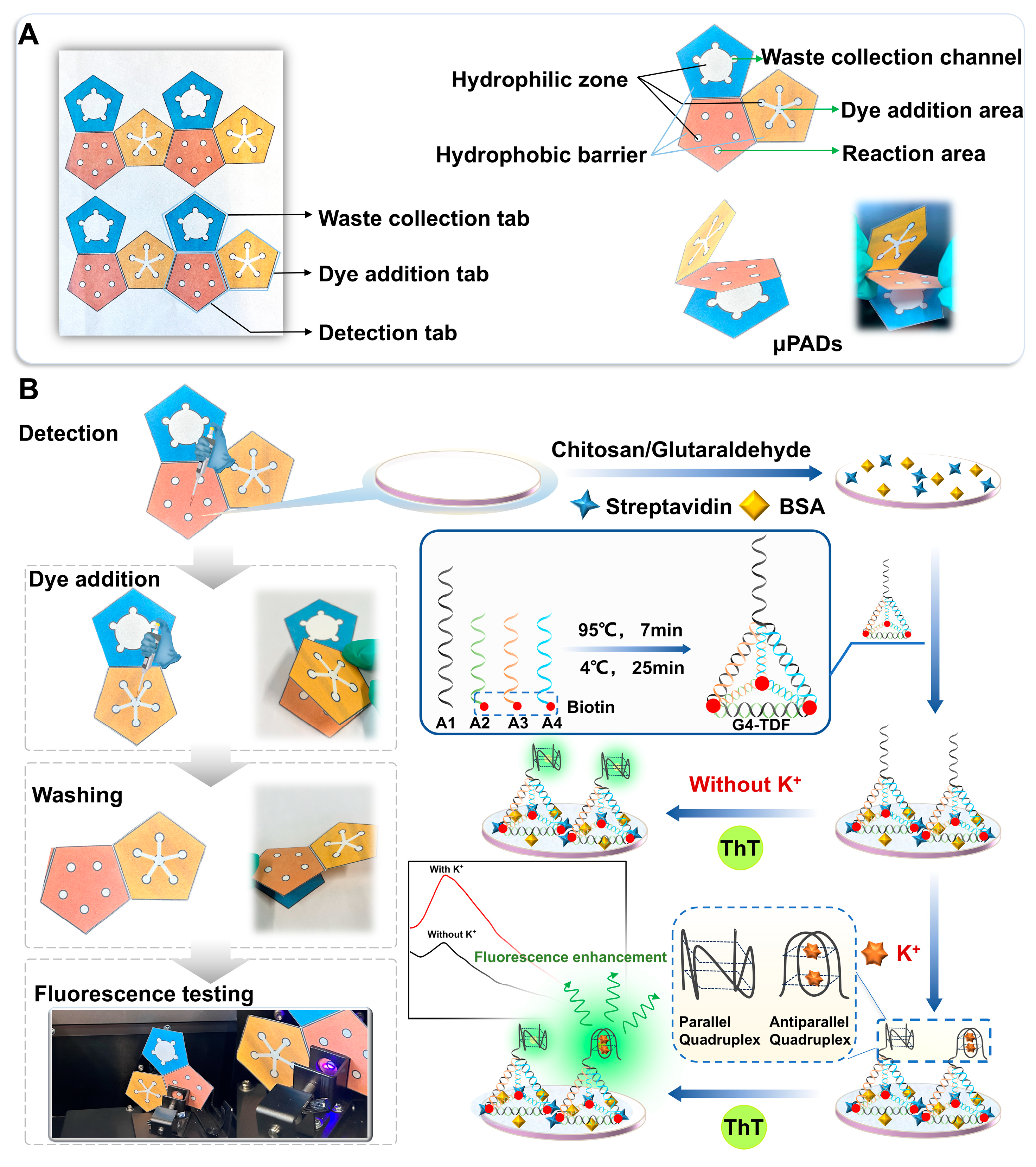
2.4. Fabrication of the G4-TDF Functionalized Origami μPADs
2.5. G4-TDF Functionalized Origami μPADs for Detection of K+
2.6. The Real Sample Analysis
3. Results and Discussion
3.1. Principle of Fluorescent Assay and Feasibility of the Origami μPADs for Detection of K+
3.2. Optimization of Experimental Conditions
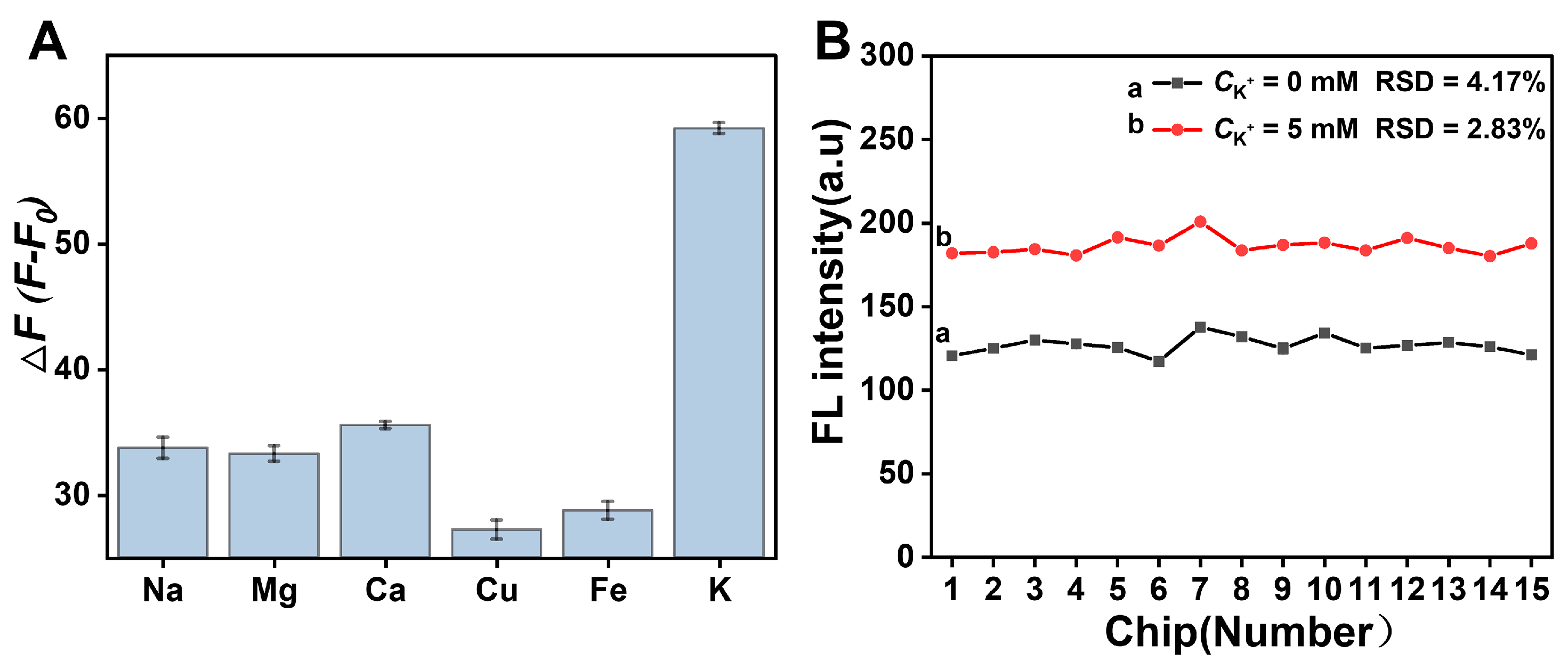
3.3. Performance Study of the Origami μPADs for Detection of K+
3.4. Detection of K+ in Actual Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, H.B.; Weaver, C.M. Rise in potassium deficiency in the US population linked to agriculture practices and dietary potassium deficits. J. Agric. Food Chem. 2020, 68, 11121–11127. [Google Scholar] [PubMed]
- Debnath, M.; Chakraborty, S.; Kumar, Y.P.; Chaudhuri, R.; Jana, B.; Dash, J. Ionophore constructed from non-covalent assembly of a G-quadruplex and liponucleoside transports K+-ion across biological membranes. Nat. Commun. 2020, 11, 469. [Google Scholar] [PubMed]
- Wang, G.F.; Chen, L.; Zhu, Y.H.; He, X.P.; Xu, G.; Zhang, X.J. Development of an electrochemical sensor based on the catalysis of ferrocene actuated hemin/G-quadruplex enzyme for the detection of potassium ions. Biosens. Bioelectron. 2014, 61, 410–416. [Google Scholar]
- Cheng, C.J.; Kuo, E.; Huang, C.L. Extracellular potassium homeostasis: Insights from hypokalemic periodic paralysis. Semin. Nephrol. 2013, 33, 237–247. [Google Scholar] [PubMed]
- Wang, Z.Q.; Pan, T.T.; Shen, M.; Liao, J.X.; Tian, Y.Q. Cross-conjugated polymers as fluorescent probes for intracellular potassium ion detection. Sens. Actuators B 2023, 390, 134008. [Google Scholar]
- Chen, Z.B.; Guo, J.X.; Ma, H.; Zhou, T.; Li, X.X. A simple colorimetric sensor for potassium ion based on DNA G-quadruplex conformation and salt-induced gold nanoparticles aggregation. Anal. Methods 2014, 6, 8018–8021. [Google Scholar] [CrossRef]
- Yan, Y.; Han, B.Q.; Zeng, J.; Zhou, W.Y.; Zhang, T.J.; Zhang, J.T.; Chen, W.X.; Zhang, C.B. A candidate reference method for serum potassium measurement by inductively coupled plasma mass spectrometry. Clin. Chem. Lab. Med. 2017, 55, 1517–1522. [Google Scholar] [CrossRef]
- Arnquist, I.J.; Hoppe, E.W. The quick and ultrasensitive determination of K in NaI using inductively coupled plasma mass Spectrometry. Nucl. Instrum. Methods Phys. Res. Sect. A 2017, 851, 15–19. [Google Scholar]
- García-Alegría, A.M.; Cáñez-Carrasco, M.G.; Serna-Félix, M.; Encinas Soto, K.K.; Gómez-Álvarez, A. Estimation of uncertainty in the determination of serum electrolytes (Na, K, Ca, Mg) by flame atomic absorption spectroscopy. MAPAN-J. Metrol. Soc. I. 2018, 33, 99–112. [Google Scholar]
- Qu, Z.C.; Steinvall, E.; Ghorbani, R.; Schmidt, F.M. Tunable diode laser atomic absorption spectroscopy for detection of potassium under optically thick conditions. Anal. Chem. 2016, 88, 3754–3760. [Google Scholar]
- Messele, H.M.; Asres, Y.H.; Hiruy, B.Z. Determination of chemical elements of barley and teff using flame atomic absorption spectroscopy (FAAS). Appl. Radiat. Isot. 2024, 211, 111401. [Google Scholar] [PubMed]
- Gamela, R.R.; Barrera, E.G.; Duarte, Á.T.; Boschetti, W.; da Silva, M.M.; Vale, M.G.R.; Dessuy, M.B. Fast sequential determination of Zn, Fe, Mg, Ca, Na, and K in infant formulas by high-resolution continuum source flame atomic absorption spectrometry using ultrasound-assisted extraction. Food Anal. Methods 2019, 12, 1420–1428. [Google Scholar] [CrossRef]
- Eum, N.S.; Lee, S.H.; Lee, D.R.; Kwon, D.K.; Shin, J.K.; Kim, J.H.; Kang, S.W. K+-ion sensing using surface plasmon resonance by NIR light source. Sens. Actuators B 2003, 96, 446–450. [Google Scholar]
- Alfonso, A.; Pazos, M.J.; Fernández-Araujo, A.; Tobio, A.; Alfonso, C.; Vieytes, M.R.; Botana, L.M. Surface plasmon resonance biosensor method for palytoxin detection based on Na+,K+-ATPase affinity. Toxins 2014, 6, 96–107. [Google Scholar]
- Kanyanee, T.; Tianrungarun, K.; Somboot, W.; Puangpila, C.; Jakmunee, J. Open tubular capillary ion chromatography with online dilution for small ions determination in drinks. Food Chem. 2022, 382, 132055. [Google Scholar]
- Michalski, R.; Lyko, A. Research onto the contents of selected inorganic ions in the dialysis fluids and dialysates by using ion chromatography. J. Liq. Chromatogr. Relat. Technol. 2016, 39, 96–103. [Google Scholar]
- Li, K.; Wang, J.; Wang, J.Q.; Zheng, Z.; Liu, X.P.; Wang, J.K.; Zhang, C.J.; He, S.S.; Wei, H.; Yu, C.Y. A programmable microfluidic paper-based analytical device for simultaneous colorimetric and photothermal visual sensing of multiple enzyme activities. Anal. Chem. 2024, 96, 12181–12188. [Google Scholar]
- Moulahoum, H. Dual chromatic laser-printed microfluidic paper-based analytical device (μPAD) for the detection of atrazine in water. ACS Omega 2023, 8, 41194–41203. [Google Scholar] [CrossRef]
- Xiong, X.L.; Zhang, J.L.; Wang, Z.; Liu, C.C.; Xiao, W.D.; Han, J.F.; Shi, Q.F. Simultaneous multiplexed detection of protein and metal ions by a colorimetric microfluidic paper-based analytical device. BioChip J. 2020, 14, 429–437. [Google Scholar] [CrossRef]
- Ming, T.; Cheng, Y.; Xing, Y.; Luo, J.P.; Mao, G.; Liu, J.T.; Sun, S.; Kong, F.L.; Jin, H.Y.; Cai, X.X. Electrochemical microfluidic paper-based aptasensor platform based on a biotin–streptavidin system for label-free detection of biomarkers. ACS Appl. Mater. Interfaces 2021, 13, 46317–46324. [Google Scholar]
- Zhuang, J.W.; Zhao, Z.Y.; Lian, K.; Yin, L.J.; Wang, J.J.; Man, S.L.; Liu, G.Z.; Ma, L. SERS-based CRISPR/Cas assay on microfluidic paper analytical devices for supersensitive detection of pathogenic bacteria in foods. Biosens. Bioelectron. 2022, 207, 114167. [Google Scholar]
- Jiang, H.E.; Zhang, Q.; Li, N.H.; Li, Z.J.; Chen, L.J.; Yang, F.Q.; Zhao, S.Q.; Liu, X.H. All-in-one strategy for the nano-engineering of paper-based bifunctional fluorescent platform for robustly-integrated real-time monitoring of food and drinking-water safety. J. Hazard. Mater. 2024, 467, 133735. [Google Scholar] [PubMed]
- Zhou, C.X.; Cui, K.; Liu, Y.; Hao, S.J.; Zhang, L.N.; Ge, S.G.; Yu, J.H. Ultrasensitive microfluidic paper-based electrochemical/visual analytical device via signal amplification of Pd@hollow Zn/Co core–shell ZIF67/ZIF8 nanoparticles for prostate-specific antigen detection. Anal. Chem. 2021, 93, 5459–5467. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.Y.; Yang, D.T.; Liu, G.Z. Signal amplification strategies for paper-based analytical devices. Biosens. Bioelectron. 2019, 136, 60–75. [Google Scholar]
- Ma, L.; Han, X.; Xia, L.; Qu, F.L.; Kong, R.M. A label-free G-quadruplex-based fluorescence assay for sensitive detection of alkaline phosphatase with the assistance of Cu2+. Spectrochim. Acta A 2020, 227, 117607. [Google Scholar]
- Khusbu, F.Y.; Zhou, X.; Chen, H.C.; Ma, C.B.; Wang, K.M. Thioflavin T as a fluorescence probe for biosensing applications. Trends Anal. Chem. 2018, 109, 1–18. [Google Scholar]
- Zhou, X.; Khusbu, F.Y.; Chen, H.C.; Ma, C.B. A turn-on fluorescence assay of alkaline phosphatase activity based on an enzyme-triggered conformational switch of G-quadruplex. Talanta 2020, 208, 120453. [Google Scholar]
- Tan, X.H.; Wang, Y.; Armitage, B.A.; Bruchez, M.P. Label-free molecular beacons for biomolecular detection. Anal. Chem. 2014, 86, 10864–10869. [Google Scholar]
- Zhang, D.C.; Tian, B.S.; Ling, Y.; Ye, L.; Xiao, M.; Yuan, K.X.; Zhang, X.Q.; Zheng, G.S.; Li, X.Y.; Zheng, J.D.; et al. CRISPR/Cas12a-powered amplification-free RNA diagnostics by integrating T7 exonuclease-assisted target recycling and split G-quadruplex catalytic signal output. Anal. Chem. 2024, 96, 10451–10458. [Google Scholar]
- Zou, G.Y.; Bi, F.; Yu, Y.L.; Liu, M.X.; Chen, S. Tetrahedral DNA-based ternary recognition ratiometric fluorescent probes for real-time in situ resolving lysosome subpopulations in living cells via Cl−, Ca2+, and pH. Anal. Chem. 2024, 96, 16639–16648. [Google Scholar]
- Kansara, K.; Singh, R.; Yadav, P.; Mansuri, A.; Kumar, A.; Bhatia, D. Lipid modification of DNA tetrahedrons enhances cellular uptake, migration, and in vivo uptake. ACS Appl. Nano Mater. 2023, 6, 13443–13452. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, Y.X.; Lu, Z.W.; Yu, H.Y.; Li, Z. Co-delivery of chemotherapeutic drugs and immune adjuvants by nanoscale DNA tetrahedrons for synergistic cancer therapy. ACS Appl. Nano Mater. 2022, 5, 101–106. [Google Scholar] [CrossRef]
- Chen, X.; Huang, J.; Zhang, S.; Mo, F.; Su, S.S.; Li, Y.; Fang, L.C.; Deng, J.; Huang, H.; Luo, Z.X.; et al. Electrochemical biosensor for DNA methylation detection through hybridization chain-amplified reaction coupled with a tetrahedral DNA nanostructure. ACS Appl. Mater. Interfaces 2019, 11, 3745–3752. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.Y.; Zhu, L.P.; Yan, M.X.; Feng, S.N.; Huang, J.S.; Yang, X.R. Electrochemiluminescence biosensor based on entropy-driven amplification and a tetrahedral DNA nanostructure for miRNA-133a detection. Anal. Chem. 2021, 93, 11809–11815. [Google Scholar] [CrossRef]
- Zhang, X.B.; Li, Z.R.; Hong, L.; Wang, X.W.; Cao, J.J. Tetrahedral DNA nanostructure-engineered paper-based electrochemical aptasensor for fumonisin B1 detection coupled with Au@Pt nanocrystals as an amplification label. J. Agric. Food Chem. 2023, 71, 19121–19128. [Google Scholar] [CrossRef]
- Pal, S.; Naik, A.; Rao, A.J.A.; Chakraborty, B.; Varma, M.M. Aptamer-DNA origami-functionalized solid-state nanopores for single-molecule sensing of G-quadruplex formation. ACS Appl. Nano Mater. 2022, 5, 8804–8810. [Google Scholar] [CrossRef]
- Chitbankluai, K.; Thavarungkul, P.; Kanatharana, P.; Kaewpet, M.; Buranachai, C. Newly found K+-thioflavin T competitive binding to DNA G-quadruplexes and the development of a label-free fluorescent biosensor with extra low detection limit for K+ determination in urine samples. Spectrochim. Acta A 2022, 276, 121244. [Google Scholar] [CrossRef]
- Yang, L.; Qing, Z.H.; Liu, C.H.; Tang, Q.; Li, J.S.; Yang, S.; Zheng, J.; Yang, R.H.; Tan, W.H. Direct fluorescent detection of blood potassium by ion-selective formation of intermolecular G-quadruplex and ligand binding. Anal. Chem. 2016, 88, 9285–9292. [Google Scholar] [CrossRef]
- Liu, H.S.; Zhang, X.; Li, X.R.; Wu, H.S.; Shi, Y.W.; Lu, W. A G-quadruplex/thioflavin T-based label-free biosensor to detect ClO− in stress-induced hypertension. Spectrochim. Acta A 2024, 314, 124231. [Google Scholar] [CrossRef]
- Hong, S.B.; Jiang, W.D.; Ding, Q.F.; Lin, K.L.; Zhao, C.C.; Wang, X.D. The current progress of tetrahedral DNA nanostructure for antibacterial application and bone tissue regeneration. Int. J. Nanomed. 2023, 18, 3761–3780. [Google Scholar] [CrossRef]
- Ren, W.J.; Pang, J.R.; Ma, R.R.; Liang, X.J.; Wei, M.; Suo, Z.G.; He, B.S.; Liu, Y. A signal on-off fluorescence sensor based on the self-assembly DNA tetrahedron for simultaneous detection of ochratoxin A and aflatoxin B1. Anal. Chim. Acta 2022, 1198, 339566. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, J.; Barooah, N.; Dhamodharan, V.; Harikrishna, S.; Pradeepkumar, P.I.; Bhasikuttan, A.C. Thioflavin T as an efficient inducer and selective fluorescent sensor for the human telomeric G-quadruplex DNA. J. Am. Chem. Soc. 2013, 135, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Dewey, H.M.; Mahmood, N.; Abello, S.M.; Sultana, N.; Jones, J.; Gluck, J.M.; Budhathoki-Uprety, J. Development of optical nanosensors for detection of potassium ions and assessment of their biocompatibility with corneal epithelial cells. ACS Omega 2024, 9, 27338–27348. [Google Scholar] [CrossRef]
- Leau, S.A.; Lete, C.; Marin, M.; del Campo, F.J.; Diaconu, I.; Lupu, S. Electrochemical sensors based on antimony tin oxide-prussian blue screen-printed electrode and PEDOT-prussian blue for potassium ion detection. J. Solid State Electrochem. 2023, 27, 1755–1766. [Google Scholar] [CrossRef]
- Cui, M.R.; Chen, L.X.; Li, X.L.; Xu, J.J.; Chen, H.Y. NIR remote-controlled “lock–unlock” nanosystem for imaging potassium ions in living cells. Anal. Chem. 2020, 92, 4558–4565. [Google Scholar] [CrossRef]
- Colella, F.; Forciniti, S.; Onesto, V.; Grasso, G.; Iuele, H.; Gigli, G.; del Mercato, L.L. A fluorescent ratiometric potassium sensor based on IPG4-silica microparticles for selective detection and fluorescence imaging of potassium cations. J. Mater. Chem. B 2024, 12, 10573–10583. [Google Scholar] [CrossRef]
- Shi, C.; Gu, H.X.; Ma, C.P. An aptamer-based fluorescent biosensor for potassium ion detection using a pyrene-labeled molecular beacon. Anal. Biochem. 2010, 400, 99–102. [Google Scholar] [CrossRef]


| Method | System | Linear Range | LOD | Reference |
|---|---|---|---|---|
| Optical | Using photoluminescent single-walled carbon nanotubes (SWCNTs) encapsulated in polymers that contain potassium chelating moieties | 5.0–7.0 mM | 0.39 mM | [43] |
| Electrochemistry | Based on antimony tin oxide (ATO)–Prussian blue (PB) screen-printed electrode (SPE) and PEDOT-PB modified glassy carbon electrode | 0.1–10 mM | 1.1 mM | [44] |
| Near-infrared ray (NIR) | Based on remote-controlled “lock−unlock” nanosystem using dual-stranded aptamer precursor as recognition molecules | 0–60 mM | 4 mM | [45] |
| Fluorescence | A ratiometric fluorescent microsensor based on IPG4–silica microparticles | 0–30 mM | 9.51 mM | [46] |
| Fluorescence | Based on aptamer and pyrene-labeled fluorescent probes | 0.6–20 mM | 0.4 mM | [47] |
| Fluorescence | G4-TDF functionalized μPADs based on intermolecular structure conversion | 0.5–5.5 mM | 0.2 mM | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Fu, X.; Liu, Y.; Zhang, Z.; Jiang, C.; Song, D. Intermolecular Structure Conversion-Based G4-TDF Nanostructures Functionalized μPADs for Fluorescent Determination of Potassium Ion in Serum. Biosensors 2025, 15, 223. https://doi.org/10.3390/bios15040223
Wang M, Fu X, Liu Y, Zhang Z, Jiang C, Song D. Intermolecular Structure Conversion-Based G4-TDF Nanostructures Functionalized μPADs for Fluorescent Determination of Potassium Ion in Serum. Biosensors. 2025; 15(4):223. https://doi.org/10.3390/bios15040223
Chicago/Turabian StyleWang, Mengqi, Xiuli Fu, Yixuan Liu, Zhiyang Zhang, Chenyu Jiang, and Dean Song. 2025. "Intermolecular Structure Conversion-Based G4-TDF Nanostructures Functionalized μPADs for Fluorescent Determination of Potassium Ion in Serum" Biosensors 15, no. 4: 223. https://doi.org/10.3390/bios15040223
APA StyleWang, M., Fu, X., Liu, Y., Zhang, Z., Jiang, C., & Song, D. (2025). Intermolecular Structure Conversion-Based G4-TDF Nanostructures Functionalized μPADs for Fluorescent Determination of Potassium Ion in Serum. Biosensors, 15(4), 223. https://doi.org/10.3390/bios15040223






