Translation of COVID-19 Serology Test on Foil-Based Lateral Flow Chips: A Journey from Injection Molding to Scalable Roll-to-Roll Nanoimprint Lithography
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Chip Designs and Simulations
2.3. Surface Characterizations and Flow Rate Measurements
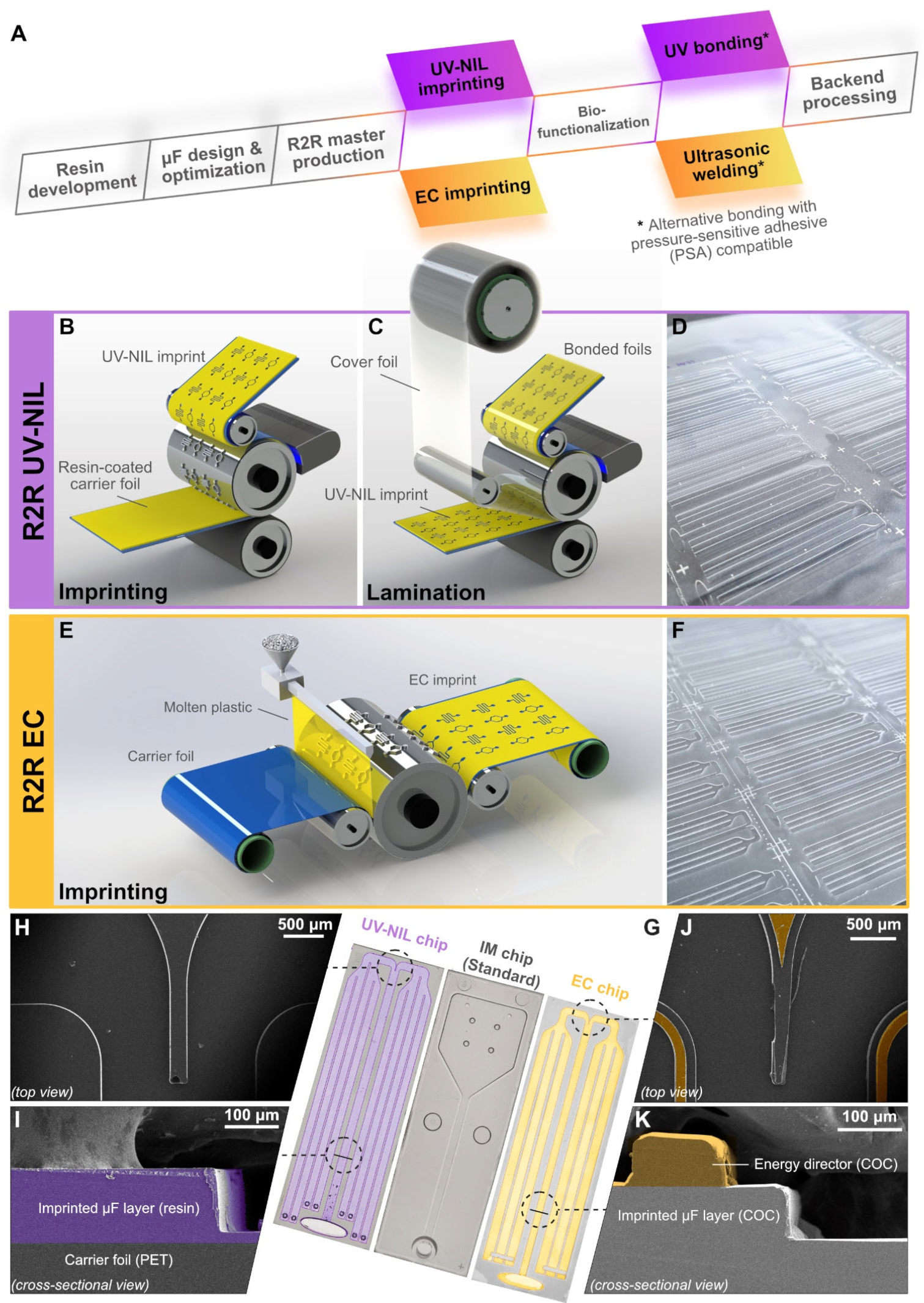
2.4. R2R Nanoimprinting
2.5. R2R Biofunctionalization
2.6. Lamination and Singulation
2.7. On-Chip SARS-CoV-2 Antibody Quantitation via Chemiluminescence
2.8. Data Collection and Statistical Analysis
3. Results and Discussion
3.1. Chip Design and Simulations
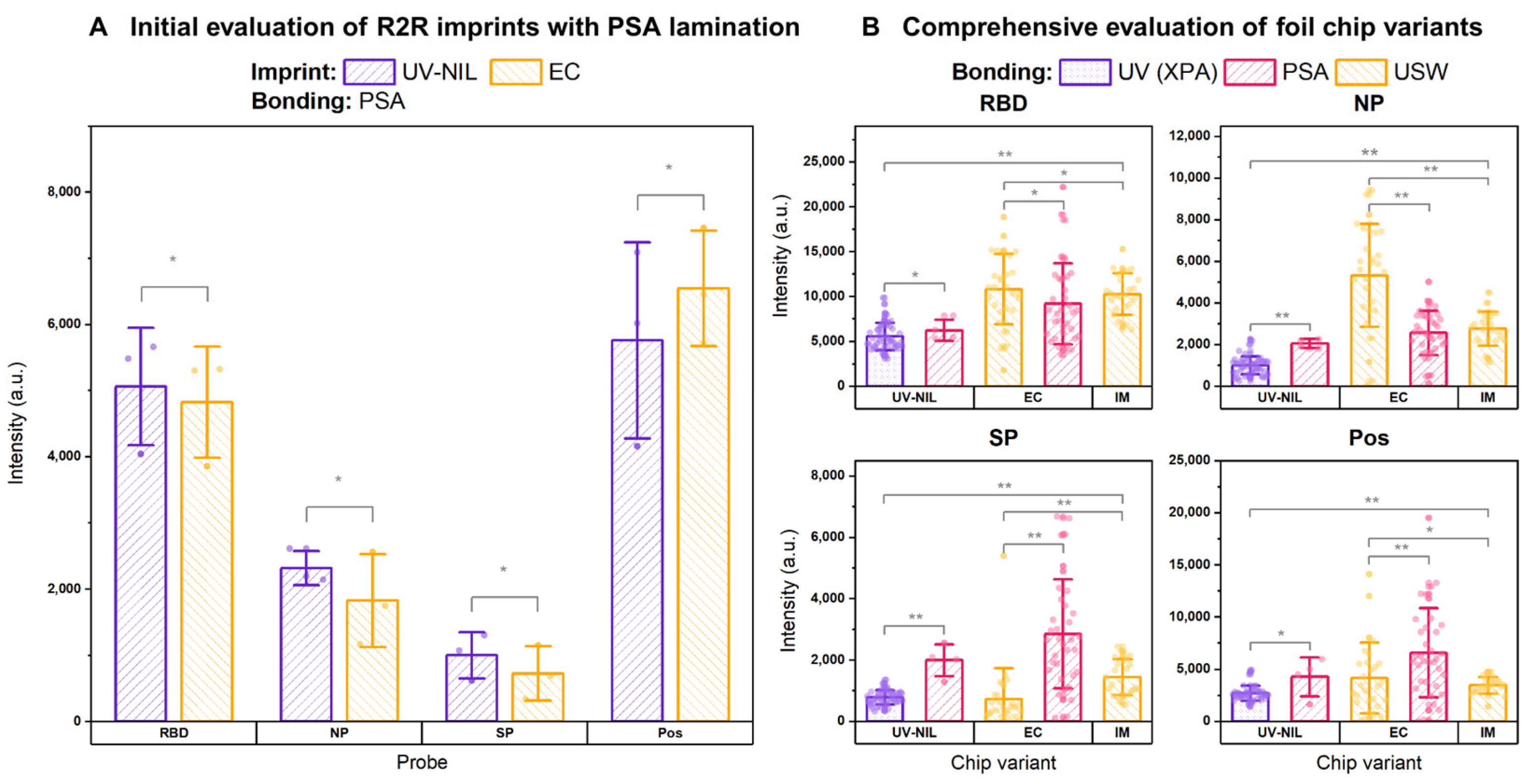
3.2. Gauging the Material Compatibility of Foil Chips for Assay Implementation
3.3. R2R Biofunctionalization: A Critical Component in High-Throughput R2R Fabrication Ecosystem
3.4. Comprehensive Evaluation of R2R Foil Chips with SARS-CoV-2 Antibody Detection
3.5. Towards Sustainable Manufacturing of the Foil Chips
4. Conclusions
5. Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mercer, T.R.; Salit, M. Testing at scale during the COVID-19 pandemic. Nat. Rev. Genet. 2021, 22, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Andryukov, B.G. Six decades of lateral flow immunoassay: From determining metabolic markers to diagnosing COVID-19. AIMS Microbiol. 2020, 6, 280–304. [Google Scholar] [CrossRef] [PubMed]
- Peto, T.; Affron, D.; Afrough, B.; Agasu, A.; Ainsworth, M.; Allanson, A.; Allen, K.; Allen, C.; Archer, L.; Ashbridge, N.; et al. COVID-19: Rapid antigen detection for SARS-CoV-2 by lateral flow assay: A national systematic evaluation of sensitivity and specificity for mass-testing. eClinicalMedicine 2021, 36, 100924. [Google Scholar] [CrossRef] [PubMed]
- Budd, J.; Miller, B.S.; Weckman, N.E.; Cherkaoui, D.; Huang, D.; Decruz, A.T.; Fongwen, N.; Han, G.-R.; Broto, M.; Estcourt, C.S.; et al. Lateral flow test engineering and lessons learned from COVID-19. Nat. Rev. Bioeng. 2023, 1, 13–31. [Google Scholar] [CrossRef]
- Lan, H. Large-Area Nanoimprint Lithography and Applications. In Micro/Nanolithography—A Heuristic Aspect on the Enduring Technology; InTech: London, UK, 2018. [Google Scholar] [CrossRef]
- Grant, B.D.; Anderson, C.E.; Williford, J.R.; Alonzo, L.F.; Glukhova, V.A.; Boyle, D.S.; Weigl, B.H.; Nichols, K.P. SARS-CoV-2 Coronavirus Nucleocapsid Antigen-Detecting Half-Strip Lateral Flow Assay Toward the Development of Point of Care Tests Using Commercially Available Reagents. Anal. Chem. 2020, 92, 11305–11309. [Google Scholar] [CrossRef]
- Mahmoudinobar, F.; Britton, D.; Montclare, J.K. Protein-based lateral flow assays for COVID-19 detection. Protein Eng. Des. Sel. 2021, 34, gzab010. [Google Scholar] [CrossRef]
- Kim, C.; Yoo, Y.K.; Lee, N.E.; Lee, J.; Kim, K.H.; Lee, S.; Kim, J.; Park, S.J.; Lee, D.; Lee, S.W.; et al. Nanoelectrokinetic-assisted lateral flow assay for COVID-19 antibody test. Biosens. Bioelectron. 2022, 212, 114385. [Google Scholar] [CrossRef]
- Ghaffari, A.; Meurant, R.; Ardakani, A. COVID-19 Serological Tests: How Well Do They Actually Perform? Diagnostics 2020, 10, 453. [Google Scholar] [CrossRef]
- Pickering, S.; Batra, R.; Merrick, B.; Snell, L.B.; Nebbia, G.; Douthwaite, S.; Reid, F.; Patel, A.; Ik, M.T.K.; Patel, B.; et al. Comparative performance of SARS-CoV-2 lateral flow antigen tests and association with detection of infectious virus in clinical specimens: A single-centre laboratory evaluation study. Lancet Microbe 2021, 2, e461–e471. [Google Scholar] [CrossRef]
- Tang, R.; Xie, M.Y.; Li, M.; Cao, L.; Feng, S.; Li, Z.; Xu, F. Nitrocellulose Membrane for Paper-based Biosensor. Appl. Mater. Today 2022, 26, 101305. [Google Scholar] [CrossRef]
- Kasetsirikul, S.; Shiddiky, M.J.A.; Nguyen, N.-T. Challenges and perspectives in the development of paper-based lateral flow assays. Microfluid. Nanofluid. 2020, 24, 17. [Google Scholar] [CrossRef]
- Nishat, S.; Jafry, A.T.; Martinez, A.W.; Awan, F.R. Paper-based microfluidics: Simplified fabrication and assay methods. Sens. Actuators B Chem. 2021, 336, 129681. [Google Scholar] [CrossRef]
- Kim, H.; Noh, H. Quantifying the fluid volumes in paper microfluidic devices for dry eye test. Macromol. Res. 2013, 21, 788–792. [Google Scholar] [CrossRef]
- Saidykhan, J.; Selevic, L.; Cinti, S.; May, J.E.; Killard, A.J. Paper-Based Lateral Flow Device for the Sustainable Measurement of Human Plasma Fibrinogen in Low-Resource Settings. Anal. Chem. 2021, 93, 14007–14013. [Google Scholar] [CrossRef]
- Xia, G.; Wang, J.; Liu, Z.; Bai, L.; Ma, L. Effect of sample volume on the sensitivity of lateral flow assays through computational modeling. Anal. Biochem. 2021, 619, 114130. [Google Scholar] [CrossRef]
- Ruppert, C.; Phogat, N.; Laufer, S.; Kohl, M.; Deigner, H.-P. A smartphone readout system for gold nanoparticle-based lateral flow assays: Application to monitoring of digoxigenin. Microchim. Acta 2019, 186, 119. [Google Scholar] [CrossRef]
- Guo, Z.; Kang, Y.; Liang, S.; Zhang, J. Detection of Hg(II) in adsorption experiment by a lateral flow biosensor based on streptavidin-biotinylated DNA probes modified gold nanoparticles and smartphone reader. Environ. Pollut. 2020, 266, 115389. [Google Scholar] [CrossRef]
- Saisin, L.; Amarit, R.; Somboonkaew, A.; Gajanandana, O.; Himananto, O.; Sutapun, B. Significant Sensitivity Improvement for Camera-Based Lateral Flow Immunoassay Readers. Sensors 2018, 18, 4026. [Google Scholar] [CrossRef]
- Zeng, N.; You, Y.; Xie, L.; Zhang, H.; Ye, L.; Hong, W.; Li, Y. A new imaged-based quantitative reader for the gold immunochromatographic assay. Optik 2018, 152, 92–99. [Google Scholar] [CrossRef]
- Berlina, A.N.; Taranova, N.A.; Zherdev, A.V.; Vengerov, Y.Y.; Dzantiev, B.B. Quantum dot-based lateral flow immunoassay for detection of chloramphenicol in milk. Anal. Bioanal. Chem. 2013, 405, 4997–5000. [Google Scholar] [CrossRef]
- Nandhakumar, P.; Muñoz San Martín, C.; Arévalo, B.; Ding, S.; Lunker, M.; Vargas, E.; Djassemi, O.; Campuzano, S.; Wang, J. Redox Cycling Amplified Electrochemical Lateral-Flow Immunoassay: Toward Decentralized Sensitive Insulin Detection. ACS Sens. 2023, 8, 3892–3901. [Google Scholar] [CrossRef] [PubMed]
- Deenin, W.; Yakoh, A.; Pimpitak, U.; Pasomsub, E.; Rengpipat, S.; Crespo, G.A.; Chaiyo, S. Electrochemical lateral-flow device for rapid COVID-19 antigen-diagnostic testing. Bioelectrochemistry 2023, 152, 108438. [Google Scholar] [CrossRef]
- Preechakasedkit, P.; Panphut, W.; Lomae, A.; Wonsawat, W.; Citterio, D.; Ruecha, N. Dual Colorimetric/Electrochemical Detection of Salmonella typhimurium Using a Laser-Induced Graphene Integrated Lateral Flow Immunoassay Strip. Anal. Chem. 2023, 95, 13904–13912. [Google Scholar] [CrossRef]
- Zhu, X.; Sarwar, M.; Zhu, J.-J.; Zhang, C.; Kaushik, A.; Li, C.-Z. Using a glucose meter to quantitatively detect disease biomarkers through a universal nanozyme integrated lateral fluidic sensing platform. Biosens. Bioelectron. 2019, 126, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Nothing, M.; Coble, B.; Zheng, H.; Park, J.; Im, H.; Weber, G.F.; Castro, C.M.; Swirski, F.K.; Weissleder, R.; et al. Integrated Biosensor for Rapid and Point-of-Care Sepsis Diagnosis. ACS Nano 2018, 12, 3378–3384. [Google Scholar] [CrossRef]
- Yafia, M.; Ymbern, O.; Olanrewaju, A.O.; Parandakh, A.; Sohrabi Kashani, A.; Renault, J.; Jin, Z.; Kim, G.; Ng, A.; Juncker, D. Microfluidic chain reaction of structurally programmed capillary flow events. Nature 2022, 605, 464–469. [Google Scholar] [CrossRef]
- Toren, P.; Smolka, M.; Haase, A.; Palfinger, U.; Nees, D.; Ruttloff, S.; Kuna, L.; Schaude, C.; Jauk, S.; Rumpler, M.; et al. High-throughput roll-to-roll production of polymer biochips for multiplexed DNA detection in point-of-care diagnostics. Lab. Chip 2020, 20, 4106–4117. [Google Scholar] [CrossRef]
- Cho, J.-H.; Han, S.-M.; Paek, E.-H.; Cho, I.-H.; Paek, S.-H. Plastic ELISA-on-a-Chip Based on Sequential Cross-Flow Chromatography. Anal. Chem. 2006, 78, 793–800. [Google Scholar] [CrossRef]
- Raj, N.; Crooks, R.M. Plastic-based lateral flow immunoassay device for electrochemical detection of NT-proBNP. Analyst 2022, 147, 2460–2469. [Google Scholar] [CrossRef]
- Daniso, E.; Maroh, B.; Feldbacher, S.; Mühlbacher, I.; Schlögl, S.; Melpignano, P. Tailoring the chemical functionalization of a transparent polyethylene foil for its application in an OLED-based DNA biosensor. Appl. Surf. Sci. 2021, 552, 149408. [Google Scholar] [CrossRef]
- Altschuh, P.; Kunz, W.; Bremerich, M.; Reiter, A.; Selzer, M.; Nestler, B. Wicking in Porous Polymeric Membranes: Determination of an Effective Capillary Radius to Predict the Flow Behavior in Lateral Flow Assays. Membranes 2022, 12, 638. [Google Scholar] [CrossRef] [PubMed]
- Mortelmans, T.; Kazazis, D.; Werder, J.; Kristiansen, P.M.; Ekinci, Y. Injection Molding of Thermoplastics for Low-Cost Nanofluidic Devices. ACS Appl. Nano Mater. 2022, 5, 17758–17766. [Google Scholar] [CrossRef]
- Liedert, C.; Rannaste, L.; Kokkonen, A.; Huttunen, O.-H.; Liedert, R.; Hiltunen, J.; Hakalahti, L. Roll-to-Roll Manufacturing of Integrated Immunodetection Sensors. ACS Sens. 2020, 5, 2010–2017. [Google Scholar] [CrossRef] [PubMed]
- Leitgeb, M.; Nees, D.; Ruttloff, S.; Palfinger, U.; Götz, J.; Liska, R.; Belegratis, M.R.; Stadlober, B. Multilength Scale Patterning of Functional Layers by Roll-to-Roll Ultraviolet-Light-Assisted Nanoimprint Lithography. ACS Nano 2016, 10, 4926–4941. [Google Scholar] [CrossRef]
- Götz, J.; Alvarez Rueda, A.; Ruttloff, S.; Kuna, L.; Belegratis, M.; Palfinger, U.; Nees, D.; Hartmann, P.; Stadlober, B. Finite Element Simulations of Filling and Demolding in Roll-to-Roll UV Nanoimprinting of Micro- and Nanopatterns. ACS Appl. Nano Mater. 2022, 5, 3434–3449. [Google Scholar] [CrossRef]
- Fruncillo, S.; Su, X.; Liu, H.; Wong, L.S. Lithographic Processes for the Scalable Fabrication of Micro- and Nanostructures for Biochips and Biosensors. ACS Sens. 2021, 6, 2002–2024. [Google Scholar] [CrossRef]
- Unno, N.; Mäkelä, T. Thermal Nanoimprint Lithography—A Review of the Process, Mold Fabrication, and Material. Nanomaterials 2023, 13, 2031. [Google Scholar] [CrossRef]
- Wechselberger, C.; Süßner, S.; Doppler, S.; Bernhard, D. Performance evaluation of serological assays to determine the immunoglobulin status in SARS-CoV-2 infected patients. J. Clin. Virol. 2020, 131, 104589. [Google Scholar] [CrossRef]
- Lee, U.N.; Su, X.; Guckenberger, D.J.; Dostie, A.M.; Zhang, T.; Berthier, E.; Theberge, A.B. Fundamentals of rapid injection molding for microfluidic cell-based assays. Lab. Chip 2018, 18, 496–504. [Google Scholar] [CrossRef]
- Lee, Y.; Choi, J.W.; Yu, J.; Park, D.; Ha, J.; Son, K.; Lee, S.; Chung, M.; Kim, H.-Y.; Jeon, N.L. Microfluidics within a well: An injection-molded plastic array 3D culture platform. Lab. Chip 2018, 18, 2433–2440. [Google Scholar] [CrossRef]
- Murthy, S.; Matschuk, M.; Huang, Q.; Mandsberg, N.K.; Feidenhans’l, N.A.; Johansen, P.; Christensen, L.; Pranov, H.; Kofod, G.; Pedersen, H.C.; et al. Fabrication of Nanostructures by Roll-to-Roll Extrusion Coating. Adv. Eng. Mater. 2016, 18, 484–489. [Google Scholar] [CrossRef]
- Okulova, N.; Johansen, P.; Christensen, L.; Taboryski, R. Replication of micro-sized pillars in polypropylene using the extrusion coating process. Microelectron. Eng. 2017, 176, 54–57. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, Y.; Wang, D.; He, F.; Rotello, V.M.; Carter, K.R.; Watkins, J.J.; Nugen, S.R. UV-nanoimprint lithography as a tool to develop flexible microfluidic devices for electrochemical detection. Lab. Chip 2015, 15, 3086–3094. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.; Pant, U.; Moirangthem, R.S. Thermal nanoimprint lithography based plasmonic nanogratings for refractive index sensing of polar solvents. Mater. Today Proc. 2020, 28, 215–217. [Google Scholar] [CrossRef]
- Toren, P.; Rumpler, M.; Smolka, M.; Haase, A.; Ruttloff, S.; Nees, D.; Stadlober, B.; Katzmayr, I.; Hierschlager, B.; Kierstein, S.; et al. High-Throughput Roll-to-Roll Production of Bio-Functionalized Polymer Components. Eur. J. Mater. Sci. Eng. 2020, 5, 11–16. [Google Scholar] [CrossRef]
- Bao, J.; Jed Harrison, D. Measurement of flow in microfluidic networks with micrometer-sized flow restrictors. AIChE J. 2006, 52, 75–85. [Google Scholar] [CrossRef]
- Bruus, H. Governing Equations in Microfluidics. Microscale Acoustofluidics; The Royal Society of Chemistry: Cambridge, UK, 2014; pp. 1–28. [Google Scholar] [CrossRef]
- Kim, D.; Herr, A.E. Protein immobilization techniques for microfluidic assays. Biomicrofluidics 2013, 7, 041501. [Google Scholar] [CrossRef]
- Dai, L.; Gao, G.F. Viral targets for vaccines against COVID-19. Nat Rev Immunol 2021, 21, 73–82. [Google Scholar] [CrossRef]
- Wagner, A.; Guzek, A.; Ruff, J.; Jasinska, J.; Scheikl, U.; Zwazl, I.; Kundi, M.; Stockinger, H.; Farcet, M.R.; Kreil, T.R.; et al. Neutralising SARS-CoV-2 RBD-specific antibodies persist for at least six months independently of symptoms in adults. Commun. Med. 2021, 1, 13. [Google Scholar] [CrossRef]
- Pattison, D.I.; Rahmanto, A.S.; Davies, M.J. Photo-oxidation of proteins. Photochem. Photobiol. Sci. 2012, 11, 38–53. [Google Scholar] [CrossRef]
- Reverberi, R.; Reverberi, L. Factors affecting the antigen-antibody reaction. Blood Transfus. 2007, 5, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Vealan, K.; Joseph, N.; Alimat, S.; Karumbati, A.S.; Thilakavathy, K. Lateral flow assay: A promising rapid point-of-care testing tool for infections and non-communicable diseases. Asian Biomed. (Res. Rev. News) 2023, 17, 250–266. [Google Scholar] [CrossRef] [PubMed]
- Ongaro, A.E.; Ndlovu, Z.; Sollier, E.; Otieno, C.; Ondoa, P.; Street, A.; Kersaudy-Kerhoas, M. Engineering a sustainable future for point-of-care diagnostics and single-use microfluidic devices. Lab. Chip 2022, 22, 3122–3137. [Google Scholar] [CrossRef] [PubMed]

| Property | Injection Molding | Roll-to-Roll Imprinting | |
|---|---|---|---|
| Fluidic patterns: | |||
| Typical number of chips per working tool | 6 | 36 and more Advantage: More design variations can be placed on same working tool for intensive parallel testing. | |
| Throughput, typical numbers for replicated chip patterns/min | 12 | 120 and more Advantage: Minimum of 10-fold increase of process throughput is possible. | |
| Post processing: | |||
| Implementation of bioprinting and lamination processes | Disadvantage: Individual chip handling required (manual or robot-based). | Processing of large-area-sheets (e.g., with 24 chips and more) or roll-to-roll processes. Advantage: increased throughput due to continuous chip processing on same substrate. | |
| Materials: | |||
| UV nano imprint lithography (UV NIL) | Extrusion Coating | ||
| Processed material | Thermoplastics. Advantage: use of known and certified polymers. Disadvantage: limited material tunability. | UV curable materials. Advantage: wide tunability of surface, mechanical and optical properties, etc. The main advantage here is material intrinsic hydrophilicity. Disadvantage: Material verification might be required (dependent on application). | Thermoplastics. Advantage: use of known and certified polymers. Disadvantage: limited material tunability. |
| Material and energy demand | Thick, rigid polymer elements. High process pressure and temperature. | Thin polymer foil chips, reduced material consumption. | |
| Process without active heating, and hence, low energy consumption. | Lower process pressure than in injection molding. | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khumwan, P.; Ruttloff, S.; Götz, J.; Nees, D.; O’Sullivan, C.; Conde, A.; Lohse, M.; Wolf, C.; Okulova, N.; Brommert, J.; et al. Translation of COVID-19 Serology Test on Foil-Based Lateral Flow Chips: A Journey from Injection Molding to Scalable Roll-to-Roll Nanoimprint Lithography. Biosensors 2025, 15, 229. https://doi.org/10.3390/bios15040229
Khumwan P, Ruttloff S, Götz J, Nees D, O’Sullivan C, Conde A, Lohse M, Wolf C, Okulova N, Brommert J, et al. Translation of COVID-19 Serology Test on Foil-Based Lateral Flow Chips: A Journey from Injection Molding to Scalable Roll-to-Roll Nanoimprint Lithography. Biosensors. 2025; 15(4):229. https://doi.org/10.3390/bios15040229
Chicago/Turabian StyleKhumwan, Pakapreud, Stephan Ruttloff, Johannes Götz, Dieter Nees, Conor O’Sullivan, Alvaro Conde, Mirko Lohse, Christian Wolf, Nastasia Okulova, Janine Brommert, and et al. 2025. "Translation of COVID-19 Serology Test on Foil-Based Lateral Flow Chips: A Journey from Injection Molding to Scalable Roll-to-Roll Nanoimprint Lithography" Biosensors 15, no. 4: 229. https://doi.org/10.3390/bios15040229
APA StyleKhumwan, P., Ruttloff, S., Götz, J., Nees, D., O’Sullivan, C., Conde, A., Lohse, M., Wolf, C., Okulova, N., Brommert, J., Benauer, R., Katzmayr, I., Ladenhauf, N., Weigel, W., Skolimowski, M., Sonnleitner, M., Smolka, M., Haase, A., Stadlober, B., & Hesse, J. (2025). Translation of COVID-19 Serology Test on Foil-Based Lateral Flow Chips: A Journey from Injection Molding to Scalable Roll-to-Roll Nanoimprint Lithography. Biosensors, 15(4), 229. https://doi.org/10.3390/bios15040229






