Atomic Pt-Layer-Coated Au Peroxidase Nanozymes with Enhanced Activity for Ultrasensitive Colorimetric Immunoassay of Interleukin-12
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of ~40 nm AuNPs
2.2. Synthesis of Au@Pt4LNPs
2.3. Investigation of Peroxidase-like Catalytic Activity of Au@Pt4LNPs
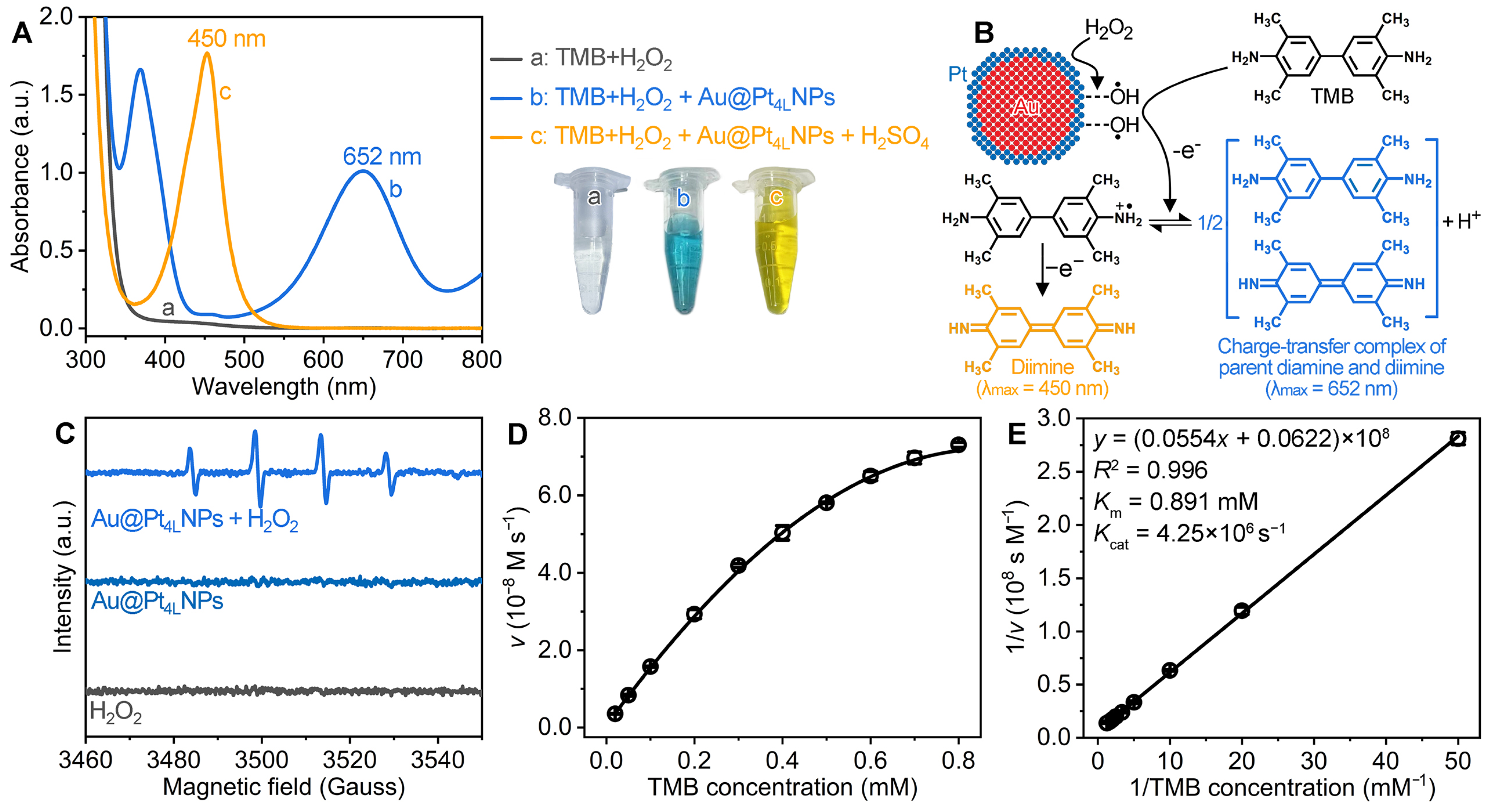
2.4. Steady-State Kinetic Analyses
2.5. Preparation of SA-Conjugated Au@Pt4LNPs (Denoted as “SA-Au@Pt4LNP Conjugates”)
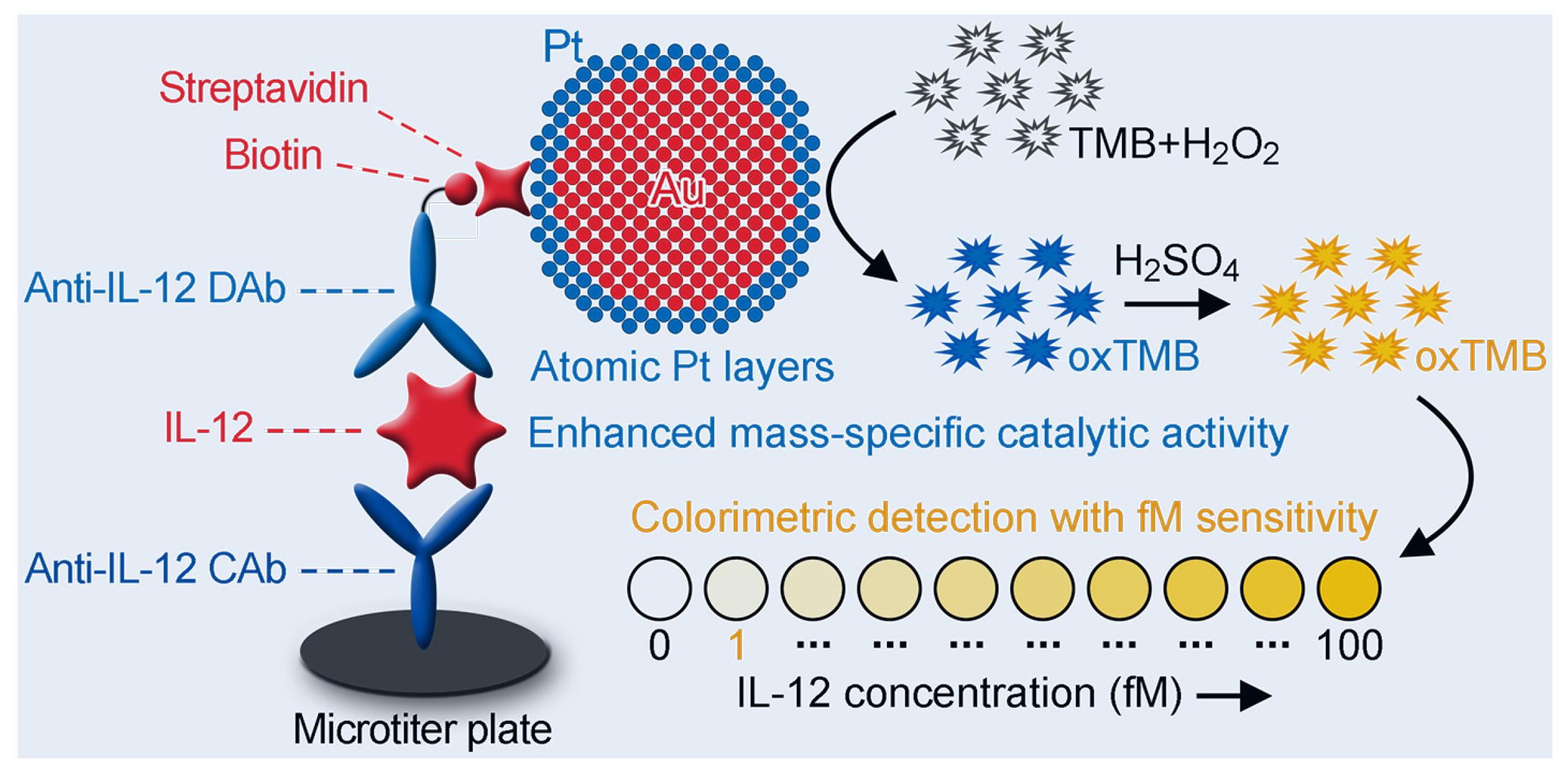
2.6. Detection of IL-12 Using Au@Pt4LNP-Enhanced CELISA
3. Results and Discussion
3.1. Synthesis and Characterization of Au@Pt4LNPs
3.2. Peroxidase-like Catalytic Properties of Au@Pt4LNPs
3.3. Influence of Pt Content on the Activity of Au@PtNPs
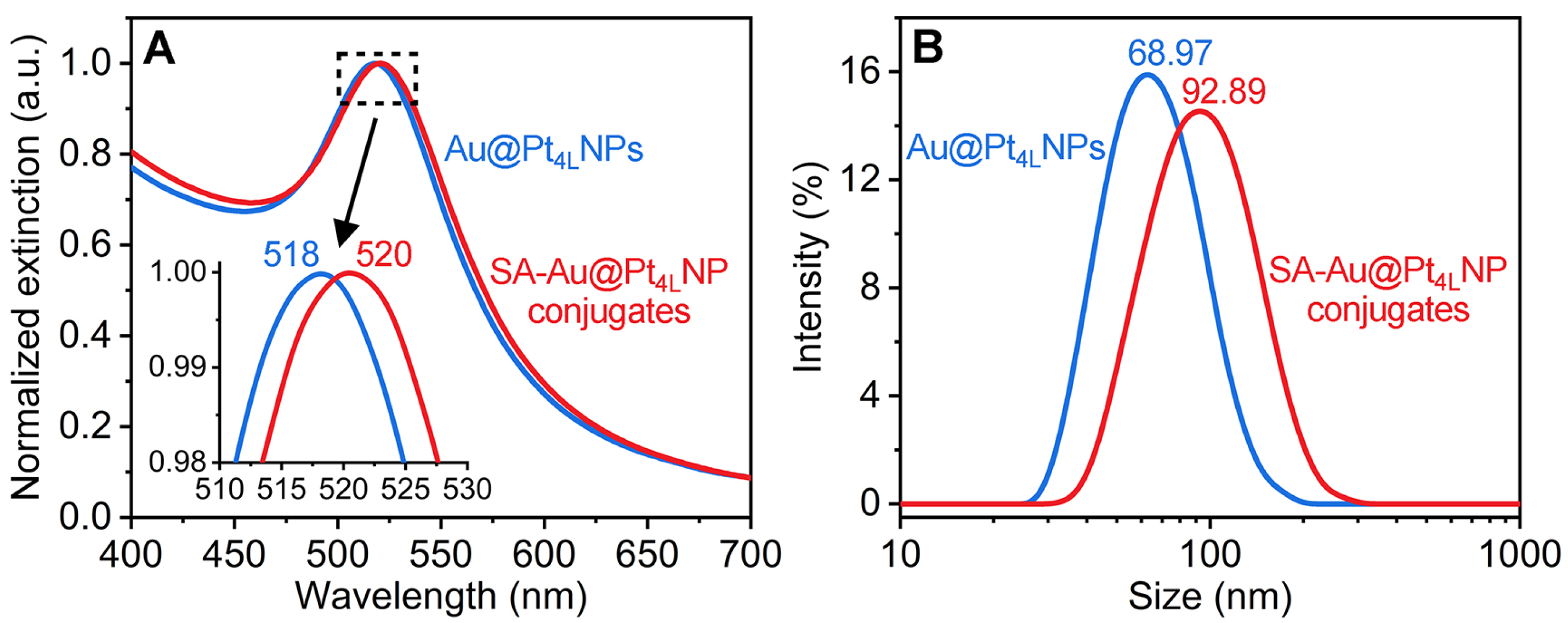
3.4. Preparation and Verification of SA-Au@Pt4LNP Conjugates
3.5. Analytical Performance of Au@Pt4LNP-Enhanced CELISA
3.6. Application in Analysis of Serum Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CELISAs | Colorimetric enzyme-linked immunosorbent assays |
| AuNPs | Au nanoparticles |
| Au@PtNPs | (Au core)@(Pt shell) nanoparticles |
| HRP | Horseradish peroxidase |
| LOD | Limit of detection |
| IL-12 | Interleukin-12 |
| Kcat | Catalytic constant |
| CAbs | Capture antibodies |
| DAbs | Detection antibodies |
| SA | Streptavidin |
| TMB | 3,3′,5,5′-Tetramethylbenzidine |
| oxTMB | Oxidized TMB |
| H2O2 | Hydrogen peroxide |
| TEM | Transmission electron microscopy |
| EDX | Energy-dispersive X-ray |
| ICP-OES | Inductively coupled plasma-optical emission spectrometry |
| fcc | Face-centered cubic |
| Km | Michaelis constant |
| Vmax | Maximal reaction velocity |
| ν | Initial reaction velocity |
| Kcat-mass-specific | Mass-specific catalytic efficiency |
| SD | Standard deviation |
| CV | Coefficient of variation |
| FBS | Fetal bovine serum |
| DI | Deionized |
| PBS | Phosphate-buffered saline |
| PBST | PBS buffer (pH 7.4) containing 0.05% Tween 20 |
| XPS | X-ray photoelectron spectroscopy |
| XRD | X-ray diffraction |
| ESR | Electron spin resonance |
References
- Zundler, S.; Neurath, M.F. Interleukin-12: Functional activities and implications for disease. Cytokine Growth Factor Rev. 2015, 26, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Hildenbrand, K.; Aschenbrenner, I.; Franke, F.C.; Devergne, O.; Feige, M.J. Biogenesis and engineering of interleukin 12 family cytokines. Trends Biochem. Sci. 2022, 47, 936–949. [Google Scholar] [CrossRef] [PubMed]
- Teng, M.W.L.; Bowman, E.P.; McElwee, J.J.; Smyth, M.J.; Casanova, J.-L.; Cooper, A.M.; Cua, D.J. IL-12 and IL-23 cytokines: From discovery to targeted therapies for immune-mediated inflammatory diseases. Nat. Med. 2015, 21, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Pope, R.M.; Shahrara, S. Possible roles of IL-12-family cytokines in rheumatoid arthritis. Nat. Rev. Rheumatol. 2013, 9, 252–256. [Google Scholar] [CrossRef]
- Kulig, P.; Musiol, S.; Freiberger, S.N.; Schreiner, B.; Gyülveszi, G.; Russo, G.; Pantelyushin, S.; Kishihara, K.; Alessandrini, F.; Kündig, T.; et al. IL-12 protects from psoriasiform skin inflammation. Nat. Commun. 2016, 7, 13466. [Google Scholar] [CrossRef]
- Verstockt, B.; Salas, A.; Sands, B.E.; Abraham, C.; Leibovitzh, H.; Neurath, M.F.; Vande Casteele, N.; Danese, S.; D’Haens, G.; Eckmann, L.; et al. IL-12 and IL-23 pathway inhibition in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 433–446. [Google Scholar] [CrossRef]
- Briukhovetska, D.; Dörr, J.; Endres, S.; Libby, P.; Dinarello, C.A.; Kobold, S. Interleukins in cancer: From biology to therapy. Nat. Rev. Cancer 2021, 21, 481–499. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, H.; Tan, Y.; Gao, Z.; Ye, H.; Dong, H. High-visual-resolution colorimetric immunoassay with attomolar sensitivity using kinetically controlled growth of Ag in AuAg nanocages and poly-enzyme-boosted tyramide signal amplification. Talanta 2025, 286, 127432. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, J.; Cai, T.; Stephens, A.; Su, S.-H.; Sandford, E.; Flora, C.; Singer, B.H.; Ghosh, M.; Choi, S.W.; et al. Machine learning-based cytokine microarray digital immunoassay analysis. Biosens. Bioelectron. 2021, 180, 113088. [Google Scholar] [CrossRef]
- Masurier, A.; Sieskind, R.; Gines, G.; Rondelez, Y. DNA circuit-based immunoassay for ultrasensitive protein pattern classification. Analyst 2024, 149, 5052–5062. [Google Scholar] [CrossRef]
- García-Rodrigo, L.; Ramos-López, C.; Sánchez-Tirado, E.; Agüí, L.; González-Cortés, A.; Yáñez-Sedeño, P.; Pingarrón, J.M. Electrochemical immunosensing of crohn’s disease biomarkers using diazonium salt-grafted crystalline nanocellulose/carbon nanotube-modified electrodes. Microchim. Acta 2024, 192, 4. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei, M.S.; Ahmed, M. Enzyme-linked immunosorbent assay (ELISA). In Cancer Cell Biology: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2022; pp. 115–134. [Google Scholar]
- Hayrapetyan, H.; Tran, T.; Tellez-Corrales, E.; Madiraju, C. Enzyme-linked immunosorbent assay: Types and applications. In Elisa: Methods and Protocols; Matson, R.S., Ed.; Springer: New York, NY, USA, 2023; pp. 1–17. [Google Scholar]
- Li, S.; Zhang, Y.; Wang, Q.; Lin, A.; Wei, H. Nanozyme-enabled analytical chemistry. Anal. Chem. 2022, 94, 312–323. [Google Scholar] [CrossRef]
- Wu, D.; Milutinovic, M.D.; Walt, D.R. Single molecule array (SIMOA) assay with optimal antibody pairs for cytokine detection in human serum samples. Analyst 2015, 140, 6277–6282. [Google Scholar] [CrossRef]
- Ye, H.; Yang, K.; Tao, J.; Liu, Y.; Zhang, Q.; Habibi, S.; Nie, Z.; Xia, X. An enzyme-free signal amplification technique for ultrasensitive colorimetric assay of disease biomarkers. ACS Nano 2017, 11, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Lu, D.; Zhang, G.; Zhang, D.; Shi, X. Recent improvements in enzyme-linked immunosorbent assays based on nanomaterials. Talanta 2021, 223, 121722. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ren, J.; Qu, X. Nanozymes: Classification, catalytic mechanisms, activity regulation, and applications. Chem. Rev. 2019, 119, 4357–4412. [Google Scholar] [CrossRef]
- Zhang, R.; Jiang, B.; Fan, K.; Gao, L.; Yan, X. Designing nanozymes for in vivo applications. Nat. Rev. Bioeng. 2024, 2, 849–868. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, G.; Liu, W.; Li, T.; Wang, Y.; Zhou, M.; Liu, Y.; Wang, X.; Wei, H. Nanozymes for nanohealthcare. Nat. Rev. Methods Primers 2024, 4, 36. [Google Scholar] [CrossRef]
- Shamsabadi, A.; Haghighi, T.; Carvalho, S.; Frenette, L.C.; Stevens, M.M. The nanozyme revolution: Enhancing the performance of medical biosensing platforms. Adv. Mater. 2024, 36, 2300184. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Bu, Z.; Tang, Z.; Diao, Q.; Tian, Q.; Li, S.; Chen, X.; Liu, J.; Liang, H.; Niu, X. Target-triggered oxygen vacancy increase of Co3O4 nanoparticles with promoted peroxidase-like activity for specific turn-on colorimetric sensing of uranyl ions. Sens. Actuators B Chem. 2024, 420, 136499. [Google Scholar] [CrossRef]
- Wu, J.; Yang, Q.; Li, Q.; Li, H.; Li, F. Two-dimensional MnO2 nanozyme-mediated homogeneous electrochemical detection of organophosphate pesticides without the interference of H2O2 and color. Anal. Chem. 2021, 93, 4084–4091. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ding, Y.; Dahlgren, R.A.; Sun, Y.; Gu, J.; Li, Y.; Liu, T.; Wang, X. Ultrafine V2O5-anchored 3d N-doped carbon nanocomposite with augmented dual-enzyme mimetic activity for evaluating total antioxidant capacity. Anal. Chim. Acta 2023, 1252, 341072. [Google Scholar] [CrossRef]
- Feng, F.; Zhang, Y.; Zhang, X.; Mu, B.; Zhang, J.; Qu, W.; Tong, W.; Liang, M.; An, Q.; Guo, Z.; et al. Enhancing the peroxidase-like activity of MoS2-based nanozymes by introducing attapulgite for antibacterial application and sensitive detection of glutathione. Nano Res. 2024, 17, 7415–7426. [Google Scholar] [CrossRef]
- Hong, C.; Chen, L.; Wu, C.; Yang, D.; Dai, J.-Y.; Huang, Z.; Cai, R.; Tan, W. Green synthesis of Au@WSe2 hybrid nanostructures with the enhanced peroxidase-like activity for sensitive colorimetric detection of glucose. Nano Res. 2022, 15, 1587–1592. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, L.; Zhang, X.; Zhang, J.; Ren, N.; Ding, L.; Wang, A.; Liu, J.; Liu, H.; Yu, X. Nitrogen vacancy modulation of tungsten nitride peroxidase-mimetic activity for bacterial infection therapy. ACS Nano 2024, 18, 24469–24483. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Zhou, S. Progress and perspective on carbon-based nanozymes for peroxidase-like applications. J. Phys. Chem. Lett. 2021, 12, 11751–11760. [Google Scholar] [CrossRef]
- Wang, W.; Cao, Q.; He, J.; Xie, Y.; Zhang, Y.; Yang, L.; Duan, M.-H.; Wang, J.; Li, W. Palladium/platinum/ruthenium trimetallic dendritic nanozymes exhibiting enhanced peroxidase-like activity for signal amplification of lateral flow immunoassays. Nano Lett. 2024, 24, 8311–8319. [Google Scholar] [CrossRef]
- Wei, Z.; Luciano, K.; Xia, X. Catalytic gold-iridium nanoparticles as labels for sensitive colorimetric lateral flow assay. ACS Nano 2022, 16, 21609–21617. [Google Scholar] [CrossRef]
- Wang, C.; Wang, L.; Nallathambi, V.; Liu, Y.; Kresse, J.; Hübner, R.; Reichenberger, S.; Gault, B.; Zhan, J.; Eychmüller, A.; et al. Structural regulation of Au-Pt bimetallic aerogels for catalyzing the glucose cascade reaction. Adv. Mater. 2024, 36, 2405200. [Google Scholar] [CrossRef]
- Zhang, M.; Guo, X. Gold/platinum bimetallic nanomaterials for immunoassay and immunosensing. Coord. Chem. Rev. 2022, 465, 214578. [Google Scholar] [CrossRef]
- Loynachan, C.N.; Thomas, M.R.; Gray, E.R.; Richards, D.A.; Kim, J.; Miller, B.S.; Brookes, J.C.; Agarwal, S.; Chudasama, V.; McKendry, R.A.; et al. Platinum nanocatalyst amplification: Redefining the gold standard for lateral flow immunoassays with ultrabroad dynamic range. ACS Nano 2018, 12, 279–288. [Google Scholar] [CrossRef]
- Gao, Z.; Ye, H.; Tang, D.; Tao, J.; Habibi, S.; Minerick, A.; Tang, D.; Xia, X. Platinum-decorated gold nanoparticles with dual functionalities for ultrasensitive colorimetric in vitro diagnostics. Nano Lett. 2017, 17, 5572–5579. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xianyu, Y. Tuning the plasmonic and catalytic signals of Au@Pt nanoparticles for dual-mode biosensing. Biosens. Bioelectron. 2023, 237, 115553. [Google Scholar] [CrossRef]
- Iglesias-Mayor, A.; Amor-Gutiérrez, O.; Novelli, A.; Fernández-Sánchez, M.-T.; Costa-García, A.; de la Escosura-Muñiz, A. Bifunctional Au@Pt/Au core@shell nanoparticles as novel electrocatalytic tags in immunosensing: Application for alzheimer’s disease biomarker detection. Anal. Chem. 2020, 92, 7209–7217. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lim, B.; Lee, E.P.; Xia, Y. Shape-controlled synthesis of platinum nanocrystals for catalytic and electrocatalytic applications. Nano Today 2009, 4, 81–95. [Google Scholar] [CrossRef]
- Kodama, K.; Nagai, T.; Kuwaki, A.; Jinnouchi, R.; Morimoto, Y. Challenges in applying highly active Pt-based nanostructured catalysts for oxygen reduction reactions to fuel cell vehicles. Nat. Nanotechnol. 2021, 16, 140–147. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, Z.; Zhang, A.; Yan, X.; Xue, W.; Peng, B.; Xin, H.L.; Pan, X.; Duan, X.; Huang, Y. Graphene-nanopocket-encaged PtCo nanocatalysts for highly durable fuel cell operation under demanding ultralow-Pt-loading conditions. Nat. Nanotechnol. 2022, 17, 968–975. [Google Scholar] [CrossRef]
- Xia, Y.; Xia, X.; Peng, H.-C. Shape-controlled synthesis of colloidal metal nanocrystals: Thermodynamic versus kinetic products. J. Am. Chem. Soc. 2015, 137, 7947–7966. [Google Scholar] [CrossRef]
- Shi, Y.; Lyu, Z.; Zhao, M.; Chen, R.; Nguyen, Q.N.; Xia, Y. Noble-metal nanocrystals with controlled shapes for catalytic and electrocatalytic applications. Chem. Rev. 2021, 121, 649–735. [Google Scholar] [CrossRef] [PubMed]
- Frens, G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Ziegler, C.; Eychmüller, A. Seeded growth synthesis of uniform gold nanoparticles with diameters of 15−300 nm. J. Phys. Chem. C 2011, 115, 4502–4506. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, C.; He, J.; Chen, P. Pd@Pt nanodendrites as peroxidase nanomimics for enhanced colorimetric ELISA of cytokines with femtomolar sensitivity. Chemosensors 2022, 10, 359. [Google Scholar] [CrossRef]
- Gao, Z.; Lv, S.; Xu, M.; Tang, D. High-index {hk0} faceted platinum concave nanocubes with enhanced peroxidase-like activity for an ultrasensitive colorimetric immunoassay of the human prostate-specific antigen. Analyst 2017, 142, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Hermanson, G.T. Bioconjugate Techniques; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Banerjee, I.; Kumaran, V.; Santhanam, V. Synthesis and characterization of Au@Pt nanoparticles with ultrathin platinum overlayers. J. Phys. Chem. C 2015, 119, 5982–5987. [Google Scholar] [CrossRef]
- Williams, B.P.; Yaguchi, M.; Lo, W.-S.; Kao, C.-R.; Lamontagne, L.K.; Sneed, B.T.; Brodsky, C.N.; Chou, L.-Y.; Kuo, C.-H.; Tsung, C.-K. Investigating lattice strain impact on the alloyed surface of small Au@PdPt core–shell nanoparticles. Nanoscale 2020, 12, 8687–8692. [Google Scholar] [CrossRef]
- Song, H.; Kim, F.; Connor, S.; Somorjai, G.A.; Yang, P. Pt nanocrystals: Shape control and langmuir−blodgett monolayer formation. J. Phys. Chem. B 2005, 109, 188–193. [Google Scholar] [CrossRef]
- Josephy, P.D.; Eling, T.; Mason, R.P. The horseradish peroxidase-catalyzed oxidation of 3, 5, 3′, 5′-tetramethylbenzidine. Free radical and charge-transfer complex intermediates. J. Biol. Chem. 1982, 257, 3669–3675. [Google Scholar] [CrossRef]
- He, W.; Liu, Y.; Yuan, J.; Yin, J.-J.; Wu, X.; Hu, X.; Zhang, K.; Liu, J.; Chen, C.; Ji, Y.; et al. Au@Pt nanostructures as oxidase and peroxidase mimetics for use in immunoassays. Biomaterials 2011, 32, 1139–1147. [Google Scholar] [CrossRef]
- Gao, Z.; Xu, M.; Hou, L.; Chen, G.; Tang, D. Irregular-shaped platinum nanoparticles as peroxidase mimics for highly efficient colorimetric immunoassay. Anal. Chim. Acta 2013, 776, 79–86. [Google Scholar] [CrossRef]
- Ge, C.; Wu, R.; Chong, Y.; Fang, G.; Jiang, X.; Pan, Y.; Chen, C.; Yin, J.-J. Synthesis of Pt hollow nanodendrites with enhanced peroxidase-like activity against bacterial: Infections implication for wound healing. Adv. Funct. Mater. 2018, 28, 1801484. [Google Scholar] [CrossRef]
- Sangwan, S.; Seth, R. Synthesis, characterization and stability of gold nanoparticles (AuNPs) in different buffer systems. J. Clust. Sci. 2022, 33, 749–764. [Google Scholar] [CrossRef]
- Mu, J.; Wang, Y.; Zhao, M.; Zhang, L. Intrinsic peroxidase-like activity and catalase-like activity of Co3O4 nanoparticles. Chem. Commun. 2012, 48, 2540–2542. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Q.; Zhao, H.; Zhang, L.; Su, Y.; Lv, Y. BSA-templated MnO2 nanoparticles as both peroxidase and oxidase mimics. Analyst 2012, 137, 4552–4558. [Google Scholar] [CrossRef]
- André, R.; Natálio, F.; Humanes, M.; Leppin, J.; Heinze, K.; Wever, R.; Schröder, H.-C.; Müller, W.E.G.; Tremel, W. V2O5 nanowires with an intrinsic peroxidase-Like activity. Adv. Funct. Mater. 2011, 21, 501–509. [Google Scholar] [CrossRef]
- Ye, H.; Mohar, J.; Wang, Q.; Catalano, M.; Kim, M.J.; Xia, X. Peroxidase-like properties of ruthenium nanoframes. Sci. Bull. 2016, 61, 1739–1745. [Google Scholar] [CrossRef]
- Xia, X.; Zhang, J.; Lu, N.; Kim, M.J.; Ghale, K.; Xu, Y.; McKenzie, E.; Liu, J.; Ye, H. Pd–Ir core–shell nanocubes: A type of highly efficient and versatile peroxidase mimic. ACS Nano 2015, 9, 9994–10004. [Google Scholar] [CrossRef]
- Wan, S.; Wang, Q.; Ye, H.; Kim, M.J.; Xia, X. Pd–Ru bimetallic nanocrystals with a porous structure and their enhanced catalytic properties. Part. Part. Syst. Charact. 2018, 35, 1700386. [Google Scholar] [CrossRef]
- Ye, H.; Liu, Y.; Chhabra, A.; Lilla, E.; Xia, X. Polyvinylpyrrolidone (PVP)-capped Pt nanocubes with superior peroxidase-like activity. ChemNanoMat 2017, 3, 33–38. [Google Scholar] [CrossRef]
- Davidson, E.; Xi, Z.; Gao, Z.; Xia, X. Ultrafast and sensitive colorimetric detection of ascorbic acid with Pd-Pt core-shell nanostructure as peroxidase mimic. Sens. Int. 2020, 1, 100031. [Google Scholar] [CrossRef]
- Liu, X.; Dai, Q.; Austin, L.; Coutts, J.; Knowles, G.; Zou, J.; Chen, H.; Huo, Q. A one-step homogeneous immunoassay for cancer biomarker detection using gold nanoparticle probes coupled with dynamic light scattering. J. Am. Chem. Soc. 2008, 130, 2780–2782. [Google Scholar] [CrossRef] [PubMed]
- Thobhani, S.; Attree, S.; Boyd, R.; Kumarswami, N.; Noble, J.; Szymanski, M.; Porter, R.A. Bioconjugation and characterisation of gold colloid-labelled proteins. J. Immunol. Methods 2010, 356, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Avvakumova, S.; Colombo, M.; Galbiati, E.; Mazzucchelli, S.; Rotem, R.; Prosperi, D. Chapter 6—Bioengineered approaches for site orientation of peptide-based ligands of nanomaterials. In Biomedical Applications of Functionalized Nanomaterials; Sarmento, B., das Neves, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 139–169. [Google Scholar]
- Kuzuya, A.; Numajiri, K.; Kimura, M.; Komiyama, M. Single-molecule accommodation of streptavidin in nanometer-scale wells formed in DNA nanostructures. Nucleic Acids Symp. Ser. 2008, 52, 681–682. [Google Scholar] [CrossRef]



| Catalyst | Size (nm) | [E] (M) | Substance | Km (M) | Vmax (M s−1) | Kcat (s−1) | Kcat-mass-specfic (s−1 mg−1Pt) | Refs. |
|---|---|---|---|---|---|---|---|---|
| HRP | N/A | 2.5 × 10−11 | TMB | 4.3 × 10−4 | 1.0 × 10−7 | 4.0 × 103 | N/A | [23] |
| Fe3O4 particles | 300 | 1.1 × 10−12 | TMB | 9.8 × 10−5 | 3.4 × 10−8 | 3.0 × 104 | N/A | [23] |
| Co3O4 cubes | 20 | 3.4 × 10−10 | TMB | 3.7 × 10−5 | 6.3 × 10−8 | 1.8 × 102 | N/A | [57] |
| MnO2 particles | 4.5 | 3.0 × 10−8 | OPD | 3.1 × 10−4 | 8.2 × 10−8 | 2.7 × 100 | N/A | [58] |
| V2O5 wires | 100 × 500 | 1.1 × 10−4 | ABTS | 4.0 × 10−7 | 2.8 × 10−1 | 2.5 × 103 | N/A | [59] |
| Au particles | 40 | 6.7 × 10−12 | TMB | N/A | 4.8 × 10−8 | 7.2 × 103 | N/A | [36] |
| Ru frames | 10 | 1.1 × 10−12 | TMB | 6.0 × 10−5 | 1.3 × 10−7 | 1.3 × 104 | N/A | [60] |
| Au@Pt rods | 30 × 70 | 1.3 × 10−11 | TMB | 2.7 × 10−5 | 1.8 × 10−7 | 1.4 × 104 | N/A | [53] |
| Pt particles | 5–7 | 8.1 × 10−11 | TMB | 1.2 × 10−4 | 1.3 × 10−6 | 2.3 × 104 | 1.64 × 1019 | [54] |
| Pd cubes | 18 | 1.4 × 10−12 | TMB | 5.4 × 10−5 | 9.7 × 10−8 | 6.9 × 104 | N/A | [61] |
| Pd-Ru cubes | 20 | N/A | TMB | N/A | N/A | 4.8 × 105 | N/A | [62] |
| Pt cubes | 7.4 | 4.1 × 10−13 | TMB | 7.3 × 10−4 | 3.3 × 10−7 | 8.2 × 105 | 9.63 × 1019 | [63] |
| Pd-Ir cubes | 19.2 | 3.4 × 10−14 | TMB | 1.3 × 10−4 | 6.5 × 10−8 | 1.9 × 106 | N/A | [61] |
| Pd@Pt cubes | 20 | 2.6 × 10−13 | TMB | 3.4 × 10−4 | 6.0 × 10−7 | 2.3 × 106 | 4.40 × 1019 | [64] |
| Concave Pt cubes | 44 | 2.5 × 10−14 | TMB | N/A | 1.5 × 10−7 | 6.0 × 106 | 3.28 × 1018 | [47] |
| Pd@Pt dendrites | 27 | 2.44 × 10−14 | TMB | 6.63 × 10−4 | 2.21 × 10−7 | 9.1 × 106 | 8.20 × 1019 | [46] |
| Au@Pt4LNPs | 42 | 3.78 × 10−14 | TMB | 8.91 × 10−4 | 1.61 × 10−7 | 4.25 × 106 | 4.13 × 1019 | This work |
| Sample No. | Spiked (pg mL−1) | Found (Mean ± SD) (pg mL−1) | CV (%, n = 3) | Recovery (%) |
|---|---|---|---|---|
| 1 | 0.2 | 0.185 ± 0.015 | 8.30 | 92.53 |
| 2 | 0.5 | 0.479 ± 0.025 | 5.25 | 95.87 |
| 3 | 1 | 1.043 ± 0.093 | 8.90 | 104.30 |
| 4 | 2 | 2.055 ± 0.176 | 8.57 | 102.75 |
| 5 | 5 | 5.118 ± 0.280 | 5.47 | 102.36 |
| 6 | 10 | 10.555 ± 0.534 | 5.06 | 105.55 |
| 7 | 20 | 19.666 ± 1.038 | 5.28 | 98.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Peng, X.; Song, H.; Tan, Y.; Xu, J.; Li, Q.; Gao, Z. Atomic Pt-Layer-Coated Au Peroxidase Nanozymes with Enhanced Activity for Ultrasensitive Colorimetric Immunoassay of Interleukin-12. Biosensors 2025, 15, 239. https://doi.org/10.3390/bios15040239
Zhang H, Peng X, Song H, Tan Y, Xu J, Li Q, Gao Z. Atomic Pt-Layer-Coated Au Peroxidase Nanozymes with Enhanced Activity for Ultrasensitive Colorimetric Immunoassay of Interleukin-12. Biosensors. 2025; 15(4):239. https://doi.org/10.3390/bios15040239
Chicago/Turabian StyleZhang, Han, Xiang Peng, Hao Song, Yongfeng Tan, Jianglian Xu, Qunfang Li, and Zhuangqiang Gao. 2025. "Atomic Pt-Layer-Coated Au Peroxidase Nanozymes with Enhanced Activity for Ultrasensitive Colorimetric Immunoassay of Interleukin-12" Biosensors 15, no. 4: 239. https://doi.org/10.3390/bios15040239
APA StyleZhang, H., Peng, X., Song, H., Tan, Y., Xu, J., Li, Q., & Gao, Z. (2025). Atomic Pt-Layer-Coated Au Peroxidase Nanozymes with Enhanced Activity for Ultrasensitive Colorimetric Immunoassay of Interleukin-12. Biosensors, 15(4), 239. https://doi.org/10.3390/bios15040239






