Recent Advances of Fluorescent Aptasensors for the Detection of Antibiotics in Food
Abstract
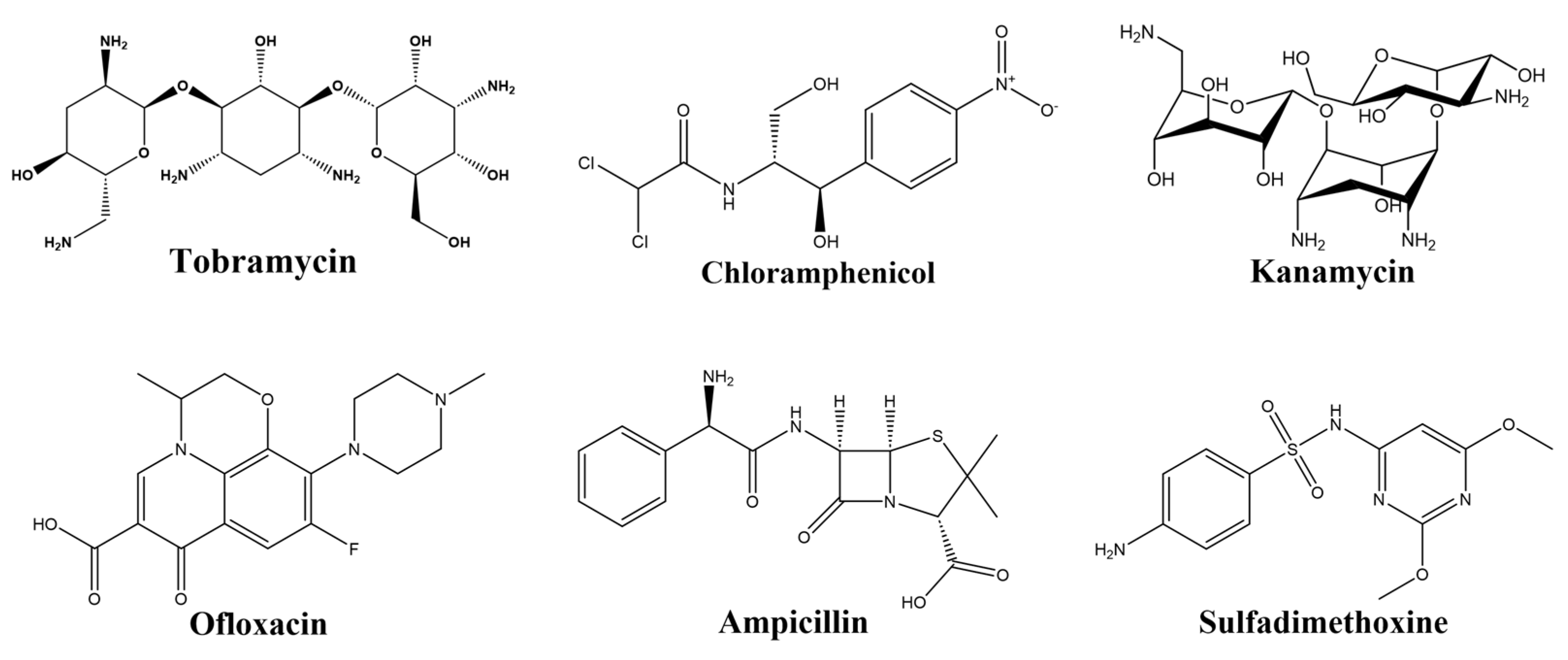
1. Introduction
| Targets | Aptamer Sequences (5′-3′) | Reference |
|---|---|---|
| Tobramycin | GGGACTTGGTTTAGGTAATGAGTCCC | [12] |
| Chloramphenicol | ACTTCAGTGAGTTGTCCCACGGTCGGCGAGTCGGTGGTAG | [16] |
| Kanamycin | TGGGGGTTGAGGCTAAGCCGA | [17] |
| Tetracycline | CGTACGGAATTCGCTAGCCCCCCGGCAGGCCACGGCTTGGGTTGGTCCCACTGCGCGTGGATCC | [18] |
| Ofloxacin | ATACCAGCTTATTCAATTGCAGGGTATCTGAGGCTTGATCTACTAAATGTCGTGGGGCATTGCTATTGGCGTTGATACGTACAATCGTAATCAGTTAG | [19] |
| Sulfadimethoxine | GAGGGCAACGAGTGTTTATAGA | [20] |
| Sulfadimidine | TTAGCTTATGCGTTGGCCGGGATAAGGATCCAGCCGTTGTAGATTTGCGTTCTAACTCTC | [21] |
| Ampicillin | CTGAATTGGATCTCTCTTCTTGAGCGATCTCCACA | [22] |
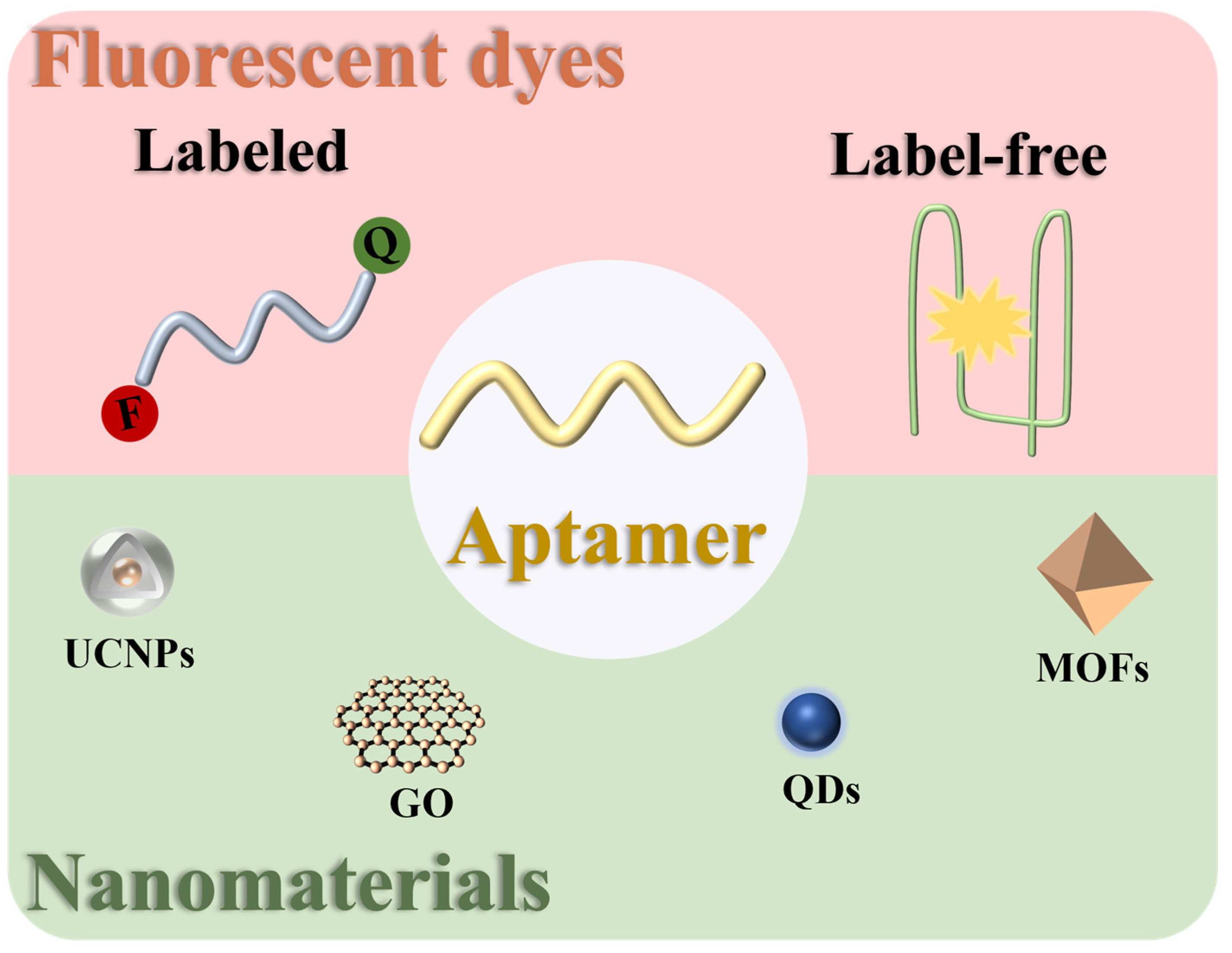
2. Fluorescent Dyes in Fluorescent Aptasensors for the Detection of Antibiotics
2.1. Labeled Type
2.2. Label-Free Type
3. Nanomaterials in Fluorescent Aptasensors for the Detection of Antibiotics
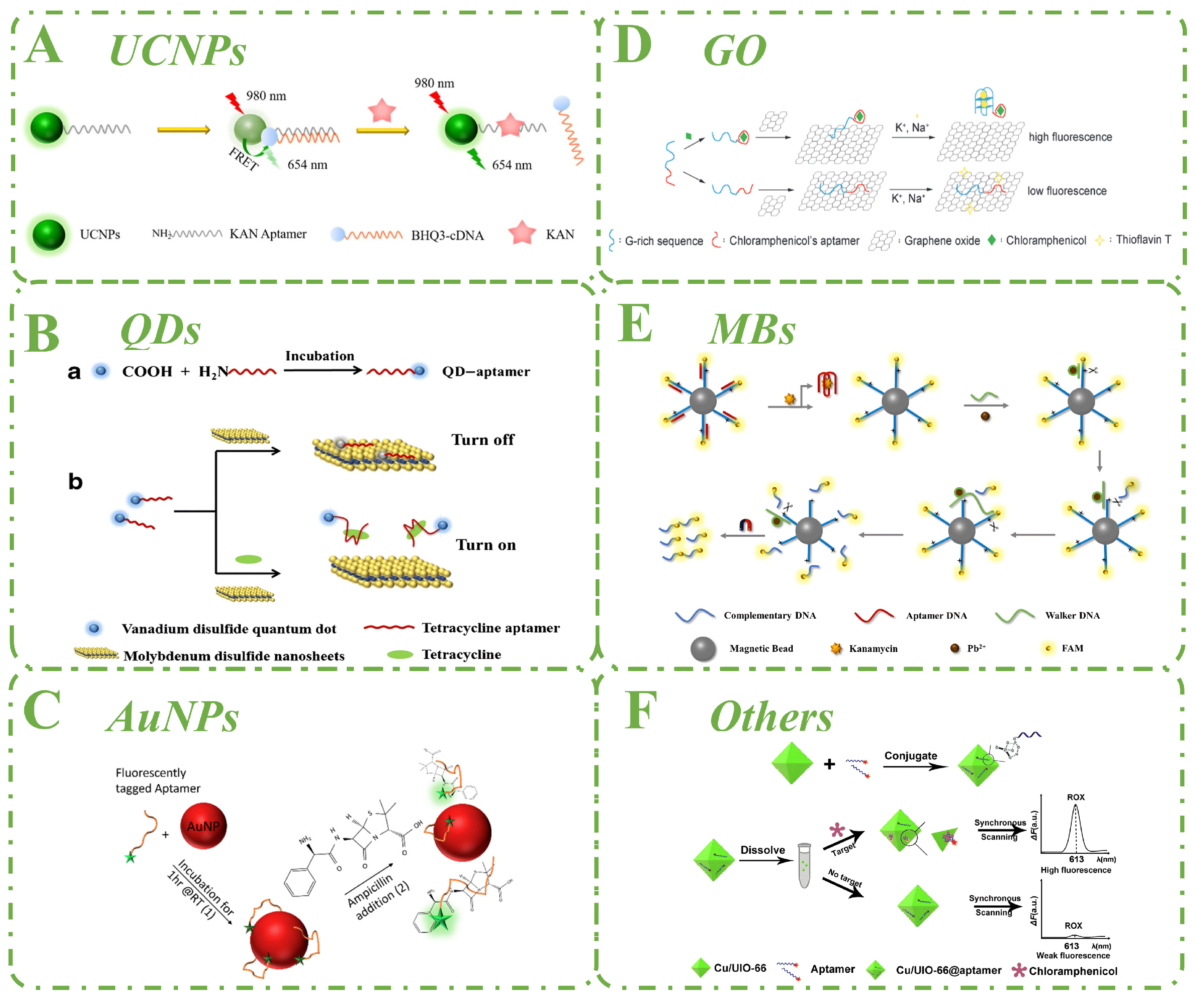
3.1. Upconversion Nanoparticles
3.2. Quantum Dots
3.3. Gold Nanoparticles
3.4. Graphene Oxide
3.5. Magnetic Beads
3.6. Other Nanomaterials
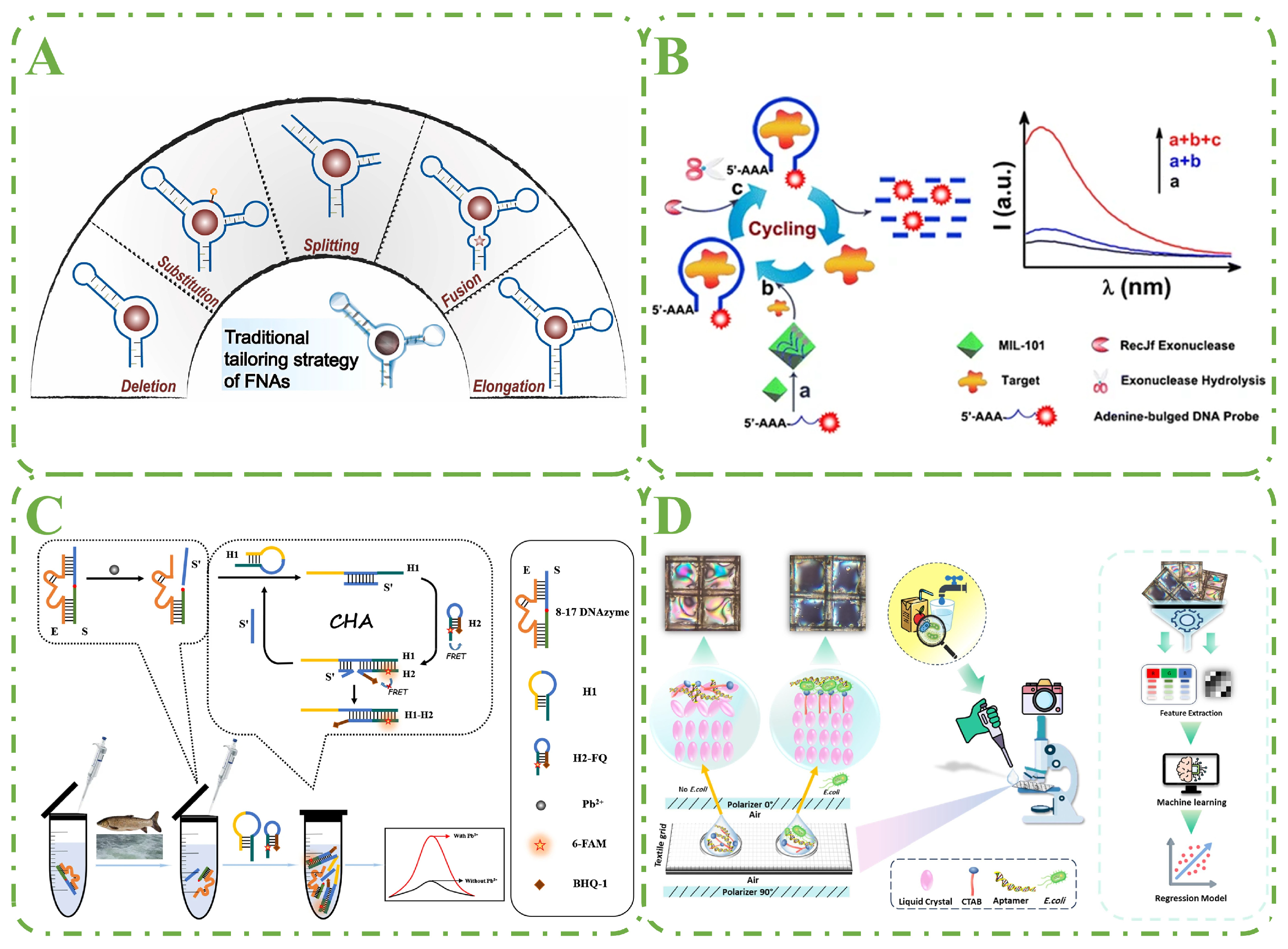
4. Aptamer Detection Technology Performance Enhancement Strategies
4.1. Nucleic Acid Tailoring Strategy
4.2. Enzyme-Assisted Signal Amplification
4.3. Enzyme-Free Signal Amplification
4.4. AI-Assisted Aptamer Performance Enhancement
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ENR | Enrofloxacin |
| MSNs | Mesoporous silica nanoparticles |
| NMM | N-methylmesoporphyrin IX |
| OFL | Ofloxacin |
| PtNPs | Platinum nanoparticles |
| RB | Rhodamine B |
| SG | SYBR gold |
| SGI | SYBR Green I |
| SLF | Sulfadimethoxine |
| SMZ | Sulfamethazine |
| TO | Thiazole orange |
References
- Abedalwafa, M.A.; Li, Y.; Ni, C.F.; Wang, L. Colorimetric sensor arrays for the detection and identification of antibiotics. Anal. Methods 2019, 11, 2836–2854. [Google Scholar] [CrossRef]
- Lan, L.; Yao, Y.; Ping, J.; Ying, Y. Recent advances in nanomaterial-based biosensors for antibiotics detection. Biosens. Bioelectron. 2017, 91, 504–514. [Google Scholar] [CrossRef]
- Alampanos, V.; Kabir, A.; Furton, K.G.; Samanidou, V.; Papadoyannis, I. Fabric phase sorptive extraction for simultaneous observation of four penicillin antibiotics from human blood serum prior to high performance liquid chromatography and photo-diode array detection. Microchem. J. 2019, 149, 103964. [Google Scholar] [CrossRef]
- Drabińska, N.; Hewett, K.; White, P.; Avison, M.B.; Persad, R.; Ratcliffe, N.M.; de Lacy Costello, B. Application of a solid-phase microextraction-gas chromatography-mass spectrometry/metal oxide sensor system for detection of antibiotic susceptibility in urinary tract infection-causing Escherichia coli—A proof of principle study. Adv. Med. Sci. 2022, 67, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tasci, F.; Canbay, H.S.; Doganturk, M. Determination of antibiotics and their metabolites in milk by liquid chromatography-tandem mass spectrometry method. Food Control 2021, 127, 108147. [Google Scholar] [CrossRef]
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef]
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef]
- Cho, E.J.; Lee, J.W.; Ellington, A.D. Applications of Aptamers as Sensors. Annu. Rev. Anal. Chem. 2009, 2, 241–264. [Google Scholar] [CrossRef]
- Yang, D.; Liu, X.; Zhou, Y.; Luo, L.; Zhang, J.; Huang, A.; Mao, Q.; Chen, X.; Tang, L. Aptamer-based biosensors for detection of lead(II) ion: A review. Anal. Methods 2017, 9, 1976–1990. [Google Scholar] [CrossRef]
- Nutiu, R.; Li, Y. Aptamers with fluorescence-signaling properties. Methods 2005, 37, 16–25. [Google Scholar] [CrossRef]
- Rowe, A.A.; Miller, E.A.; Plaxco, K.W. Reagentless measurement of aminoglycoside antibiotics in blood serum via an electrochemical, ribonucleic acid aptamer-based biosensor. Anal. Chem. 2010, 82, 7090–7095. [Google Scholar] [CrossRef] [PubMed]
- Naeeminejad, S.; Abnous, K.; Taghdisi, S.M. A simple and label-free fluorescent aptasensor for detection of tobramycin: Appropriate for on-site antibiotic monitoring. Microchem. J. 2021, 165, 106128. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, Y.; Jia, J.; Xiang, Y. Colorimetric aptasensors for determination of tobramycin in milk and chicken eggs based on DNA and gold nanoparticles. Food Chem. 2018, 249, 98–103. [Google Scholar] [CrossRef]
- Mehlhorn, A.; Rahimi, P.; Joseph, Y. Aptamer-Based Biosensors for Antibiotic Detection: A Review. Biosensors 2018, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Mahapatra, C.; Chen, H.; Peng, X.; Ramakrishna, S.; Nanda, H.S. Recent developments in fluorescent aptasensors for the detection of antibiotics. Curr. Opin. Biomed. Eng. 2020, 13, 16–24. [Google Scholar] [CrossRef]
- Ma, X.; Li, H.; Qiao, S.; Huang, C.; Liu, Q.; Shen, X.; Geng, Y.; Xu, W.; Sun, C. A simple and rapid sensing strategy based on structure-switching signaling aptamers for the sensitive detection of chloramphenicol. Food Chem. 2020, 302, 125359. [Google Scholar] [CrossRef]
- Ma, X.; Qiao, S.; Sun, H.; Su, R.; Sun, C.; Zhang, M. Development of Structure-Switching Aptamers for Kanamycin Detection Based on Fluorescence Resonance Energy Transfer. Front. Chem. 2019, 7, 29. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, W.; Tan, S.; Chen, T. Label-Free and Simple G-quadruplex-based Turn-Off Fluorescence Assay for the Detection of Kanamycin. Anal. Lett. 2018, 51, 1718–1729. [Google Scholar] [CrossRef]
- Sun, C.; Su, R.; Bie, J.; Sun, H.; Qiao, S.; Ma, X.; Sun, R.; Zhang, T. Label-free fluorescent sensor based on aptamer and thiazole orange for the detection of tetracycline. Dyes Pigment. 2018, 149, 867–875. [Google Scholar] [CrossRef]
- Ouyang, Q.; Liu, Y.; Chen, Q.; Guo, Z.; Zhao, J.; Li, H.; Hu, W. Rapid and specific sensing of tetracycline in food using a novel upconversion aptasensor. Food Control. 2017, 81, 156–163. [Google Scholar] [CrossRef]
- Liu, X.; Gao, T.; Gao, X.; Ma, T.; Tang, Y.; Zhu, L.; Li, J. An aptamer based sulfadimethoxine assay that uses magnetized upconversion nanoparticles. Microchim. Acta 2017, 184, 3557–3563. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, R.; Hassan, M.; Li, H.; Ouyang, Q.; Chen, Q. Fluorescence resonance energy transfer-based aptasensor for sensitive detection of kanamycin in food. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2021, 262, 120147. [Google Scholar] [CrossRef] [PubMed]
- Babaei, M.; Jalalian, S.H.; Bakhtiari, H.; Ramezani, M.; Abnous, K.; Taghdisi, S.M. Aptamer-Based Fluorescent Switch for Sensitive Detection of Oxytetracycline. Aust. J. Chem. 2017, 70, 718–723. [Google Scholar] [CrossRef]
- Umrao, S.; Anusha, S.; Jain, V.; Chakraborty, B.; Roy, R. Smartphone-based kanamycin sensing with ratiometric FRET. RSC Adv. 2019, 9, 6143–6151. [Google Scholar] [CrossRef]
- Tu, C.; Dai, Y.; Zhang, Y.; Wang, W.; Wu, L. A simple fluorescent strategy based on triple-helix molecular switch for sensitive detection of chloramphenicol. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2020, 224, 117415. [Google Scholar] [CrossRef] [PubMed]
- Ben Aissa, S.; Mastouri, M.; Catanante, G.; Raouafi, N.; Marty, J.L. Investigation of a Truncated Aptamer for Ofloxacin Detection Using a Rapid FRET-Based Apta-Assay. Antibiotics 2020, 9, 860. [Google Scholar] [CrossRef]
- He, H.; Xie, C.; Yao, L.; Ning, G.; Wang, Y. A Sensitive Fluorescent Assay for Tetracycline Detection Based on Triple-helix Aptamer Probe and Cyclodextrin Supramolecular Inclusion. J. Fluoresc. 2021, 31, 63–71. [Google Scholar] [CrossRef]
- Yang, H.; Wu, Q.; Su, D.; Wang, Y.; Li, L.; Zhang, X. A Label-free and Turn-on Fluorescence Strategy for Kanamycin Detection Based on the NMM/G-quadruplex Structure. Anal. Sci. 2017, 33, 133–135. [Google Scholar] [CrossRef]
- Ma, L.; Sun, N.; Tu, C.; Zhang, Q.; Diao, A. Design of an aptamer—Based fluorescence displacement biosensor for selective and sensitive detection of kanamycin in aqueous samples. RSC Adv. 2017, 7, 38512–38518. [Google Scholar] [CrossRef]
- Chen, T.-X.; Ning, F.; Liu, H.-S.; Wu, K.-F.; Li, W.; Ma, C.-B. Label-free fluorescent strategy for sensitive detection of tetracycline based on triple-helix molecular switch and G-quadruplex. Chin. Chem. Lett. 2017, 28, 1380–1384. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, W.; Tan, S.; Chen, T. A Label-free and Functional Fluorescent Oligonucleotide Probe Based on a G-Quadruplex Molecular Beacon for the Detection of Kanamycin. Chem. Res. Chin. Univ. 2018, 34, 541–545. [Google Scholar] [CrossRef]
- Yang, C.; Bie, J.; Zhang, X.; Yan, C.; Li, H.; Zhang, M.; Su, R.; Zhang, X.; Sun, C. A label-free aptasensor for the detection of tetracycline based on the luminescence of SYBR Green I. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2018, 202, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Yan, Z.; Wang, L.; Zhou, X.; Yan, R.; Zhang, D.; Shen, G.; Zhou, S. Fluorometric determination for ofloxacin by using an aptamer and SYBR Green I. Microchim. Acta 2019, 186, 668. [Google Scholar] [CrossRef]
- Zhou, Y.; Zuo, L.; Wei, Y.; Dong, C. Development of fluorescent aptasensing system for ultrasensitive analysis of kanamycin. J. Lumin. 2020, 222, 117124. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, Y.; Liao, W.; Wang, W.; Wu, L. G-quadruplex specific thioflavin T-based label-free fluorescence aptasensor for rapid detection of tetracycline. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2020, 238, 118406. [Google Scholar] [CrossRef] [PubMed]
- Khajavian, Z.; Esmaelpourfarkhani, M.; Ramezani, M.; Alibolandi, M.; Abnous, K.; Taghdisi, S.M. A highly sensitive, simple and label-free fluorescent aptasensor for tobramycin sensing based on PicoGreen intercalation into DNA duplex regions of three-way junction origami. Microchem. J. 2021, 160, 105657. [Google Scholar] [CrossRef]
- Wang, C.; Li, J. Fluorescence method for kanamycin detection based on the conversion of G-triplex and G-quadruplex. Anal. Bioanal. Chem. 2021, 413, 7073–7080. [Google Scholar] [CrossRef]
- Wei, Y.; Zhou, Y.; Wei, Y.; Dong, C.; Wang, L. A fluorescent aptasensor based on berberine for ultrasensitive detection of bisphenol A in tap water. Anal. Methods 2021, 13, 1816–1822. [Google Scholar] [CrossRef]
- Taghdisi, S.M.; Danesh, N.M.; Nameghi, M.A.; Ramezani, M.; Alibolandi, M.; Abnous, K. A DNA triangular prism-based fluorescent aptasensor for ultrasensitive detection of prostate-specific antigen. Anal. Chim. Acta 2020, 1120, 36–42. [Google Scholar] [CrossRef]
- Bayraç, A.T.; Acar, Y. Label-free G-Quadruplex aptamer and Thioflavin-T based turn-off fluorescent detection of ethanolamine. Dyes Pigment. 2020, 172, 107788. [Google Scholar] [CrossRef]
- Khusbu, F.Y.; Zhou, X.; Chen, H.; Ma, C.; Wang, K. Thioflavin T as a fluorescence probe for biosensing applications. TrAC-Trends Anal. Chem. 2018, 109, 1–18. [Google Scholar] [CrossRef]
- Tao, X.; Peng, Y.; Liu, J. Nanomaterial-based fluorescent biosensors for veterinary drug detection in foods. J. Food Drug Anal. 2020, 28, 575–594. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gao, D.; Sun, F.; Li, Z.; Wang, Y.; Qiu, C.; He, K.; Wang, J. Nanomaterial-based aptamer biosensors for ochratoxin A detection: A review. Anal. Bioanal. Chem. 2022, 414, 2953–2969. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Cui, B. Simultaneous fluorometric and chirality based aptasensing of sulfamethazine by using upconversion nanoparticles and Au@Ag@Au core-shell nanoparticles. Microchim. Acta 2019, 186, 555. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, B.; Bao, Q.; Yang, T.; Wei, T.; Wang, J.; Mao, C.; Zhang, C.; Yang, M. Aptamer-modified sensitive nanobiosensors for the specific detection of antibiotics. J. Mater. Chem. B 2020, 8, 8607–8613. [Google Scholar] [CrossRef]
- Zhang, Y.; Hassan, M.M.; Rong, Y.; Liu, R.; Li, H.; Ouyang, Q.; Chen, Q. A solid-phase capture probe based on upconvertion nanoparticles and inner filter effect for the determination of ampicillin in food. Food Chem. 2022, 386, 132739. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hassan, M.; Rong, Y.; Liu, R.; Li, H.; Ouyang, Q.; Chen, Q. An upconversion nanosensor for rapid and sensitive detection of tetracycline in food based on magnetic-field-assisted separation. Food Chem. 2022, 373, 131497. [Google Scholar] [CrossRef]
- Liu, R.; Haruna, S.A.; Ali, S.; Xu, J.; Ouyang, Q.; Li, H.; Chen, Q. An Up-conversion signal probe-MnO2 nanosheet sensor for rapid and sensitive detection of tetracycline in food. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2022, 270, 120855. [Google Scholar] [CrossRef]
- Wang, Y.; Gan, N.; Zhou, Y.; Li, T.; Cao, Y.; Chen, Y. Novel single-stranded DNA binding protein-assisted fluorescence aptamer switch based on FRET for homogeneous detection of antibiotics. Biosens. Bioelectron. 2017, 87, 508–513. [Google Scholar] [CrossRef]
- He, Y.; Wen, X.; Zhang, B.; Fan, Z. Novel aptasensor for the ultrasensitive detection of kanamycin based on grapheneoxide quantum-dot-linked single-stranded DNA-binding protein. Sens. Actuators B-Chem. 2018, 265, 20–26. [Google Scholar] [CrossRef]
- Chen, X.-X.; Lin, Z.-Z.; Yao, Q.-H.; Huang, Z.-Y. A practical aptaprobe for sulfadimethoxine residue detection in water and fish based on the fluorescence quenching of CdTe QDs by poly (diallyldimethylammonium chloride). J. Food Compos. Anal. 2020, 91, 103526. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, J.; Deng, J.; Wang, S. A novel fluorescent “turn-on” aptasensor based on nitrogen-doped graphene quantum dots and hexagonal cobalt oxyhydroxide nanoflakes to detect tetracycline. Anal. Bioanal. Chem. 2020, 412, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Zhang, H.; Sun, X.; Huang, X.; Li, H.; Li, F.; Guo, Y.; Yang, Q. Aptasensor based on fluorescence resonance energy transfer for the determination of kanamycin. Eur. Food Res. Technol. 2022, 248, 1563–1572. [Google Scholar] [CrossRef]
- Ma, X.; Du, C.; Zhang, J.; Shang, M.; Song, W. A system composed of vanadium(IV) disulfide quantum dots and molybdenum(IV) disulfide nanosheets for use in an aptamer-based fluorometric tetracycline assay. Microchim. Acta 2019, 186, 837. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Yi, H.; Wang, L.; Zhou, X.; Yan, R.; Zhang, D.; Wang, S.; Su, L.; Zhou, S. Fluorescent aptasensor for ofloxacin detection based on the aggregation of gold nanoparticles and its effect on quenching the fluorescence of Rhodamine B. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2019, 221, 117203. [Google Scholar] [CrossRef]
- Esmaelpourfarkhani, M.; Abnous, K.; Taghdisi, S.M.; Chamsaz, M. A novel turn-off fluorescent aptasensor for ampicillin detection based on perylenetetracarboxylic acid diimide and gold nanoparticles. Biosens. Bioelectron. 2020, 164, 112329. [Google Scholar] [CrossRef]
- Chen, X.-X.; Lin, Z.-Z.; Hong, C.-Y.; Yao, Q.-H.; Huang, Z.-Y. Adichromatic label-free aptasensor for sulfadimethoxine detection in fish and water based on AuNPs color and fluorescent dyeing of double-stranded DNA with SYBR Green I. Food Chem. 2020, 309, 125712. [Google Scholar] [CrossRef]
- Sun, Y.; Qi, T.; Jin, Y.; Liang, L.; Zhao, J. A signal-on fluorescent aptasensor based on gold nanoparticles for kanamycin detection. RSC Adv. 2021, 11, 10054–10060. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, X.; Kou, Q.; Sun, Q.; Wang, Y.; Wu, P.; Yang, L.; Tang, J.; Le, T. An Ultrasensitive Label-Free Fluorescent Aptasensor Platform for Detection of Sulfamethazine. Int. J. Nanomed. 2021, 16, 2751–2759. [Google Scholar] [CrossRef]
- Simmons, M.D.; Miller, L.M.; Sundström, M.O.; Johnson, S. Aptamer-Based Detection of Ampicillin in Urine Samples. Antibiotics 2020, 9, 655. [Google Scholar] [CrossRef]
- Tan, J.; Wang, F.; Wang, Z.; Lu, Q.; Deng, L. An enzyme-free fluorometric nanoprobe for chloramphenicol based on signal amplification using graphene oxide sheets. Microchim. Acta 2020, 187, 319. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liu, M.; Li, B. DNAzyme-powered DNA walking machine for ultrasensitive fluorescence aptasensing of kanamycin. Microchim. Acta 2020, 187, 678. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhou, L.; Wu, Y.-X.; Zhang, K.; Cao, Y.; Zhou, Y.; Wu, D.; Hu, F.; Gan, N. A two dimensional metal-organic framework nanosheets-based fluorescence resonance energy transfer aptasensor with circular strand-replacement DNA polymerization target-triggered amplification strategy for homogenous detection of antibiotics. Anal. Chim. Acta 2018, 1020, 1–8. [Google Scholar] [CrossRef]
- Dehghani, S.; Danesh, N.M.; Ramezani, M.; Alibolandi, M.; Lavaee, P.; Nejabat, M.; Abnous, K.; Taghdisi, S.M. A label-free fluorescent aptasensor for detection of kanamycin based on dsDNA-capped mesoporous silica nanoparticles and Rhodamine B. Anal. Chim. Acta 2018, 1030, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Jiang, Y.; Wang, P.; Xiong, W.; Qi, B.; Zhang, Y.; Xiang, D.; Zhai, K. Bimetallic organic framework-based aptamer sensors: A new platform for fluorescence detection of chloramphenicol. Anal. Bioanal. Chem. 2020, 412, 5273–5281. [Google Scholar] [CrossRef]
- Chen, K.; Zhu, L.; Li, J.; Zhang, Y.; Yu, Y.; Wang, X.; Wei, W.; Huang, K.; Xu, W. High-content tailoring strategy to improve the multifunctionality of functional nucleic acids. Biosens. Bioelectron. 2024, 261, 116494. [Google Scholar] [CrossRef]
- He, J.; Li, G.; Hu, Y. Aptamer-involved fluorescence amplification strategy facilitated by directional enzymatic hydrolysis for bioassays based on a metal-organic framework platform: Highly selective and sensitive determination of thrombin and oxytetracycline. Microchim. Acta 2017, 184, 2365–2373. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Du, C.; Li, Y.; Ma, X.; Yang, C.; Xu, W.; Sun, C. Structure-switching aptamer triggering signal amplification strategy for tobramycin detection based on hybridization chain reaction and fluorescence synergism. Talanta 2022, 243, 123318. [Google Scholar] [CrossRef]
- Mostajabodavati, S.; Mousavizadegan, M.; Hosseini, M.; Mohammadimasoudi, M.; Mohammadi, J. Machine learning-assisted liquid crystal-based aptasensor for the specific detection of whole-cell Escherichia coli in water and food. Food Chem. 2024, 448, 139113. [Google Scholar] [CrossRef]
- Zhou, W.; Xu, L.; Jiang, B. Target-initiated autonomous synthesis of metal-ion dependent DNAzymes for label-free and amplified fluorescence detection of kanamycin in milk samples. Anal. Chim. Acta 2021, 1148, 238195. [Google Scholar] [CrossRef]
- Zhou, C.; Zou, H.; Sun, C.; Ren, D.; Xiong, W.; Li, Y. Fluorescent aptasensor for detection of four tetracycline veterinary drugs in milk based on catalytic hairpin assembly reaction and displacement of G-quadruplex. Anal. Bioanal. Chem. 2018, 410, 2981–2989. [Google Scholar] [CrossRef] [PubMed]
- Khabbazian, M.; Jabbari, H. AI-powered aptamer generation. Nat. Comput. Sci. 2022, 2, 356–357. [Google Scholar] [CrossRef] [PubMed]

| Analyte | Materials | Linear Range | LOD | Sample | Reference | |
|---|---|---|---|---|---|---|
| Labeled | TOB | SGI | 0.1–6 μM | 0.063 μM | Milk | [12] |
| CAP | FAM/BHQ1 | 3–309 nM | 2.16 nM | Milk | [16] | |
| KAN | FAM/Dabcyl | 100–600 nM | 13.52 nM | Milk | [17] | |
| KAN | ThT | 0.6–20 nM | 0.33 nM | Milk | [18] | |
| TET | TO | 0.11–225 μM | 0.07 μM | Milk | [19] | |
| OTC | FAM/BHQ1 | 0–250 nM | 1.67 nM | Milk | [23] | |
| KAN | Cy5/Cy3 | 0.05–5 nM | 0.18 nM | Milk | [24] | |
| CAP | FAM/BHQ1 | 5–200 nM | 1.2 nM | Honey | [25] | |
| OFL | FAM/TAMRA | 0.2–20 μM | 0.12 μM | Milk | [26] | |
| TET | Pyrenes | 5.0–100 nM | 1.6 nM | Milk | [27] | |
| Label-free | KAN | NMM | 0.5–100 nM | 0.5 nM | Milk | [28] |
| KAN | ThT | 1 nM–300 uM | 300 pM | Milk | [29] | |
| TET | ThT | 0.2–20.0 nM | 970.0 pM | Serum | [30] | |
| KAN | ThT | 0.7–10 nM | 0.37 nM | Milk | [31] | |
| TET | SGI | 11–56 μM | 0.23 μM | Milk | [32] | |
| OFL | SGI | 1.1–200 nM | 0.34 nM | Water | [33] | |
| KAN | Berberine | 5.0–71.0 nM | 2.3 nM | Milk | [34] | |
| TET | ThT | 0.01–1.0 μM | 0.001 μM | Honey | [35] | |
| TOB | PG | 80 nM–2 μM | 21.86 nM | Serum | [36] | |
| KAN | ThT | 50–2000 nM | 1.05 nM | Pork | [37] |
| Analyte | Materials | Linear Range | LOD | Sample | Reference |
|---|---|---|---|---|---|
| TET | UCNPs/MNPs | 0.02–225 nM | 0.014 nM | Pork | [20] |
| SLF | UCNPs/MNPs | 3–29 nM | 0.35 nM | Perch | [21] |
| SMZ | UCNPs/Au@Ag/AuNPs | 0.36–359 nM | 0.07 nM | Milk | [44] |
| ENR | UCNPs/GO | 2–173 nM | 1.3 nM | Milk | [45] |
| KAN | UCNPs/BHQ3 | 0.005–50 uM | 18.9 nM | Milk | [22] |
| AMP | UCNPs/PtNPs | 1–247 nM | 0.79 nM | Pork | [46] |
| TET | UCNPs/MNPs | 1–2250 nM | 0.38 nM | Pork | [47] |
| TET | UCNPs/MnO2 nanosheets | 0.02–225 nM | 0.019 nM | Milk | [48] |
| CAP | QDs/AuNPs | 0.015–309 nM | 9 pM | Milk | [49] |
| KAN | QDs/BHQ1 | 0.02–185 nM | 12 pM | Milk | [50] |
| SDM | QDs/PDDA | 80–966 nM | 7.21 nM | Fish | [51] |
| TET | QDs/CoOOH nanoflakes | 2–225 nM | 2.13 nM | Milk | [52] |
| KAN | QDs/AuNPs | 0.01–500 nM | 5.7 pM | Milk | [53] |
| TET | QDs/MoS2 nanosheets | 2–562 nM | 0.13 nM | Milk | [54] |
| OFL | RB/AuNPs | 20–300 nM | 1.66 nM | Milk | [55] |
| AMP | PTCDI/AuNPs | 100–1000 pM | 29.2 pM | Serum | [56] |
| SDM | SGI/AuNPs | 6–966 nM | 10.98 nM | Fish | [57] |
| KAN | FAM/AuNPs | 0.1 pM–0.1 uM | 0.1 pM | Water | [58] |
| SMZ | RB/AuNPs | 4–143 nM | 2.94 nM | Water | [59] |
| AMP | FAM/AuNPs | 100 nM–100 μM | 20.6 nM | Urine | [60] |
| CAP | FAM/GO | 6–618 nM | 0.9 nM | urine | [61] |
| CAP | ThT/GO | 2–20 nM | 1.45 nM | Milk | [62] |
| CAP | SG/MOFs | 0.003–30 nM | 0.9 pM | Milk | [63] |
| KAN | RB/MSNs | 24.75–137.15 nM | 7.5 nM | Serum | [64] |
| CAP | ROX/MOFs | 0.2–10 nM | 0.09 nM | Fish | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Yang, W.; Lin, H.; Zhang, M.; Sun, C. Recent Advances of Fluorescent Aptasensors for the Detection of Antibiotics in Food. Biosensors 2025, 15, 252. https://doi.org/10.3390/bios15040252
Liu Z, Yang W, Lin H, Zhang M, Sun C. Recent Advances of Fluorescent Aptasensors for the Detection of Antibiotics in Food. Biosensors. 2025; 15(4):252. https://doi.org/10.3390/bios15040252
Chicago/Turabian StyleLiu, Zheng, Wenyi Yang, Huikai Lin, Mingdi Zhang, and Chunyan Sun. 2025. "Recent Advances of Fluorescent Aptasensors for the Detection of Antibiotics in Food" Biosensors 15, no. 4: 252. https://doi.org/10.3390/bios15040252
APA StyleLiu, Z., Yang, W., Lin, H., Zhang, M., & Sun, C. (2025). Recent Advances of Fluorescent Aptasensors for the Detection of Antibiotics in Food. Biosensors, 15(4), 252. https://doi.org/10.3390/bios15040252




