Organic Bioelectronics in Microphysiological Systems: Bridging the Gap Between Biological Systems and Electronic Technologies
Abstract
1. Introduction
2. Biology to Be Studied with Organic Bioelectronics
2.1. Transmembrane Potential
2.2. Electrogenic Cells
2.2.1. AP Propagation
2.2.2. Ca2+-Dependent Neurotransmitter Release at Synaptic Terminals
2.2.3. Intracellular Ion Channel Modulation
2.3. Non-Electrogenic Cells
2.3.1. Ion Transport, Signaling, and Homeostasis
2.3.2. Metabolic Pathways and Redox Signaling
2.3.3. Tight Junction Integrity and Paracellular Transport
2.3.4. Cellular Functions
2.3.5. Biocompatibility
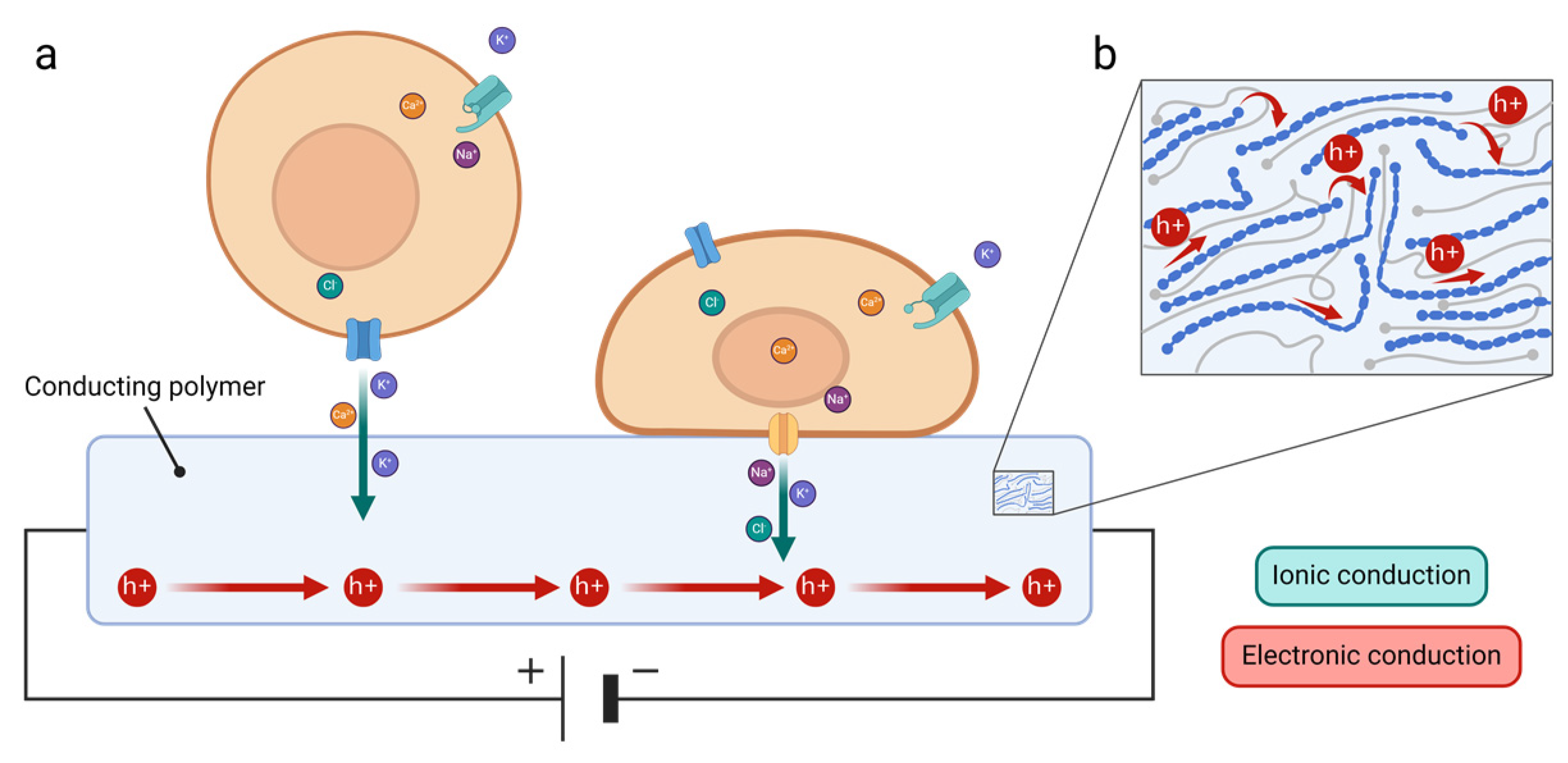
3. Organic Materials for Biological Applications
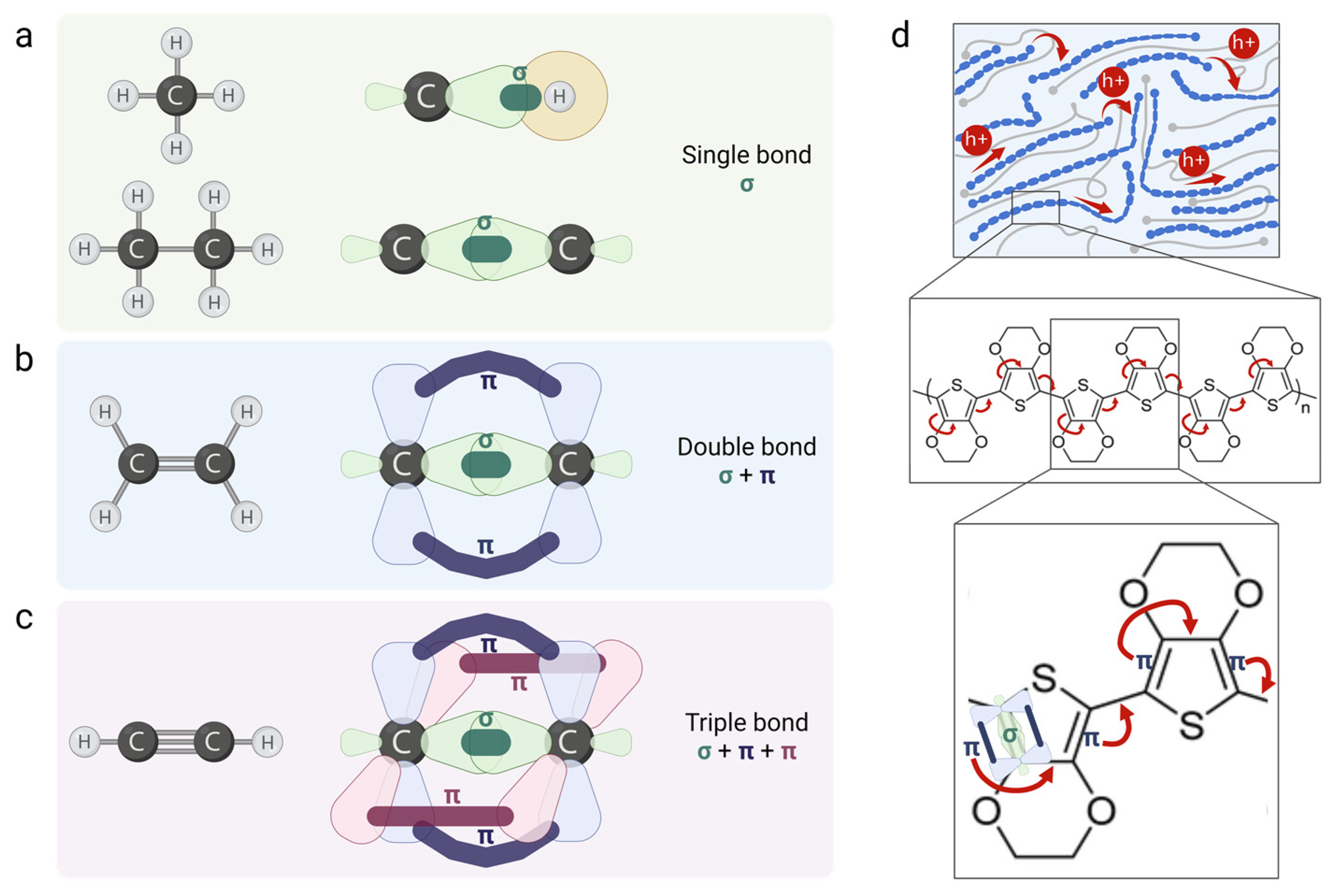
3.1. Organic Materials
3.2. Conducting Polymers
- Oxidative polymerization: chemical synthesis where monomers are oxidized by an oxidant to generate reactive intermediates that polymerize to form the polymer.
- Oxidative electrochemical polymerization: electrochemical synthesis where monomers undergo electrochemical oxidation on an electrode surface under an applied electrical potential.
- Vapor-phase polymerization: a monomer in the vapor phase reacts only with the oxidant deposited on a substrate to form the polymer.
- Plasma polymerization: gas-phase monomers are introduced into a plasma environment, leading to the formation of a polymer film on a substrate. The plasma (containing energetic species like ions or electrons) creates free radicals on the surface of the polymer that initiate polymerization.
- Solid-state polymerization: monomers are exposed to heat until the end groups mobilize enough to initiate polymerization in the absence of oxygen or water.
- Enzymatic polymerization: enzymes catalyze the in vitro polymerization of monomers via non-biosynthetic pathways.
3.3. Types of Conducting Polymers
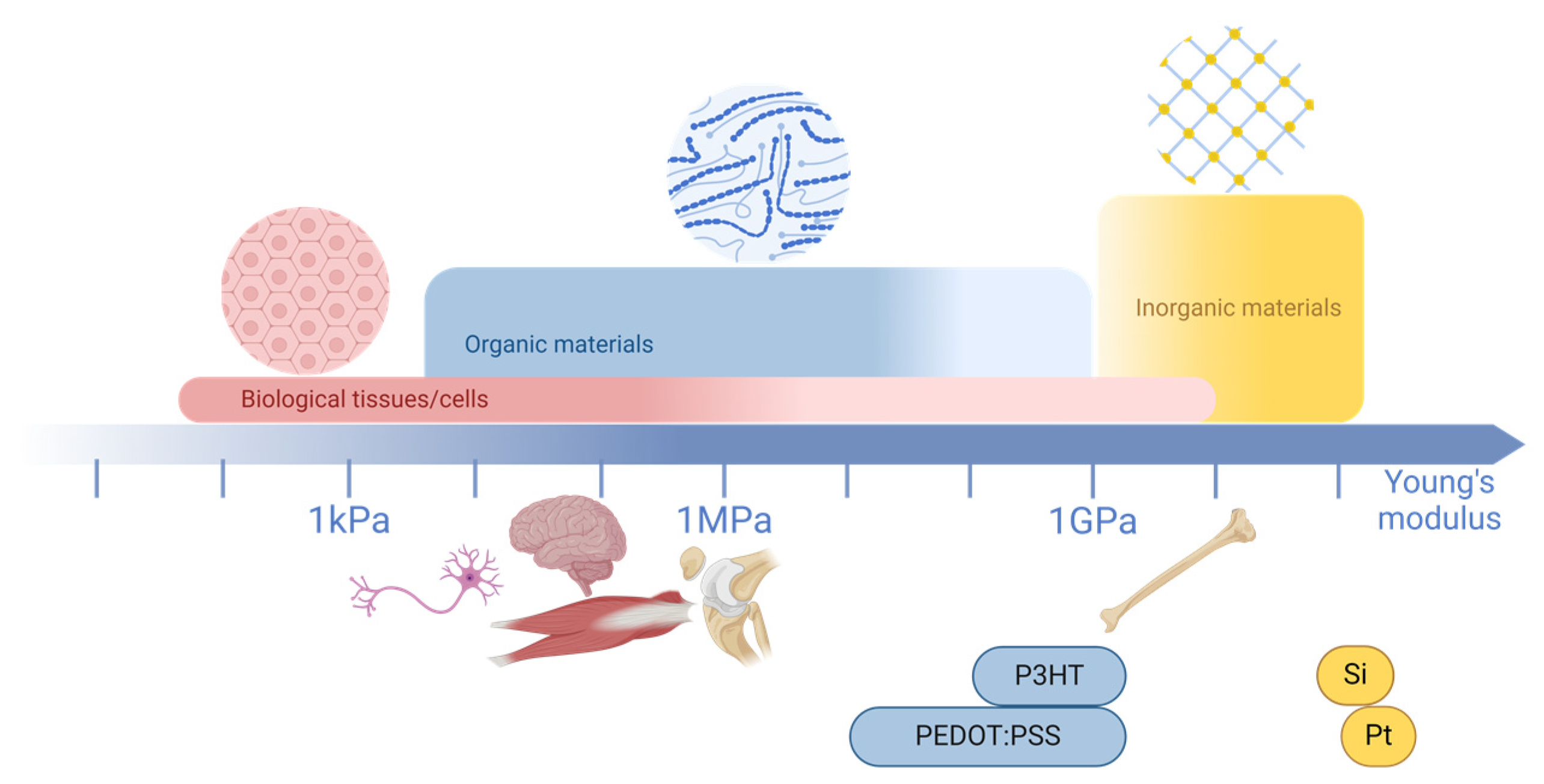
3.4. Advantages of Organic Materials
| CPs | Metals | Graphene | MXenes | ||
|---|---|---|---|---|---|
| Sensitivity | Electrical conductivity | 10−10 to 4380 S/cm [86,87,102] | 105 to 6.8 × 105 S/cm [134] | 106 S/cm [135] | 100 to 24,000 S/cm [136] |
| Electron mobility | 10−6 to 10−4 cm2/Vs [137,138,139] | 5460 to 37,590 cm2/Vs [140] | 200 to 2 × 105 cm2/Vs [135,141] | 106 cm2/Vs [142] | |
| Mechanical properties | Young’s modulus | 2 MPa to 5 GPa [85,99,100] | 72 GPa to 410 GPa [143] | 1 TPa [135,144] | 0.33 ± 0.03 TPa [145] |
| Flexibility | + + | − − | −/+ | −/+ | |
| Biocompatibility | + + + | + + | + | + + | |
| Cost | USD 0.30 to 10 per gram [146] | USD 30 to 96 per gram (subject to fluctuations due to market dynamics) [147,148,149] | USD 1.12 per gram [150] | USD 20.33 per gram [151] | |
4. Organic Bioelectronic Devices and Their Fabrication
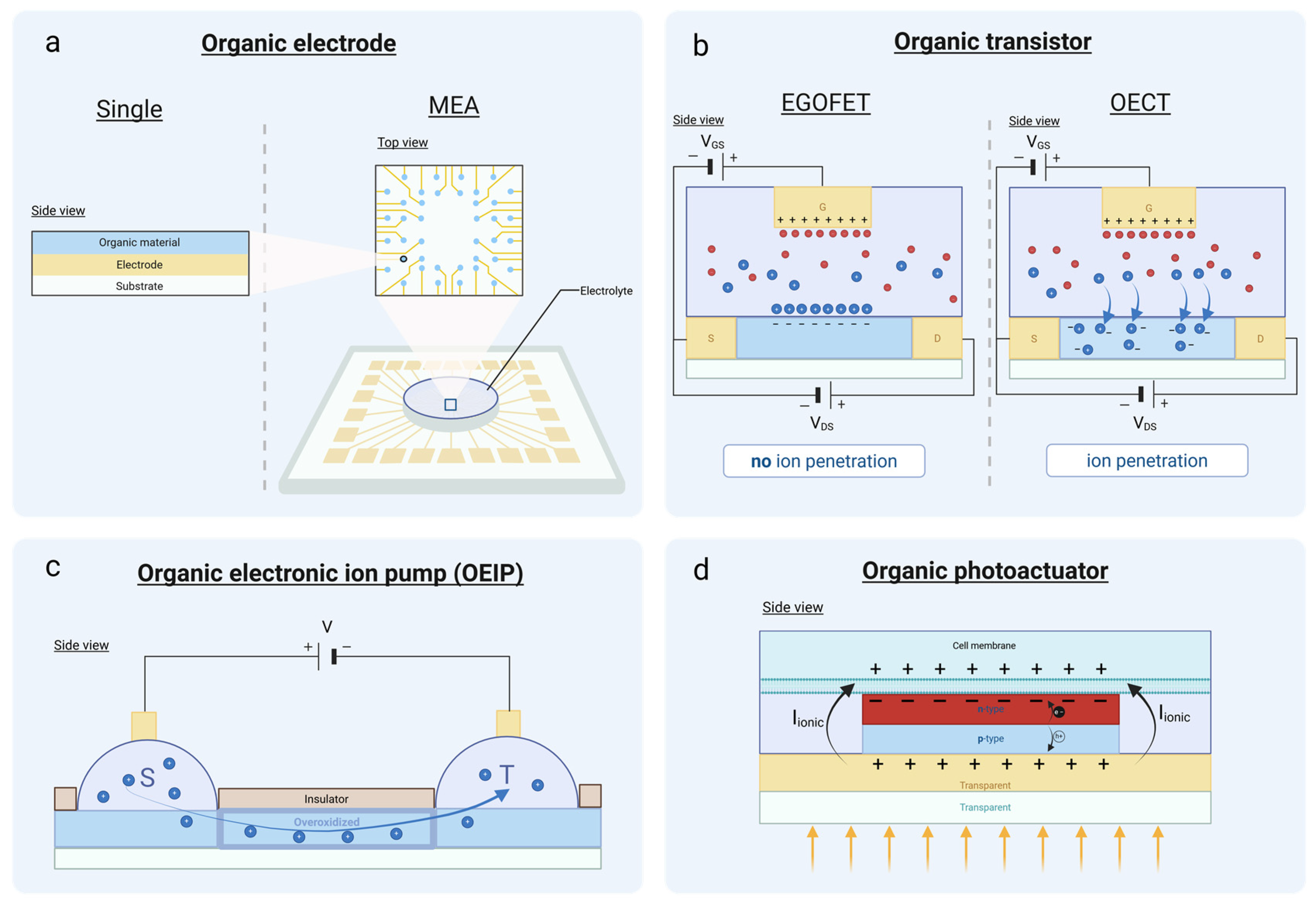
4.1. Organic Electrodes
4.2. Organic Transistors
4.2.1. EGOFET
4.2.2. OECT
4.3. OEIPs
4.4. Organic Photoactuators
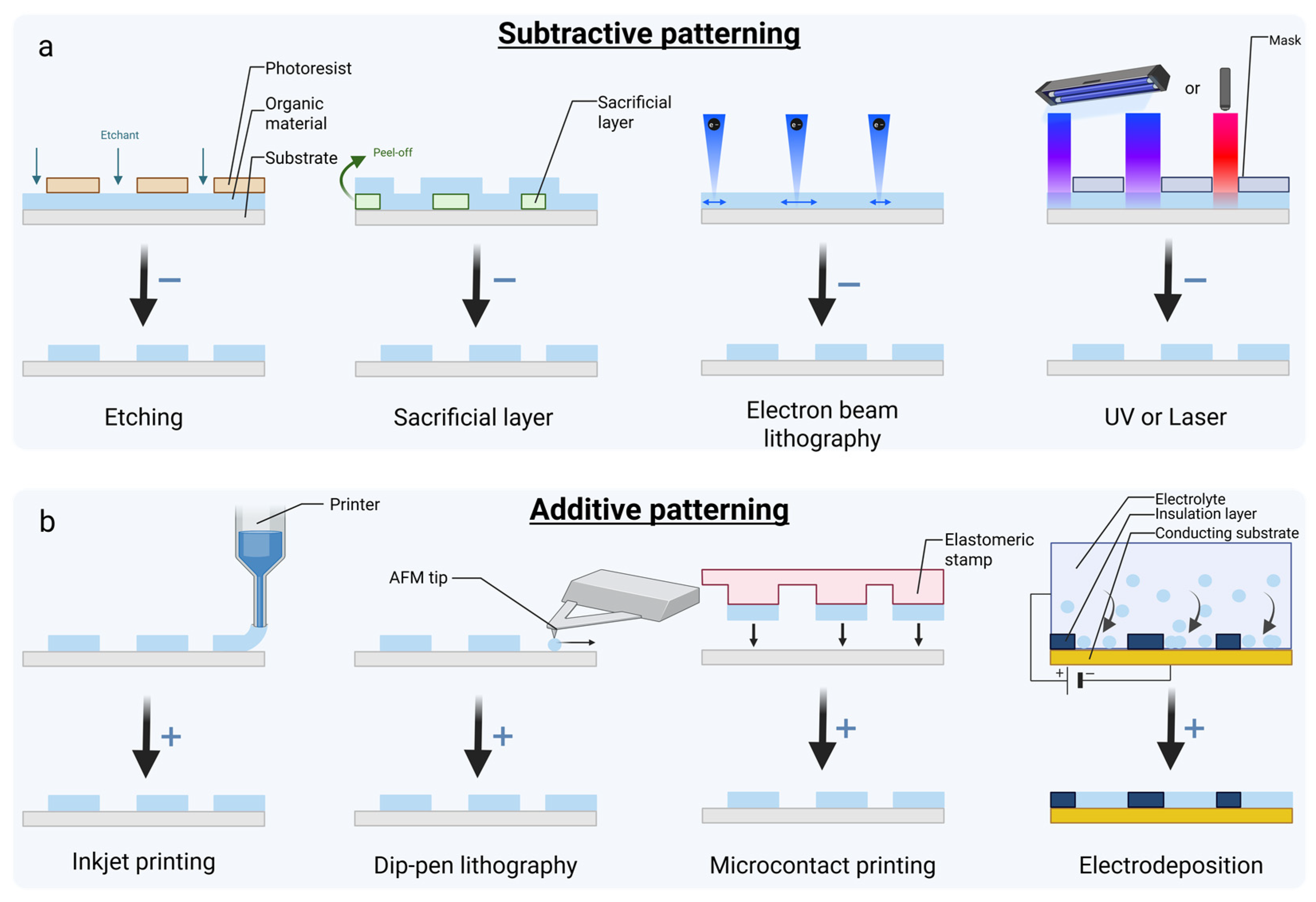
5. Organic Material Patterning and Biofunctionalization Methods
5.1. Organic Material Patterning
5.2. Biofunctionalization
6. In Vivo Applications
6.1. Implantable Devices for Electrical Recording
6.2. Wearable Devices for Electrical Recording
6.3. Implantable Devices for Electrical Stimulation
6.4. Wearable Devices for Electrical Stimulation
7. In Vitro Applications
7.1. Electrical Stimulation
7.2. Electrical Monitoring
7.3. Cellular Function and Tight Junction Monitoring
7.4. Biosensing
8. Conclusions and Future Visions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sleeman, K.E.; de Brito, M.; Etkind, S.; Nkhoma, K.; Guo, P.; Higginson, I.J.; Gomes, B.; Harding, R. The escalating global burden of serious health-related suffering: Projections to 2060 by world regions, age groups, and health conditions. Lancet Glob. Health 2019, 7, e883–e892. [Google Scholar] [CrossRef] [PubMed]
- Abdul Kadir, L.; Stacey, M.; Barrett-Jolley, R. Emerging Roles of the Membrane Potential: Action Beyond the Action Potential. Front. Physiol. 2018, 9, 1661. [Google Scholar] [CrossRef]
- Simon, D.T.; Gabrielsson, E.O.; Tybrandt, K.; Berggren, M. Organic Bioelectronics: Bridging the Signaling Gap between Biology and Technology. Chem. Rev. 2016, 116, 13009–13041. [Google Scholar] [CrossRef] [PubMed]
- Owens, R.M.; Malliaras, G.G. Organic Electronics at the Interface with Biology. MRS Bull. 2010, 35, 449–456. [Google Scholar] [CrossRef]
- Gkoupidenis, P.; Zhang, Y.; Kleemann, H.; Ling, H.; Santoro, F.; Fabiano, S.; Salleo, A.; van de Burgt, Y. Organic mixed conductors for bioinspired electronics. Nat. Rev. Mater. 2024, 9, 134–149. [Google Scholar] [CrossRef]
- Namsheer, K.; Rout, C.S. Conducting polymers: A comprehensive review on recent advances in synthesis, properties and applications. RSC Adv. 2021, 11, 5659–5697. [Google Scholar] [CrossRef]
- Syama, S.; Mohanan, P.V. Safety and biocompatibility of graphene: A new generation nanomaterial for biomedical application. Int. J. Biol. Macromol. 2016, 86, 546–555. [Google Scholar] [CrossRef]
- Szuplewska, A.; Kulpińska, D.; Jakubczak, M.; Dybko, A.; Chudy, M.; Olszyna, A.; Brzózka, Z.; Jastrzębska, A.M. The 10th anniversary of MXenes: Challenges and prospects for their surface modification toward future biotechnological applications. Adv. Drug Deliv. Rev. 2022, 182, 114099. [Google Scholar] [CrossRef]
- Pitsalidis, C.; Pappa, A.-M.; Boys, A.J.; Fu, Y.; Moysidou, C.-M.; van Niekerk, D.; Saez, J.; Savva, A.; Iandolo, D.; Owens, R.M. Organic Bioelectronics for In Vitro Systems. Chem. Rev. 2022, 122, 4700–4790. [Google Scholar] [CrossRef]
- Bettucci, O.; Matrone, G.M.; Santoro, F. Conductive Polymer-Based Bioelectronic Platforms toward Sustainable and Biointegrated Devices: A Journey from Skin to Brain across Human Body Interfaces. Adv. Mater. Technol. 2022, 7, 2100293. [Google Scholar] [CrossRef]
- Zeglio, E.; Rutz, A.L.; Winkler, T.E.; Malliaras, G.G.; Herland, A. Conjugated Polymers for Assessing and Controlling Biological Functions. Adv. Mater. 2019, 31, 1806712. [Google Scholar] [CrossRef] [PubMed]
- McCormick, D.A. Chapter 12—Membrane Potential and Action Potential. In From Molecules to Networks, 3rd ed.; Byrne, J.H., Heidelberger, R., Waxham, M.N., Eds.; Academic Press: Boston, MA, USA, 2014; pp. 351–376. ISBN 978-0-12-397179-1. [Google Scholar]
- Kulbacka, J.; Choromańska, A.; Rossowska, J.; Weżgowiec, J.; Saczko, J.; Rols, M.-P. Cell Membrane Transport Mechanisms: Ion Channels and Electrical Properties of Cell Membranes. In Transport Across Natural and Modified Biological Membranes and Its Implications in Physiology and Therapy; Kulbacka, J., Satkauskas, S., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 39–58. ISBN 978-3-319-56895-9. [Google Scholar]
- Dimitrov, A.G. Resting membrane state as an interplay of electrogenic transporters with various pumps. Pflug. Arch.-Eur. J. Physiol. 2023, 475, 1113–1128. [Google Scholar] [CrossRef]
- Jana, S. Action Potential. In Textbook of Veterinary Physiology; Das, P.K., Sejian, V., Mukherjee, J., Banerjee, D., Eds.; Springer Nature: Singapore, 2023; pp. 37–44. ISBN 978-981-19941-0-4. [Google Scholar]
- Fletcher, A. Action potential: Generation and propagation. Anaesth. Intensive Care Med. 2019, 20, 243–247. [Google Scholar] [CrossRef]
- Roth, R.H.; Ding, J.B. From Neurons to Cognition: Technologies for Precise Recording of Neural Activity Underlying Behavior. BME Front. 2020, 2020, 7190517. [Google Scholar] [CrossRef] [PubMed]
- Uguz, I.; Ohayon, D.; Yilmaz, S.; Griggs, S.; Sheelamanthula, R.; Fabbri, J.D.; McCulloch, I.; Inal, S.; Shepard, K.L. Complementary integration of organic electrochemical transistors for front-end amplifier circuits of flexible neural implants. Sci. Adv. 2024, 10, eadi9710. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.E.; Joo, H.R.; Lan Fan, J.; Liu, D.F.; Barnett, A.H.; Chen, S.; Geaghan-Breiner, C.; Karlsson, M.P.; Karlsson, M.; Lee, K.Y.; et al. High-density, long-lasting, and multi-region electrophysiological recordings using polymer electrode arrays. Neuron 2019, 101, 21–31.e5. [Google Scholar] [CrossRef]
- de Camp, N.V.; Kalinka, G.; Bergeler, J. Light-cured polymer electrodes for non-invasive EEG recordings. Sci. Rep. 2018, 8, 14041. [Google Scholar] [CrossRef]
- Yang, H.; Ji, S.; Chaturvedi, I.; Xia, H.; Wang, T.; Chen, G.; Pan, L.; Wan, C.; Qi, D.; Ong, Y.-S.; et al. Adhesive Biocomposite Electrodes on Sweaty Skin for Long-Term Continuous Electrophysiological Monitoring. ACS Mater. Lett. 2020, 2, 478–484. [Google Scholar] [CrossRef]
- Zheng, X.; Woeppel, K.M.; Griffith, A.Y.; Chang, E.; Looker, M.J.; Fisher, L.E.; Clapsaddle, B.J.; Cui, X.T. Soft Conducting Elastomer for Peripheral Nerve Interface. Adv. Healthc. Mater. 2019, 8, 1801311. [Google Scholar] [CrossRef]
- Vara, H.; Collazos-Castro, J.E. Enhanced spinal cord microstimulation using conducting polymer-coated carbon microfibers. Acta Biomater. 2019, 90, 71–86. [Google Scholar] [CrossRef]
- Yang, B.; Yao, F.; Ye, L.; Hao, T.; Zhang, Y.; Zhang, L.; Dong, D.; Fang, W.; Wang, Y.; Zhang, X.; et al. A conductive PEDOT/alginate porous scaffold as a platform to modulate the biological behaviors of brown adipose-derived stem cells. Biomater. Sci. 2020, 8, 3173–3185. [Google Scholar] [CrossRef] [PubMed]
- Mihic, A.; Cui, Z.; Wu, J.; Vlacic, G.; Miyagi, Y.; Li, S.-H.; Lu, S.; Sung, H.-W.; Weisel, R.D.; Li, R.-K. A Conductive Polymer Hydrogel Supports Cell Electrical Signaling and Improves Cardiac Function After Implantation into Myocardial Infarct. Circulation 2015, 132, 772–784. [Google Scholar] [CrossRef]
- Zhang, C.; Hsieh, M.-H.; Wu, S.-Y.; Li, S.-H.; Wu, J.; Liu, S.-M.; Wei, H.-J.; Weisel, R.D.; Sung, H.-W.; Li, R.-K. A self-doping conductive polymer hydrogel that can restore electrical impulse propagation at myocardial infarct to prevent cardiac arrhythmia and preserve ventricular function. Biomaterials 2020, 231, 119672. [Google Scholar] [CrossRef] [PubMed]
- Dolphin, A.C.; Lee, A. Presynaptic calcium channels: Specialized control of synaptic neurotransmitter release. Nat. Rev. Neurosci. 2020, 21, 213–229. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Wang, N.; Lin, X.; Wang, Z.; Zhao, X.; Fang, P.; Yue, H.; Kim, J.; Luo, J.; Cui, S.; et al. Organic electrochemical transistor arrays for real-time mapping of evoked neurotransmitter release in vivo. eLife 2020, 9, e50345. [Google Scholar] [CrossRef]
- Kim, D.; Yokota, T.; Suzuki, T.; Lee, S.; Woo, T.; Yukita, W.; Koizumi, M.; Tachibana, Y.; Yawo, H.; Onodera, H.; et al. Ultraflexible organic light-emitting diodes for optogenetic nerve stimulation. Proc. Natl. Acad. Sci. USA 2020, 117, 21138–21146. [Google Scholar] [CrossRef]
- Sessler, C.D.; Zhou, Y.; Wang, W.; Hartley, N.D.; Fu, Z.; Graykowski, D.; Sheng, M.; Wang, X.; Liu, J. Optogenetic polymerization and assembly of electrically functional polymers for modulation of single-neuron excitability. Sci. Adv. 2022, 8, eade1136. [Google Scholar] [CrossRef]
- Rost, B.R.; Wietek, J.; Yizhar, O.; Schmitz, D. Optogenetics at the presynapse. Nat. Neurosci. 2022, 25, 984–998. [Google Scholar] [CrossRef]
- Jentsch, T.J. VRACs and other ion channels and transporters in the regulation of cell volume and beyond. Nat. Rev. Mol. Cell Biol. 2016, 17, 293–307. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Wang, M.; Zhao, S.; Zhao, Z.; Fang, J. Mechanotransduction pathways in the regulation of cartilage chondrocyte homoeostasis. J. Cell. Mol. Med. 2020, 24, 5408–5419. [Google Scholar] [CrossRef]
- Bootman, M.D.; Bultynck, G. Fundamentals of Cellular Calcium Signaling: A Primer. Cold Spring Harb. Perspect. Biol. 2020, 12, a038802. [Google Scholar] [CrossRef]
- Boulware, M.J.; Marchant, J.S. Timing in Cellular Ca2+ Signaling. Curr. Biol. 2008, 18, R769–R776. [Google Scholar] [CrossRef]
- Inácio, P.M.C.; Medeiros, M.C.R.; Carvalho, T.; Félix, R.C.; Mestre, A.; Hubbard, P.C.; Ferreira, Q.; Morgado, J.; Charas, A.; Freire, C.S.R.; et al. Ultra-low noise PEDOT:PSS electrodes on bacterial cellulose: A sensor to access bioelectrical signals in non-electrogenic cells. Org. Electron. 2020, 85, 105882. [Google Scholar] [CrossRef]
- Li, C.; Li, Z.; Nguyen, T.D.D.; Ingebrandt, S.; Vu, X.T. Real-time and multiplexed detection of sodium and potassium ions using PEDOT:PSS OECT microarrays integrated with ion-selective membranes. Electrochim. Acta 2024, 507, 145111. [Google Scholar] [CrossRef]
- Coppedè, N.; Giannetto, M.; Villani, M.; Lucchini, V.; Battista, E.; Careri, M.; Zappettini, A. Ion selective textile organic electrochemical transistor for wearable sweat monitoring. Org. Electron. 2020, 78, 105579. [Google Scholar] [CrossRef]
- Fabri, M.; Villa, M.; Stanczak, M.A.; Edwards-Hicks, J.; Corrado, M.; Pearce, E.L. Research Techniques Made Simple: Profiling Cellular Energy Metabolism. J. Investig. Dermatol. 2021, 141, 2767–2774.e2. [Google Scholar] [CrossRef]
- Lennicke, C.; Cochemé, H.M. Redox metabolism: ROS as specific molecular regulators of cell signaling and function. Mol. Cell 2021, 81, 3691–3707. [Google Scholar] [CrossRef]
- Chandel, N.S. Glycolysis. Cold Spring Harb. Perspect. Biol. 2021, 13, a040535. [Google Scholar] [CrossRef]
- Ahmad, M.; Wolberg, A.; Kahwaji, C.I. Biochemistry, Electron Transport Chain. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK526105/ (accessed on 8 October 2024).
- Fujisaki, H.; Watcharawittayakul, T.; Matsumoto, A.; Miyahara, Y.; Goda, T. In-situ chemical modification of printed conducting polymer films for specific glucose biosensing. Sens. Actuators B Chem. 2021, 349, 130829. [Google Scholar] [CrossRef]
- Naveen, M.H.; Gurudatt, N.G.; Shim, Y.-B. Applications of conducting polymer composites to electrochemical sensors: A review. Appl. Mater. Today 2017, 9, 419–433. [Google Scholar] [CrossRef]
- Sehit, E.; Altintas, Z. Significance of nanomaterials in electrochemical glucose sensors: An updated review (2016–2020). Biosens. Bioelectron. 2020, 159, 112165. [Google Scholar] [CrossRef]
- Rathee, K.; Dhull, V.; Dhull, R.; Singh, S. Biosensors based on electrochemical lactate detection: A comprehensive review. Biochem. Biophys. Rep. 2016, 5, 35–54. [Google Scholar] [CrossRef]
- Thirumalai, D.; Santhamoorthy, M.; Kim, S.-C.; Lim, H.-R. Conductive Polymer-Based Hydrogels for Wearable Electrochemical Biosensors. Gels 2024, 10, 459. [Google Scholar] [CrossRef]
- Lin, P.-H.; Sheu, S.-C.; Chen, C.-W.; Huang, S.-C.; Li, B.-R. Wearable hydrogel patch with noninvasive, electrochemical glucose sensor for natural sweat detection. Talanta 2022, 241, 123187. [Google Scholar] [CrossRef]
- Babeli, I.; Puiggalí-Jou, A.; Roa, J.J.; Ginebra, M.-P.; García-Torres, J.; Alemán, C. Hybrid conducting alginate-based hydrogel for hydrogen peroxide detection from enzymatic oxidation of lactate. Int. J. Biol. Macromol. 2021, 193, 1237–1248. [Google Scholar] [CrossRef]
- Aycan, D.; Karaca, F.; Alemdar, N. Development of hyaluronic acid-based electroconductive hydrogel as a sensitive non-enzymatic glucose sensor. Mater. Today Commun. 2023, 35, 105745. [Google Scholar] [CrossRef]
- Yao, C.-Y.; Qin, Y.; Fan, W.-T.; Yan, L.-P.; Chen, M.; Liu, Y.-L.; Huang, W.-H. A three-dimensional electrochemical biosensor integrated with hydrogel for cells culture and lactate release monitoring. J. Electroanal. Chem. 2022, 915, 116338. [Google Scholar] [CrossRef]
- Omar, F.S.; Duraisamy, N.; Ramesh, K.; Ramesh, S. Conducting polymer and its composite materials based electrochemical sensor for Nicotinamide Adenine Dinucleotide (NADH). Biosens. Bioelectron. 2016, 79, 763–775. [Google Scholar] [CrossRef]
- Xu, Z.; Qiao, X.; Tao, R.; Li, Y.; Zhao, S.; Cai, Y.; Luo, X. A wearable sensor based on multifunctional conductive hydrogel for simultaneous accurate pH and tyrosine monitoring in sweat. Biosens. Bioelectron. 2023, 234, 115360. [Google Scholar] [CrossRef]
- Marrero, D.; Guimera, A.; Maes, L.; Villa, R.; Alvarez, M.; Illa, X. Organ-on-a-chip with integrated semitransparent organic electrodes for barrier function monitoring. Lab. A Chip 2023, 23, 1825–1834. [Google Scholar] [CrossRef]
- Barron, S.L.; Oldroyd, S.V.; Saez, J.; Chernaik, A.; Guo, W.; McCaughan, F.; Bulmer, D.; Owens, R.M. A Conformable Organic Electronic Device for Monitoring Epithelial Integrity at the Air Liquid Interface. Adv. Mater. 2024, 36, 2306679. [Google Scholar] [CrossRef]
- Yeung, S.Y.; Gu, X.; Tsang, C.M.; Tsao, S.W.G.; Hsing, I. Organic electrochemical transistor array for monitoring barrier integrity of epithelial cells invaded by nasopharyngeal carcinoma. Sens. Actuators B Chem. 2019, 297, 126761. [Google Scholar] [CrossRef]
- Sun, C.; Bu, N.; Hu, X. Recent trends in electronic skin for transdermal drug delivery. Intell. Pharm. 2023, 1, 183–191. [Google Scholar] [CrossRef]
- Dong, R.; Ma, P.X.; Guo, B. Conductive biomaterials for muscle tissue engineering. Biomaterials 2020, 229, 119584. [Google Scholar] [CrossRef]
- Petty, A.J.I.; Keate, R.L.; Jiang, B.; Ameer, G.A.; Rivnay, J. Conducting Polymers for Tissue Regeneration in Vivo. Chem. Mater. 2020, 32, 4095–4115. [Google Scholar] [CrossRef]
- Min, J.H.; Patel, M.; Koh, W.-G. Incorporation of Conductive Materials into Hydrogels for Tissue Engineering Applications. Polymers 2018, 10, 1078. [Google Scholar] [CrossRef]
- Nasser, R.A.; Arya, S.S.; Alshehhi, K.H.; Teo, J.C.M.; Pitsalidis, C. Conducting polymer scaffolds: A new frontier in bioelectronics and bioengineering. Trends Biotechnol. 2024, 42, 760–779. [Google Scholar] [CrossRef]
- Saberi, A.; Jabbari, F.; Zarrintaj, P.; Saeb, M.R.; Mozafari, M. Electrically Conductive Materials: Opportunities and Challenges in Tissue Engineering. Biomolecules 2019, 9, 448. [Google Scholar] [CrossRef]
- Savva, A.; Saez, J.; Withers, A.; Barberio, C.; Stoeger, V.; Elias-Kirma, S.; Lu, Z.; Moysidou, C.-M.; Kallitsis, K.; Pitsalidis, C.; et al. 3D organic bioelectronics for electrical monitoring of human adult stem cells. Mater. Horiz. 2023, 10, 3589–3600. [Google Scholar] [CrossRef]
- Pitsalidis, C.; van Niekerk, D.; Moysidou, C.-M.; Boys, A.J.; Withers, A.; Vallet, R.; Owens, R.M. Organic electronic transmembrane device for hosting and monitoring 3D cell cultures. Sci. Adv. 2022, 8, eabo4761. [Google Scholar] [CrossRef]
- Kwon, H.J.; Lee, G.S.; Chun, H. Electrical stimulation drives chondrogenesis of mesenchymal stem cells in the absence of exogenous growth factors. Sci. Rep. 2016, 6, 39302. [Google Scholar] [CrossRef]
- Prasopthum, A.; Deng, Z.; Khan, I.M.; Yin, Z.; Guo, B.; Yang, J. Three dimensional printed degradable and conductive polymer scaffolds promote chondrogenic differentiation of chondroprogenitor cells. Biomater. Sci. 2020, 8, 4287–4298. [Google Scholar] [CrossRef]
- Björninen, M.; Gilmore, K.; Pelto, J.; Seppänen-Kaijansinkko, R.; Kellomäki, M.; Miettinen, S.; Wallace, G.; Grijpma, D.; Haimi, S. Electrically Stimulated Adipose Stem Cells on Polypyrrole-Coated Scaffolds for Smooth Muscle Tissue Engineering. Ann. Biomed. Eng. 2017, 45, 1015–1026. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Y.; Zhang, J.; Hu, X.; Yang, Z.; Guo, Y.; Wang, Y. In-situ doping of a conductive hydrogel with low protein absorption and bacterial adhesion for electrical stimulation of chronic wounds. Acta Biomater. 2019, 89, 217–226. [Google Scholar] [CrossRef]
- Feron, K.; Lim, R.; Sherwood, C.; Keynes, A.; Brichta, A.; Dastoor, P.C. Organic Bioelectronics: Materials and Biocompatibility. Int. J. Mol. Sci. 2018, 19, 2382. [Google Scholar] [CrossRef]
- Sukumaran, A.; Sweety, V.K.; Vikas, B.; Joseph, B.; Sukumaran, A.; Sweety, V.K.; Vikas, B.; Joseph, B. Cytotoxicity and Cell Viability Assessment of Biomaterials. In Cytotoxicity—Understanding Cellular Damage and Response; IntechOpen: London, UK, 2023; ISBN 978-1-80356-246-9. [Google Scholar]
- Asplund, M.; Thaning, E.; Lundberg, J.; Sandberg-Nordqvist, A.C.; Kostyszyn, B.; Inganäs, O.; von Holst, H. Toxicity evaluation of PEDOT/biomolecular composites intended for neural communication electrodes. Biomed. Mater. 2009, 4, 045009. [Google Scholar] [CrossRef]
- ISO 10993-1:2018; Biological Evaluation of Medical Devices. ISO: Geneva, Switzerland, 2018.
- Gad, S.C. Device Safety Evaluation. In Safety Evaluation of Pharmaceuticals and Medical Devices: International Regulatory Guidelines; Gad, S.C., Ed.; Springer US: Boston, MA, USA, 2011; pp. 91–112. ISBN 978-1-4419-7449-5. [Google Scholar]
- Wang, C.; Yokota, T.; Someya, T. Natural Biopolymer-Based Biocompatible Conductors for Stretchable Bioelectronics. Chem. Rev. 2021, 121, 2109–2146. [Google Scholar] [CrossRef]
- Kenry; Liu, B. Recent Advances in Biodegradable Conducting Polymers and Their Biomedical Applications. Biomacromolecules 2018, 19, 1783–1803. [Google Scholar] [CrossRef]
- Nezakati, T.; Seifalian, A.; Tan, A.; Seifalian, A.M. Conductive Polymers: Opportunities and Challenges in Biomedical Applications. Chem. Rev. 2018, 118, 6766–6843. [Google Scholar] [CrossRef]
- Zahabi, N.; Zozoulenko, I. Band Versus Hopping Transport in Conducting Polymers by Ab Initio Molecular Dynamics: Exploring the Effect of Electric Field, Trapping and Temperature. Adv. Electron. Mater. 2024, 11, 2400239. [Google Scholar] [CrossRef]
- Kim, J.; Guo, J.; Sini, G.; Sørensen, M.K.; Andreasen, J.W.; Woon, K.L.; Coropceanu, V.; Paleti, S.H.K.; Wei, H.; Peralta, S.; et al. Remarkable conductivity enhancement in P-doped polythiophenes via rational engineering of polymer-dopant interactions. Mater. Today Adv. 2023, 18, 100360. [Google Scholar] [CrossRef]
- Ding, H.; Hussein, A.M.; Ahmad, I.; Latef, R.; Abbas, J.K.; Ali, A.T.A.; Saeed, S.M.; Abdulwahid, A.S.; Ramadan, M.F.; Rasool, H.A.; et al. Conducting polymers in industry: A comprehensive review on the characterization, synthesis and application. Alex. Eng. J. 2024, 88, 253–267. [Google Scholar] [CrossRef]
- Letheby, H. XXIX.—On the production of a blue substance by the electrolysis of sulphate of aniline. J. Chem. Soc. 1862, 15, 161–163. [Google Scholar] [CrossRef]
- Kayser, L.V.; Lipomi, D.J. Stretchable Conductive Polymers and Composites Based on PEDOT and PEDOT:PSS. Adv. Mater. 2019, 31, 1806133. [Google Scholar] [CrossRef]
- Sun, K.; Zhang, S.; Li, P.; Xia, Y.; Zhang, X.; Du, D.; Isikgor, F.; Ouyang, J. Review on application of PEDOTs and PEDOT:PSS in energy conversion and storage devices. J. Mater. Sci. Mater. Electron. 2015, 26, 1–25. [Google Scholar] [CrossRef]
- Seiti, M.; Giuri, A.; Corcione, C.E.; Ferraris, E. Advancements in tailoring PEDOT: PSS properties for bioelectronic applications: A comprehensive review. Biomater. Adv. 2023, 154, 213655. [Google Scholar] [CrossRef]
- Lu, B.; Yuk, H.; Lin, S.; Jian, N.; Qu, K.; Xu, J.; Zhao, X. Pure PEDOT:PSS hydrogels. Nat. Commun. 2019, 10, 1043. [Google Scholar] [CrossRef]
- Kim, S.-M.; Kim, C.-H.; Kim, Y.; Kim, N.; Lee, W.-J.; Lee, E.-H.; Kim, D.; Park, S.; Lee, K.; Rivnay, J.; et al. Influence of PEDOT:PSS crystallinity and composition on electrochemical transistor performance and long-term stability. Nat. Commun. 2018, 9, 3858. [Google Scholar] [CrossRef]
- Ahmad Shahrim, N.; Ahmad, Z.; Azman, A.W.; Buys, Y.F.; Sarifuddin, N. Mechanisms for doped PEDOT:PSS electrical conductivity improvement. Mater. Adv. 2021, 2, 7118–7138. [Google Scholar] [CrossRef]
- Yeon, C.; Yun, S.J.; Kim, J.; Lim, J.W. PEDOT:PSS Films with Greatly Enhanced Conductivity via Nitric Acid Treatment at Room Temperature and Their Application as Pt/TCO-Free Counter Electrodes in Dye-Sensitized Solar Cells. Adv. Electron. Mater. 2015, 1, 1500121. [Google Scholar] [CrossRef]
- Stříteský, S.; Marková, A.; Víteček, J.; Šafaříková, E.; Hrabal, M.; Kubáč, L.; Kubala, L.; Weiter, M.; Vala, M. Printing inks of electroactive polymer PEDOT:PSS: The study of biocompatibility, stability, and electrical properties. J. Biomed. Mater. Res. Part. A 2018, 106, 1121–1128. [Google Scholar] [CrossRef]
- Yazdimamaghani, M.; Razavi, M.; Mozafari, M.; Vashaee, D.; Kotturi, H.; Tayebi, L. Biomineralization and biocompatibility studies of bone conductive scaffolds containing poly(3,4-ethylenedioxythiophene):poly(4-styrene sulfonate) (PEDOT:PSS). J. Mater. Sci. Mater. Med. 2015, 26, 274. [Google Scholar] [CrossRef]
- Medagoda, D.I.; Ghezzi, D. Organic semiconductors for light-mediated neuromodulation. Commun. Mater. 2021, 2, 1–7. [Google Scholar] [CrossRef]
- Yang, K.; Oh, J.Y.; Lee, J.S.; Jin, Y.; Chang, G.-E.; Chae, S.S.; Cheong, E.; Baik, H.K.; Cho, S.-W. Photoactive Poly(3-hexylthiophene) Nanoweb for Optoelectrical Stimulation to Enhance Neurogenesis of Human Stem Cells. Theranostics 2017, 7, 4591–4604. [Google Scholar] [CrossRef]
- Giovannitti, A.; Nielsen, C.B.; Sbircea, D.-T.; Inal, S.; Donahue, M.; Niazi, M.R.; Hanifi, D.A.; Amassian, A.; Malliaras, G.G.; Rivnay, J.; et al. N-type organic electrochemical transistors with stability in water. Nat. Commun. 2016, 7, 13066. [Google Scholar] [CrossRef]
- Giovannitti, A.; Sbircea, D.-T.; Inal, S.; Nielsen, C.B.; Bandiello, E.; Hanifi, D.A.; Sessolo, M.; Malliaras, G.G.; McCulloch, I.; Rivnay, J. Controlling the mode of operation of organic transistors through side-chain engineering. Proc. Natl. Acad. Sci. USA 2016, 113, 12017–12022. [Google Scholar] [CrossRef]
- Lang, U.; Naujoks, N.; Dual, J. Mechanical characterization of PEDOT:PSS thin films. Synth. Met. 2009, 159, 473–479. [Google Scholar] [CrossRef]
- Kaur, G.; Adhikari, R.; Cass, P.; Bown, M.; Gunatillake, P. Electrically conductive polymers and composites for biomedical applications. RSC Adv. 2015, 5, 37553–37567. [Google Scholar] [CrossRef]
- Pang, A.L.; Arsad, A.; Ahmadipour, M. Synthesis and factor affecting on the conductivity of polypyrrole: A short review. Polym. Adv. Technol. 2021, 32, 1428–1454. [Google Scholar] [CrossRef]
- Guanggui, C.; Jianning, D.; Zhongqiang, Z.; Zhiyong, L.; Huasheng, P. Study on the preparation and multiproperties of the polypyrrole films doped with different ions. Surf. Interface Anal. 2012, 44, 844–850. [Google Scholar] [CrossRef]
- Otero, T.F.; López Cascales, J.J.; Vázquez Arenas, G. Mechanical characterization of free-standing polypyrrole film. Mater. Sci. Eng. C 2007, 27, 18–22. [Google Scholar] [CrossRef]
- Ramtin, A.; Seyfoddin, A.; Coutinho, F.P.; Waterhouse, G.I.N.; Rupenthal, I.D.; Svirskis, D. Cytotoxicity considerations and electrically tunable release of dexamethasone from polypyrrole for the treatment of back-of-the-eye conditions. Drug Deliv. Transl. Res. 2016, 6, 793–799. [Google Scholar] [CrossRef]
- Zhang, B.G.X.; Spinks, G.M.; Gorkin, R.; Sangian, D.; Di Bella, C.; Quigley, A.F.; Kapsa, R.M.I.; Wallace, G.G.; Choong, P.F.M. In vivo biocompatibility of porous and non-porous polypyrrole based trilayered actuators. J. Mater. Sci. Mater. Med. 2017, 28, 172. [Google Scholar] [CrossRef]
- Kumar, A.M.; Suresh, B.; Das, S.; Obot, I.B.; Adesina, A.Y.; Ramakrishna, S. Promising bio-composites of polypyrrole and chitosan: Surface protective and in vitro biocompatibility performance on 316L SS implants. Carbohydr. Polym. 2017, 173, 121–130. [Google Scholar] [CrossRef]
- Majeed, A.H.; Mohammed, L.A.; Hammoodi, O.G.; Sehgal, S.; Alheety, M.A.; Saxena, K.K.; Dadoosh, S.A.; Mohammed, I.K.; Jasim, M.M.; Salmaan, N.U. A Review on Polyaniline: Synthesis, Properties, Nanocomposites, and Electrochemical Applications. Int. J. Polym. Sci. 2022, 2022, 9047554. [Google Scholar] [CrossRef]
- Beygisangchin, M.; Abdul Rashid, S.; Shafie, S.; Sadrolhosseini, A.R.; Lim, H.N. Preparations, Properties, and Applications of Polyaniline and Polyaniline Thin Films—A Review. Polymers 2021, 13, 2003. [Google Scholar] [CrossRef]
- Bahramian, A. A molecular view on a polyaniline–TiO2 nanostructured thin film: Effect of temperature and pressure on the thermal, mechanical, and dynamical properties. Thin Solid. Film. 2015, 592, 39–53. [Google Scholar] [CrossRef]
- Kašpárková, V.; Humpolíček, P.; Stejskal, J.; Capáková, Z.; Bober, P.; Skopalová, K.; Lehocký, M. Exploring the Critical Factors Limiting Polyaniline Biocompatibility. Polymers 2019, 11, 362. [Google Scholar] [CrossRef]
- Neusser, D.; Malacrida, C.; Kern, M.; Gross, Y.M.; van Slageren, J.; Ludwigs, S. High Conductivities of Disordered P3HT Films by an Electrochemical Doping Strategy. Chem. Mater. 2020, 32, 6003–6013. [Google Scholar] [CrossRef]
- Mefferd, B.E.; Nambiar, V.V.; Lu, H.; Stefan, M.C. Viscoelastic Characterization of Poly(3-hexylthiophene): Determination of Young’s Modulus. ACS Appl. Polym. Mater. 2023, 5, 6318–6324. [Google Scholar] [CrossRef]
- Šafaříková, E.; Švihálková Šindlerová, L.; Stříteský, S.; Kubala, L.; Vala, M.; Weiter, M.; Víteček, J. Evaluation and improvement of organic semiconductors’ biocompatibility towards fibroblasts and cardiomyocytes. Sens. Actuators B Chem. 2018, 260, 418–425. [Google Scholar] [CrossRef]
- Scarpa, G.; Idzko, A.-L.; Götz, S.; Thalhammer, S. Biocompatibility Studies of Functionalized Regioregular Poly(3-hexylthiophene) Layers for Sensing Applications. Macromol. Biosci. 2010, 10, 378–383. [Google Scholar] [CrossRef]
- Kiefer, D.; Kroon, R.; Hofmann, A.I.; Sun, H.; Liu, X.; Giovannitti, A.; Stegerer, D.; Cano, A.; Hynynen, J.; Yu, L.; et al. Double doping of conjugated polymers with monomer molecular dopants. Nat. Mater. 2019, 18, 149–155. [Google Scholar] [CrossRef]
- Yoon, S.E.; Kang, Y.; Im, J.; Lee, J.; Lee, S.Y.; Park, J.; Gao, Y.J.; Jeon, D.; Son, J.Y.; Kim, J.; et al. Enhancing dopant diffusion for ultrahigh electrical conductivity and efficient thermoelectric conversion in conjugated polymers. Joule 2023, 7, 2291–2317. [Google Scholar] [CrossRef]
- Uguz, I.; Ohayon, D.; Arslan, V.; Sheelamanthula, R.; Griggs, S.; Hama, A.; Stanton, J.W.; McCulloch, I.; Inal, S.; Shepard, K.L. Flexible switch matrix addressable electrode arrays with organic electrochemical transistor and pn diode technology. Nat. Commun. 2024, 15, 533. [Google Scholar] [CrossRef]
- Inal, S.; Malliaras, G.G.; Rivnay, J. Benchmarking organic mixed conductors for transistors. Nat. Commun. 2017, 8, 1767. [Google Scholar] [CrossRef]
- Irimia-Vladu, M.; Glowacki, E.D.; Sariciftci, N.S.; Bauer, S. Green Materials for Electronics, 1st ed.; Wiley: Hoboken, NJ, USA, 2017; ISBN 978-3-527-33865-8. [Google Scholar]
- Rivnay, J.; Owens, R.M.; Malliaras, G.G. The Rise of Organic Bioelectronics. Chem. Mater. 2014, 26, 679–685. [Google Scholar] [CrossRef]
- Kim, D.-H.; Richardson-Burns, S.; Povlich, L.; Abidian, M.R.; Spanninga, S.; Hendricks, J.L.; Martin, D.C. Soft, Fuzzy, and Bioactive Conducting Polymers for Improving the Chronic Performance of Neural Prosthetic Devices. In Indwelling Neural Implants: Strategies for Contending with the In Vivo Environment; Reichert, W.M., Ed.; Frontiers in Neuroengineering; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2008; ISBN 978-0-8493-9362-4. Available online: http://www.ncbi.nlm.nih.gov/books/NBK3940/ (accessed on 18 November 2024).
- Hopcroft, M.A.; Nix, W.D.; Kenny, T.W. What is the Young’s Modulus of Silicon? J. Microelectromech. Syst. 2010, 19, 229–238. [Google Scholar] [CrossRef]
- Isogai, K.; Okamoto, S.; Asaba, T.; Ogusu, S.; Shimizu, Y.; Watanabe, T.; Yamada, Y. Young’s moduli of subcutaneous tissues and muscles under different loads at the gluteal region calculated using ultrasonography. J. Phys. Ther. Sci. 2022, 34, 777–783. [Google Scholar] [CrossRef]
- Jazvinšćak Jembrek, M.; Simic, G.; Hof, P.; Šegota, S. Atomic force microscopy as an advanced tool in neuroscience. Transl. Neurosci. 2015, 6, 117. [Google Scholar] [CrossRef]
- Jeong, J.-W.; Shin, G.; Park, S.I.; Yu, K.J.; Xu, L.; Rogers, J.A. Soft materials in neuroengineering for hard problems in neuroscience. Neuron 2015, 86, 175–186. [Google Scholar] [CrossRef]
- Kabir, W.; Di Bella, C.; Choong, P.F.M.; O’Connell, C.D. Assessment of Native Human Articular Cartilage: A Biomechanical Protocol. Cartilage 2021, 13, 427S–437S. [Google Scholar] [CrossRef]
- Kim, N.; Lienemann, S.; Petsagkourakis, I.; Alemu Mengistie, D.; Kee, S.; Ederth, T.; Gueskine, V.; Leclère, P.; Lazzaroni, R.; Crispin, X.; et al. Elastic conducting polymer composites in thermoelectric modules. Nat. Commun. 2020, 11, 1424. [Google Scholar] [CrossRef]
- Maddali, H.; House, K.L.; Emge, T.J.; O’Carroll, D.M. Identification of the local electrical properties of crystalline and amorphous domains in electrochemically doped conjugated polymer thin films. RSC Adv. 2020, 10, 21454–21463. [Google Scholar] [CrossRef]
- Qu, J.; Ouyang, L.; Kuo, C.; Martin, D.C. Stiffness, strength and adhesion characterization of electrochemically deposited conjugated polymer films. Acta Biomater. 2016, 31, 114–121. [Google Scholar] [CrossRef]
- Rho, J.Y.; Ashman, R.B.; Turner, C.H. Young’s modulus of trabecular and cortical bone material: Ultrasonic and microtensile measurements. J. Biomech. 1993, 26, 111–119. [Google Scholar] [CrossRef]
- Rivnay, J.; Wang, H.; Fenno, L.; Deisseroth, K.; Malliaras, G.G. Next-generation probes, particles, and proteins for neural interfacing. Sci. Adv. 2017, 3, e1601649. [Google Scholar] [CrossRef]
- Salvadori, M.C.; Brown, I.G.; Vaz, A.R.; Melo, L.L.; Cattani, M. Measurement of the elastic modulus of nanostructured gold and platinum thin films. Phys. Rev. B 2003, 67, 153404. [Google Scholar] [CrossRef]
- Liao, C.; Zhang, M.; Yao, M.Y.; Hua, T.; Li, L.; Yan, F. Flexible Organic Electronics in Biology: Materials and Devices. Adv. Mater. 2015, 27, 7493–7527. [Google Scholar] [CrossRef]
- Schwartz, G.; Tee, B.C.-K.; Mei, J.; Appleton, A.L.; Kim, D.H.; Wang, H.; Bao, Z. Flexible polymer transistors with high pressure sensitivity for application in electronic skin and health monitoring. Nat. Commun. 2013, 4, 1859. [Google Scholar] [CrossRef]
- Chen, M.; Qin, Y.; Fan, W.-T.; Yan, J.; Hong, F.; Huang, W.-H.; Liu, Y.-L. Three-Dimensional Stretchable Sensor-Hydrogel Integrated Platform for Cardiomyocyte Culture and Mechanotransduction Monitoring. Anal. Chem. 2023, 95, 12859–12866. [Google Scholar] [CrossRef]
- Airaghi Leccardi, M.J.I.; Desbiolles, B.X.E.; Haddad, A.Y.; Joy, B.C.; Song, C.; Sarkar, D. Light-induced rolling of azobenzene polymer thin films for wrapping subcellular neuronal structures. Commun. Chem. 2024, 7, 249. [Google Scholar] [CrossRef]
- Inal, S.; Hama, A.; Ferro, M.; Pitsalidis, C.; Oziat, J.; Iandolo, D.; Pappa, A.-M.; Hadida, M.; Huerta, M.; Marchat, D.; et al. Conducting Polymer Scaffolds for Hosting and Monitoring 3D Cell Culture. Adv. Biosyst. 2017, 1, 1700052. [Google Scholar] [CrossRef]
- Jayaram, A.K.; Pitsalidis, C.; Tan, E.; Moysidou, C.-M.; De Volder, M.F.L.; Kim, J.-S.; Owens, R.M. 3D Hybrid Scaffolds Based on PEDOT:PSS/MWCNT Composites. Front. Chem. 2019, 7, 363. [Google Scholar] [CrossRef]
- Taherian, R. The Theory of Electrical Conductivity. In Electrical Conductivity in Polymer-Based Composites: Experiments, Modelling and Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–18. ISBN 978-0-12-812541-0. [Google Scholar]
- Mbayachi, V.B.; Ndayiragije, E.; Sammani, T.; Taj, S.; Mbuta, E.R.; Khan, A. ullah Graphene synthesis, characterization and its applications: A review. Results Chem. 2021, 3, 100163. [Google Scholar] [CrossRef]
- Jia, L.; Zhou, S.; Ahmed, A.; Yang, Z.; Liu, S.; Wang, H.; Li, F.; Zhang, M.; Zhang, Y.; Sun, L. Tuning MXene electrical conductivity towards multifunctionality. Chem. Eng. J. 2023, 475, 146361. [Google Scholar] [CrossRef]
- Kokil, A.; Yang, K.; Kumar, J. Techniques for characterization of charge carrier mobility in organic semiconductors. J. Polym. Sci. Part. B Polym. Phys. 2012, 50, 1130–1144. [Google Scholar] [CrossRef]
- Babel, A.; Jenekhe, S.A. High Electron Mobility in Ladder Polymer Field-Effect Transistors. J. Am. Chem. Soc. 2003, 125, 13656–13657. [Google Scholar] [CrossRef]
- Cai, H.-T.; Xu, H.; Tang, C.; Li, J.; Yang, Z.-Y.; Ye, S.-H.; Huang, W. Intrinsic ambipolar transport for the traditional conducting or hole transport ionic blend polymer PEDOT:PSS. Polymer 2019, 180, 121732. [Google Scholar] [CrossRef]
- Adesakin, G.E.; Akande, T.H.; Olubosede, O.; Edema, O.G.; Akinbolusere, A.O.; Aliyu, E.O.; Adekoya, M.A.; Fatigun, A.T. Current Density, Electron Mobility and Drift Velocity Of Metals. J. Adv. Res. Dyn. Control Syst. 2019, 11, 1986–1995. [Google Scholar]
- Urade, A.R.; Lahiri, I.; Suresh, K.S. Graphene Properties, Synthesis and Applications: A Review. JOM 2023, 75, 614–630. [Google Scholar] [CrossRef] [PubMed]
- Salim, O.; Mahmoud, K.A.; Pant, K.K.; Joshi, R.K. Introduction to MXenes: Synthesis and characteristics. Mater. Today Chem. 2019, 14, 100191. [Google Scholar] [CrossRef]
- Kalkman, A.J.; Verbruggen, A.H.; Janssen, G.C.A.M. Young’s modulus measurements and grain boundary sliding in free-standing thin metal films. Appl. Phys. Lett. 2001, 78, 2673–2675. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Lipatov, A.; Lu, H.; Alhabeb, M.; Anasori, B.; Gruverman, A.; Gogotsi, Y.; Sinitskii, A. Elastic properties of 2D Ti3C2Tx MXene monolayers and bilayers. Sci. Adv. 2018, 4, eaat0491. [Google Scholar] [CrossRef]
- Guo, X.; Facchetti, A. The journey of conducting polymers from discovery to application. Nat. Mater. 2020, 19, 922–928. [Google Scholar] [CrossRef]
- Kang, S.; Rahman, A.; McGinnis, S.; Vikesland, P. Toward environmentally favorable nano-sensing by production of reusable gold nanoparticles from gold nano-waste: Life cycle and nanocircular economy implications. Environ. Sci. Nano 2024, 11, 1499–1507. [Google Scholar] [CrossRef]
- Tang, X.; Ng, H.Y. Cobalt and nitrogen-doped carbon catalysts for enhanced oxygen reduction and power production in microbial fuel cells. Electrochim. Acta 2017, 247, 193–199. [Google Scholar] [CrossRef]
- Firmansyah, M.L.; Yoshida, W.; Hanada, T.; Goto, M. Application of Ionic Liquids in Solvent Extraction of Platinum Group Metals. SERDJ 2020, 27, 1–24. [Google Scholar] [CrossRef]
- Islam, A.; Mukherjee, B.; Pandey, K.K.; Keshri, A.K. Ultra-Fast, Chemical-Free, Mass Production of High Quality Exfoliated Graphene. ACS Nano 2021, 15, 1775–1784. [Google Scholar] [CrossRef]
- Zaed, M.A.; Tan, K.H.; Abdullah, N.; Saidur, R.; Pandey, A.K.; Saleque, A.M. Cost analysis of MXene for low-cost production, and pinpointing of its economic footprint. Open Ceram. 2024, 17, 100526. [Google Scholar] [CrossRef]
- Amdursky, N.; Głowacki, E.D.; Meredith, P. Macroscale Biomolecular Electronics and Ionics. Adv. Mater. 2019, 31, 1802221. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, B.D.; Tybrandt, K.; Stavrinidou, E.; Rivnay, J. Organic mixed ionic–electronic conductors. Nat. Mater. 2020, 19, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Savva, A.; Wustoni, S.; Inal, S. Ionic-to-electronic coupling efficiency in PEDOT:PSS films operated in aqueous electrolytes. J. Mater. Chem. C 2018, 6, 12023–12030. [Google Scholar] [CrossRef]
- Rivnay, J.; Inal, S.; Collins, B.A.; Sessolo, M.; Stavrinidou, E.; Strakosas, X.; Tassone, C.; Delongchamp, D.M.; Malliaras, G.G. Structural control of mixed ionic and electronic transport in conducting polymers. Nat. Commun. 2016, 7, 11287. [Google Scholar] [CrossRef]
- Ruiz, C.; García-Frutos, E.M.; Hennrich, G.; Gómez-Lor, B. Organic Semiconductors toward Electronic Devices: High Mobility and Easy Processability. J. Phys. Chem. Lett. 2012, 3, 1428–1436. [Google Scholar] [CrossRef]
- Bao, Z.; Feng, Y.; Dodabalapur, A.; Raju, V.R.; Lovinger, A.J. High-Performance Plastic Transistors Fabricated by Printing Techniques. Chem. Mater. 1997, 9, 1299–1301. [Google Scholar] [CrossRef]
- Won, D.; Bang, J.; Choi, S.H.; Pyun, K.R.; Jeong, S.; Lee, Y.; Ko, S.H. Transparent Electronics for Wearable Electronics Application. Chem. Rev. 2023, 123, 9982–10078. [Google Scholar] [CrossRef]
- Koutsouras, D.A.; Malliaras, G.G.; Langereis, G. The rise of bioelectronic medicine. Bioelectron. Med. 2024, 10, 19. [Google Scholar] [CrossRef]
- Harris, A.R.; Morgan, S.J.; Chen, J.; Kapsa, R.M.I.; Wallace, G.G.; Paolini, A.G. Conducting polymer coated neural recording electrodes. J. Neural Eng. 2013, 10, 016004. [Google Scholar] [CrossRef]
- Traficante, D.D. Impedance: What it is, and why it must be matched. Concepts Magn. Reson. 1989, 1, 73–92. [Google Scholar] [CrossRef]
- Koutsouras, D.A.; Hama, A.; Pas, J.; Gkoupidenis, P.; Hivert, B.; Faivre-Sarrailh, C.; Di Pasquale, E.; Owens, R.M.; Malliaras, G.G. PEDOT:PSS microelectrode arrays for hippocampal cell culture electrophysiological recordings. MRS Commun. 2017, 7, 259–265. [Google Scholar] [CrossRef]
- Martinez, J.; Pedreira, C.; Ison, M.J.; Quian Quiroga, R. Realistic simulation of extracellular recordings. J. Neurosci. Methods 2009, 184, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Hierlemann, A.; Frey, U. Extracellular Recording of Entire Neural Networks Using a Dual-Mode Microelectrode Array With 19 584 Electrodes and High SNR. IEEE J. Solid-State Circuits 2021, 56, 2466–2475. [Google Scholar] [CrossRef]
- Lu, Z.; Pavia, A.; Savva, A.; Kergoat, L.; Owens, R.M. Organic microelectrode arrays for bioelectronic applications. Mater. Sci. Eng. R Rep. 2023, 153, 100726. [Google Scholar] [CrossRef]
- Rowley-Neale, S.J.; Banks, C.E. Biosensors—Microelectrode Design and Operation. In Encyclopedia of Interfacial Chemistry; Wandelt, K., Ed.; Elsevier: Oxford, UK, 2018; pp. 72–80. ISBN 978-0-12-809894-3. [Google Scholar]
- Heuschkel, M.O.; Fejtl, M.; Raggenbass, M.; Bertrand, D.; Renaud, P. A three-dimensional multi-electrode array for multi-site stimulation and recording in acute brain slices. J. Neurosci. Methods 2002, 114, 135–148. [Google Scholar] [CrossRef]
- Ferrea, E.; Maccione, A.; Medrihan, L.; Nieus, T.; Ghezzi, D.; Baldelli, P.; Benfenati, F.; Berdondini, L. Large-scale, high-resolution electrophysiological imaging of field potentials in brain slices with microelectronic multielectrode arrays. Front. Neural Circuits 2012, 6, 36627. [Google Scholar] [CrossRef]
- Aqrawe, Z.; Montgomery, J.; Travas-Sejdic, J.; Svirskis, D. Conducting polymers for neuronal microelectrode array recording and stimulation. Sens. Actuators B Chem. 2018, 257, 753–765. [Google Scholar] [CrossRef]
- Koutsouras, D.A.; Gkoupidenis, P.; Stolz, C.; Subramanian, V.; Malliaras, G.G.; Martin, D.C. Impedance Spectroscopy of Spin-Cast and Electrochemically Deposited PEDOT:PSS Films on Microfabricated Electrodes with Various Areas. ChemElectroChem 2017, 4, 2321–2327. [Google Scholar] [CrossRef]
- Newman, J. Resistance for Flow of Current to a Disk. J. Electrochem. Soc. 1966, 113, 501. [Google Scholar] [CrossRef]
- Ludwig, K.A.; Uram, J.D.; Yang, J.; Martin, D.C.; Kipke, D.R. Chronic neural recordings using silicon microelectrode arrays electrochemically deposited with a poly(3,4-ethylenedioxythiophene) (PEDOT) film. J. Neural Eng. 2006, 3, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.M.; Boehler, C.; Liljemalm, R.; Fries, P.; Stieglitz, T.; Asplund, M. Recording Quality Is Systematically Related to Electrode Impedance. Adv. Healthc. Mater. 2024, 13, 2303401. [Google Scholar] [CrossRef]
- Ludwig, K.A.; Langhals, N.B.; Joseph, M.D.; Richardson-Burns, S.M.; Hendricks, J.L.; Kipke, D.R. Poly(3,4-ethylenedioxythiophene) (PEDOT) polymer coatings facilitate smaller neural recording electrodes. J. Neural Eng. 2011, 8, 014001. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Bu, H.; Vinet, M.; Cao, M.; Takagi, S.; Hwang, S.; Ghani, T.; Banerjee, K. The future transistors. Nature 2023, 620, 501–515. [Google Scholar] [CrossRef]
- Torricelli, F.; Adrahtas, D.Z.; Bao, Z.; Berggren, M.; Biscarini, F.; Bonfiglio, A.; Bortolotti, C.A.; Frisbie, C.D.; Macchia, E.; Malliaras, G.G.; et al. Electrolyte-gated transistors for enhanced performance bioelectronics. Nat. Rev. Methods Primers 2021, 1, 66. [Google Scholar] [CrossRef]
- Kang, H.; Kim, J.-Y.; Choi, Y.-K.; Nam, Y. Feasibility Study of Extended-Gate-Type Silicon Nanowire Field-Effect Transistors for Neural Recording. Sensors 2017, 17, 705. [Google Scholar] [CrossRef] [PubMed]
- Rivnay, J.; Inal, S.; Salleo, A.; Owens, R.M.; Berggren, M.; Malliaras, G.G. Organic electrochemical transistors. Nat. Rev. Mater. 2018, 3, 1–14. [Google Scholar] [CrossRef]
- Devoret, M.H.; Schoelkopf, R.J. Amplifying quantum signals with the single-electron transistor. Nature 2000, 406, 1039–1046. [Google Scholar] [CrossRef]
- Kim, S.H.; Hong, K.; Xie, W.; Lee, K.H.; Zhang, S.; Lodge, T.P.; Frisbie, C.D. Electrolyte-Gated Transistors for Organic and Printed Electronics. Adv. Mater. 2013, 25, 1822–1846. [Google Scholar] [CrossRef]
- Spanu, A.; Martines, L.; Bonfiglio, A. Interfacing cells with organic transistors: A review of in vitro and in vivo applications. Lab. Chip 2021, 21, 795–820. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, S.-M.; Kim, G.; Yoon, M.-H. Designing Polymeric Mixed Conductors and Their Application to Electrochemical-Transistor-Based Biosensors. Macromol. Biosci. 2020, 20, e2000211. [Google Scholar] [CrossRef] [PubMed]
- Mohd Razip Wee, M.F.; Al-Hardan, N.H.; Masood, A.; Hamid, M.A.A.; Jalar, A.; Ahmed, N.M. The Physics and Operating Principles of Field-effect Transistor-based Biosensors. In Field-Effect Transistor Biosensors for Rapid Pathogen Detection; Royal Society of Chemistry: London, UK, 2024. [Google Scholar] [CrossRef]
- Shinagawa, T.; Takanabe, K. Towards Versatile and Sustainable Hydrogen Production through Electrocatalytic Water Splitting: Electrolyte Engineering. ChemSusChem 2017, 10, 1318–1336. [Google Scholar] [CrossRef]
- Berto, M.; Diacci, C.; D’Agata, R.; Pinti, M.; Bianchini, E.; Lauro, M.D.; Casalini, S.; Cossarizza, A.; Berggren, M.; Simon, D.; et al. EGOFET Peptide Aptasensor for Label-Free Detection of Inflammatory Cytokines in Complex Fluids. Adv. Biosyst. 2018, 2, 1700072. [Google Scholar] [CrossRef]
- Khodagholy, D.; Rivnay, J.; Sessolo, M.; Gurfinkel, M.; Leleux, P.; Jimison, L.H.; Stavrinidou, E.; Herve, T.; Sanaur, S.; Owens, R.M.; et al. High transconductance organic electrochemical transistors. Nat. Commun. 2013, 4, 2133. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, J.B.; Raut, P.; Kumar, S. Organic Electronics in Biosensing: A Promising Frontier for Medical and Environmental Applications. Biosensors 2023, 13, 976. [Google Scholar] [CrossRef]
- Donahue, M.J.; Williamson, A.; Strakosas, X.; Friedlein, J.T.; McLeod, R.R.; Gleskova, H.; Malliaras, G.G. High-Performance Vertical Organic Electrochemical Transistors. Adv. Mater. 2018, 30, 1705031. [Google Scholar] [CrossRef]
- Huang, W.; Chen, J.; Yao, Y.; Zheng, D.; Ji, X.; Feng, L.-W.; Moore, D.; Glavin, N.R.; Xie, M.; Chen, Y.; et al. Vertical organic electrochemical transistors for complementary circuits. Nature 2023, 613, 496–502. [Google Scholar] [CrossRef]
- Koutsouras, D.A.; Torricelli, F.; Blom, P.W.M. Submicron Vertical Channel Organic Electrochemical Transistors with Ultrahigh Transconductance. Adv. Electron. Mater. 2023, 9, 2200868. [Google Scholar] [CrossRef]
- Lin, P.; Yan, F.; Yu, J.; Chan, H.L.W.; Yang, M. The Application of Organic Electrochemical Transistors in Cell-Based Biosensors. Adv. Mater. 2010, 22, 3655–3660. [Google Scholar] [CrossRef]
- Berggren, M.; Głowacki, E.D.; Simon, D.T.; Stavrinidou, E.; Tybrandt, K. In Vivo Organic Bioelectronics for Neuromodulation. Chem. Rev. 2022, 122, 4826–4846. [Google Scholar] [CrossRef]
- Strakosas, X.; Seitanidou, M.; Tybrandt, K.; Berggren, M.; Simon, D.T. An electronic proton-trapping ion pump for selective drug delivery. Sci. Adv. 2021, 7, eabd8738. [Google Scholar] [CrossRef]
- Isaksson, J.; Kjäll, P.; Nilsson, D.; Robinson, N.; Berggren, M.; Richter-Dahlfors, A. Electronic control of Ca2+ signalling in neuronal cells using an organic electronic ion pump. Nat. Mater. 2007, 6, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, A.; Song, Z.; Nilsson, D.; Meyerson, B.A.; Simon, D.T.; Linderoth, B.; Berggren, M. Therapy using implanted organic bioelectronics. Sci. Adv. 2015, 1, e1500039. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, A.; Sjöström, T.A.; Tybrandt, K.; Berggren, M.; Simon, D.T. Chemical delivery array with millisecond neurotransmitter release. Sci. Adv. 2016, 2, e1601340. [Google Scholar] [CrossRef]
- Martino, N.; Feyen, P.; Porro, M.; Bossio, C.; Zucchetti, E.; Ghezzi, D.; Benfenati, F.; Lanzani, G.; Antognazza, M.R. Photothermal cellular stimulation in functional bio-polymer interfaces. Sci. Rep. 2015, 5, 8911. [Google Scholar] [CrossRef]
- Ðerek, V.; Rand, D.; Migliaccio, L.; Hanein, Y.; Głowacki, E.D. Untangling Photofaradaic and Photocapacitive Effects in Organic Optoelectronic Stimulation Devices. Front. Bioeng. Biotechnol. 2020, 8, 284. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, J.; Travaglini, L.; Lauto, A.; Cramer, T.; Fraboni, B.; Seidel, J.; Mawad, D. Photoactive Organic Substrates for Cell Stimulation: Progress and Perspectives. Adv. Mater. Technol. 2019, 4, 1800744. [Google Scholar] [CrossRef]
- Park, S.; Kang, Y.J.; Majd, S. A Review of Patterned Organic Bioelectronic Materials and Their Biomedical Applications. Adv. Mater. 2015, 27, 7583–7619. [Google Scholar] [CrossRef]
- Baker, C.; Wagner, K.; Wagner, P.; Officer, D.L.; Mawad, D. Biofunctional conducting polymers: Synthetic advances, challenges, and perspectives towards their use in implantable bioelectronic devices. Adv. Phys. X 2021, 6, 1899850. [Google Scholar] [CrossRef]
- Fenoy, G.E.; Azzaroni, O.; Knoll, W.; Marmisollé, W.A. Functionalization Strategies of PEDOT and PEDOT:PSS Films for Organic Bioelectronics Applications. Chemosensors 2021, 9, 212. [Google Scholar] [CrossRef]
- Ferlauto, L.; D’Angelo, A.N.; Vagni, P.; Airaghi Leccardi, M.J.I.; Mor, F.M.; Cuttaz, E.A.; Heuschkel, M.O.; Stoppini, L.; Ghezzi, D. Development and Characterization of PEDOT:PSS/Alginate Soft Microelectrodes for Application in Neuroprosthetics. Front. Neurosci. 2018, 12, 648. [Google Scholar] [CrossRef]
- Liu, X.; Yue, Z.; Higgins, M.J.; Wallace, G.G. Conducting polymers with immobilised fibrillar collagen for enhanced neural interfacing. Biomaterials 2011, 32, 7309–7317. [Google Scholar] [CrossRef]
- Lee, J.; Choi, Y.; Song, J.; Seong, D.; Jin, S.; Ju, J.; Son, D.; Shin, M. Nerve-Mimetic Adhesive Hydrogel Electroceuticals: Tailoring In Situ Physically Entangled Domains in Singular Polymers. ACS Nano 2024, 18, 34949–34961. [Google Scholar] [CrossRef]
- Obidin, N.; Tasnim, F.; Dagdeviren, C. The Future of Neuroimplantable Devices: A Materials Science and Regulatory Perspective. Adv. Mater. 2020, 32, 1901482. [Google Scholar] [CrossRef]
- Magaziner, J.; Mangione, K.K.; Orwig, D.; Baumgarten, M.; Magder, L.; Terrin, M.; Fortinsky, R.H.; Gruber-Baldini, A.L.; Beamer, B.A.; Tosteson, A.N.A.; et al. Effect of a Multicomponent Home-Based Physical Therapy Intervention on Ambulation After Hip Fracture in Older Adults: The CAP Randomized Clinical Trial. JAMA 2019, 322, 946. [Google Scholar] [CrossRef] [PubMed]
- Momin, M.; Feng, L.; Ahmed, S.; Ren, J.; Hossain, A.; Zhang, S.; Zhou, T. 3D-printed flexible neural probes for recordings at single-neuron level. Device 2024, 2, 100519. [Google Scholar] [CrossRef]
- Middya, S.; Carnicer-Lombarte, A.; Sawiak, S.; Hilton, S.; Curto, V.F.; Barone, D.G.; Schierle, G.S.K.; Malliaras, G.G. Conducting Polymer Microelectrode Arrays for Simultaneous Electrophysiology and Advanced Brain Imaging. Adv. Funct. Mater. 2025, 2417312. [Google Scholar] [CrossRef]
- Li, Y.; Yang, X.; Ding, Y.; Zhang, H.; Cheng, Y.; Li, X.; Sun, J.; Liu, Y.; Li, Y.; Fan, D. A Wireless Health Monitoring System Accomplishing Bimodal Decoupling Based on an “IS”-Shaped Multifunctional Conductive Hydrogel. Small 2025, 2411046. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, Z.; Li, M.; Fang, L.; Chen, F.; Han, S.; Lan, L.; Chen, J.; Chen, Q.; Wang, H.; et al. High-Transconductance, Highly Elastic, Durable and Recyclable All-Polymer Electrochemical Transistors with 3D Micro-Engineered Interfaces. Nano-Micro Lett. 2022, 14, 184. [Google Scholar] [CrossRef]
- Zhong, T.; Zhang, M.; Fu, Y.; Han, Y.; Guan, H.; He, H.; Zhao, T.; Xing, L.; Xue, X.; Zhang, Y.; et al. An artificial triboelectricity-brain-behavior closed loop for intelligent olfactory substitution. Nano Energy 2019, 63, 103884. [Google Scholar] [CrossRef]
- Maya-Vetencourt, J.F.; Manfredi, G.; Mete, M.; Colombo, E.; Bramini, M.; Di Marco, S.; Shmal, D.; Mantero, G.; Dipalo, M.; Rocchi, A.; et al. Subretinally injected semiconducting polymer nanoparticles rescue vision in a rat model of retinal dystrophy. Nat. Nanotechnol. 2020, 15, 698–708. [Google Scholar] [CrossRef]
- Silverå Ejneby, M.; Jakešová, M.; Ferrero, J.J.; Migliaccio, L.; Sahalianov, I.; Zhao, Z.; Berggren, M.; Khodagholy, D.; Đerek, V.; Gelinas, J.N.; et al. Chronic electrical stimulation of peripheral nerves via deep-red light transduced by an implanted organic photocapacitor. Nat. Biomed. Eng. 2022, 6, 741–753. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, L.M.; Ismailov, U.; Badier, J.-M.; Greco, F.; Ismailova, E. Conducting polymer tattoo electrodes in clinical electro- and magneto-encephalography. Npj Flex. Electron. 2020, 4, 1–9. [Google Scholar] [CrossRef]
- Ferrari, L.M.; Ismailov, U.; Greco, F.; Ismailova, E. Capacitive Coupling of Conducting Polymer Tattoo Electrodes with the Skin. Adv. Mater. Interfaces 2021, 8, 2100352. [Google Scholar] [CrossRef]
- Zushin, P.-J.H.; Mukherjee, S.; Wu, J.C. FDA Modernization Act 2.0: Transitioning beyond animal models with human cells, organoids, and AI/ML-based approaches. J. Clin. Investig. 2023, 133, e175824. [Google Scholar] [CrossRef]
- Perel, P.; Roberts, I.; Sena, E.; Wheble, P.; Briscoe, C.; Sandercock, P.; Macleod, M.; Mignini, L.E.; Jayaram, P.; Khan, K.S. Comparison of treatment effects between animal experiments and clinical trials: Systematic review. BMJ 2007, 334, 197. [Google Scholar] [CrossRef]
- Wikswo, J.P. The relevance and potential roles of microphysiological systems in biology and medicine. Exp. Biol. Med. 2014, 239, 1061–1072. [Google Scholar] [CrossRef]
- Wang, K.; Man, K.; Liu, J.; Liu, Y.; Chen, Q.; Zhou, Y.; Yang, Y. Microphysiological Systems: Design, Fabrication, and Applications. ACS Biomater. Sci. Eng. 2020, 6, 3231–3257. [Google Scholar] [CrossRef]
- Li, T.L.; Liu, Y.; Forro, C.; Yang, X.; Beker, L.; Bao, Z.; Cui, B.; Pașca, S.P. Stretchable mesh microelectronics for the biointegration and stimulation of human neural organoids. Biomaterials 2022, 290, 121825. [Google Scholar] [CrossRef]
- Zhang, F.; Cheng, H.; Qu, K.; Qian, X.; Lin, Y.; Zhang, Y.; Qian, S.; Huang, N.; Cui, C.; Chen, M. Continuous contractile force and electrical signal recordings of 3D cardiac tissue utilizing conductive hydrogel pillars on a chip. Mater. Today Bio 2023, 20, 100626. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, P.; O’Leary, G.; Zhao, Y.; Xu, Y.; Rafatian, N.; Okhovatian, S.; Landau, S.; Valiante, T.A.; Travas-Sejdic, J.; et al. Flexible 3D printed microwires and 3D microelectrodes for heart-on-a-chip engineering. Biofabrication 2023, 15, 035023. [Google Scholar] [CrossRef] [PubMed]
- Waafi, A.K.; Gaio, N.; Quiros-Solano, W.F.; Dijkstra, P.; Sarro, P.M.; Dekker, R. Low-Impedance PEDOT:PSS MEA Integrated in a Stretchable Organ-on-Chip Device. IEEE Sens. J. 2020, 20, 1150–1157. [Google Scholar] [CrossRef]
- Huang, Q.; Tang, B.; Romero, J.C.; Yang, Y.; Elsayed, S.K.; Pahapale, G.; Lee, T.-J.; Morales Pantoja, I.E.; Han, F.; Berlinicke, C.; et al. Shell microelectrode arrays (MEAs) for brain organoids. Sci. Adv. 2022, 8, eabq5031. [Google Scholar] [CrossRef]
- Kalmykov, A.; Huang, C.; Bliley, J.; Shiwarski, D.; Tashman, J.; Abdullah, A.; Rastogi, S.K.; Shukla, S.; Mataev, E.; Feinberg, A.W.; et al. Organ-on-e-chip: Three-dimensional self-rolled biosensor array for electrical interrogations of human electrogenic spheroids. Sci. Adv. 2019, 5, eaax0729. [Google Scholar] [CrossRef]
- Wu, Y.; Cheng, J.; Qi, J.; Hang, C.; Dong, R.; Low, B.C.; Yu, H.; Jiang, X. Three-dimensional liquid metal-based neuro-interfaces for human hippocampal organoids. Nat. Commun. 2024, 15, 4047. [Google Scholar] [CrossRef] [PubMed]
- Moysidou, C.-M.; Pitsalidis, C.; Al-Sharabi, M.; Withers, A.M.; Zeitler, J.A.; Owens, R.M. 3D Bioelectronic Model of the Human Intestine. Adv. Biol. 2021, 5, 2000306. [Google Scholar] [CrossRef]
- Mou, L.; Mandal, K.; Mecwan, M.M.; Hernandez, A.L.; Maity, S.; Sharma, S.; Herculano, R.D.; Kawakita, S.; Jucaud, V.; Dokmeci, M.R.; et al. Integrated Biosensors for Monitoring Microphysiological Systems. Lab. Chip 2022, 22, 3801–3816. [Google Scholar] [CrossRef] [PubMed]
- Koklu, A.; Wustoni, S.; Musteata, V.-E.; Ohayon, D.; Moser, M.; McCulloch, I.; Nunes, S.P.; Inal, S. Microfluidic Integrated Organic Electrochemical Transistor with a Nanoporous Membrane for Amyloid-β Detection. ACS Nano 2021, 15, 8130–8141. [Google Scholar] [CrossRef]
- Lee, M.-H.; Liu, K.-T.; Thomas, J.L.; Su, Z.-L.; O’Hare, D.; van Wuellen, T.; Chamarro, J.M.; Bolognin, S.; Luo, S.-C.; Schwamborn, J.C.; et al. Peptide-Imprinted Poly(hydroxymethyl 3,4-ethylenedioxythiophene) Nanotubes for Detection of α Synuclein in Human Brain Organoids. ACS Appl. Nano Mater. 2020, 3, 8027–8036. [Google Scholar] [CrossRef]
- Oldroyd, P.; Gurke, J.; Malliaras, G.G. Stability of Thin Film Neuromodulation Electrodes under Accelerated Aging Conditions. Adv. Funct. Mater. 2023, 33, 2208881. [Google Scholar] [CrossRef]
- Roh, H.; Cunin, C.; Samal, S.; Gumyusenge, A. Towards organic electronics that learn at the body-machine interface: A materials journey. MRS Commun. 2022, 12, 565–577. [Google Scholar] [CrossRef]
- Adilbekova, B.; Scaccabarozzi, A.D.; Faber, H.; Nugraha, M.I.; Bruevich, V.; Kaltsas, D.; Naphade, D.R.; Wehbe, N.; Emwas, A.-H.; Alshareef, H.N.; et al. Enhancing the Electrical Conductivity and Long-Term Stability of PEDOT:PSS Electrodes through Sequential Treatment with Nitric Acid and Cesium Chloride. Adv. Mater. 2024, 36, 2405094. [Google Scholar] [CrossRef] [PubMed]
- Yuk, H.; Lu, B.; Lin, S.; Qu, K.; Xu, J.; Luo, J.; Zhao, X. 3D printing of conducting polymers. Nat. Commun. 2020, 11, 1604. [Google Scholar] [CrossRef]
- Yin, Y.; Stecke, K.E.; Li, D. The evolution of production systems from Industry 2.0 through Industry 4.0. Int. J. Prod. Res. 2018, 56, 848–861. [Google Scholar] [CrossRef]
- Zheng, Y.-Q.; Liu, Y.; Zhong, D.; Nikzad, S.; Liu, S.; Yu, Z.; Liu, D.; Wu, H.-C.; Zhu, C.; Li, J.; et al. Monolithic optical microlithography of high-density elastic circuits. Science 2021, 373, 88–94. [Google Scholar] [CrossRef]
- Gumyusenge, A.; Tran, D.T.; Luo, X.; Pitch, G.M.; Zhao, Y.; Jenkins, K.A.; Dunn, T.J.; Ayzner, A.L.; Savoie, B.M.; Mei, J. Semiconducting polymer blends that exhibit stable charge transport at high temperatures. Science 2018, 362, 1131–1134. [Google Scholar] [CrossRef] [PubMed]
- Curto, V.F.; Marchiori, B.; Hama, A.; Pappa, A.-M.; Ferro, M.P.; Braendlein, M.; Rivnay, J.; Fiocchi, M.; Malliaras, G.G.; Ramuz, M.; et al. Organic transistor platform with integrated microfluidics for in-line multi-parametric in vitro cell monitoring. Microsyst. Nanoeng. 2017, 3, 1–12. [Google Scholar] [CrossRef]
- Gao, S.; Fang, A.; Huang, Y.; Giunchiglia, V.; Noori, A.; Schwarz, J.R.; Ektefaie, Y.; Kondic, J.; Zitnik, M. Empowering biomedical discovery with AI agents. Cell 2024, 187, 6125–6151. [Google Scholar] [CrossRef]
- Ha, T.; Lee, D.; Kwon, Y.; Park, M.S.; Lee, S.; Jang, J.; Choi, B.; Jeon, H.; Kim, J.; Choi, H.; et al. AI-driven robotic chemist for autonomous synthesis of organic molecules. Sci. Adv. 2023, 9, eadj0461. [Google Scholar] [CrossRef]

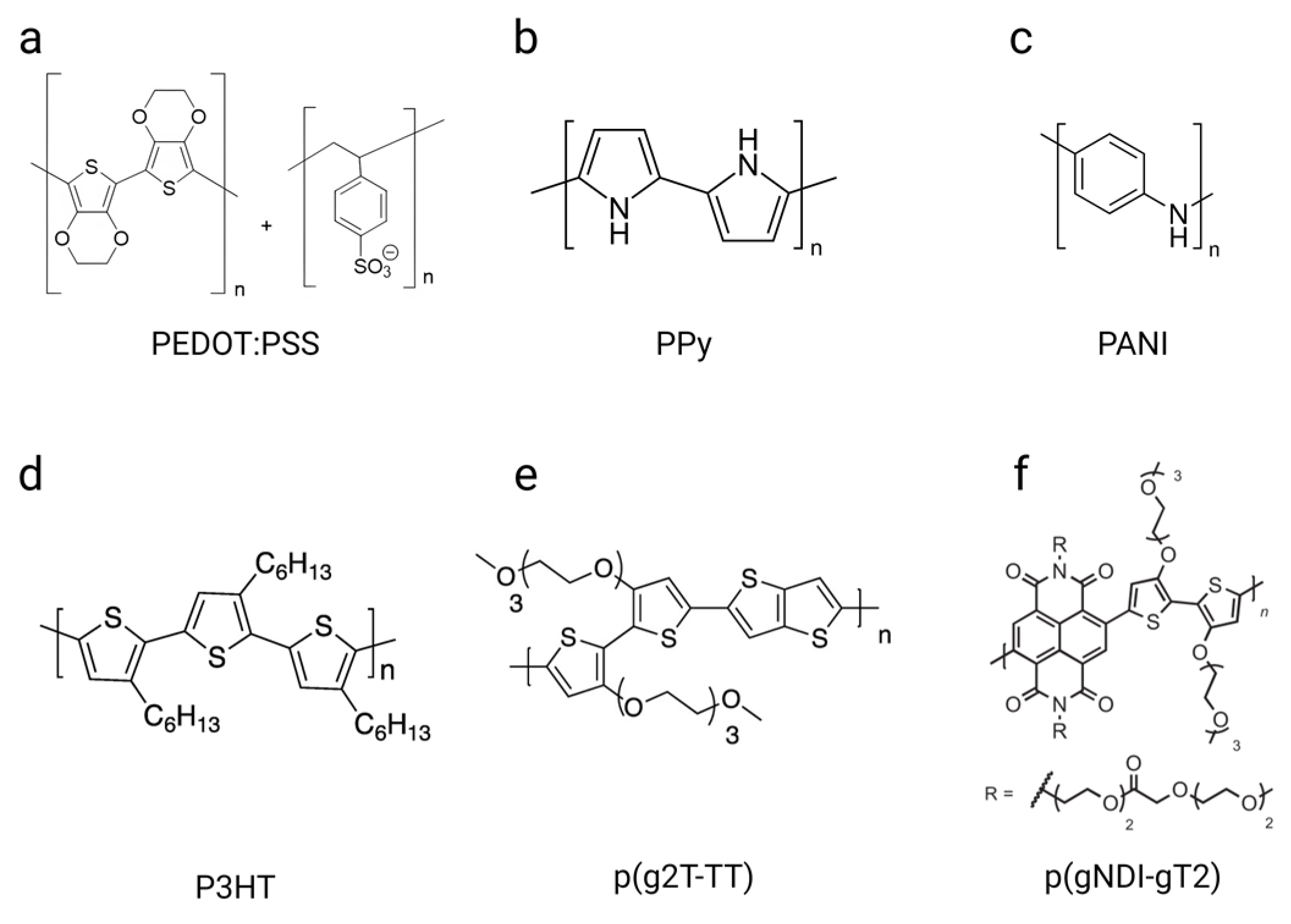
| Polymer Name | Abbreviation | Type of Doping | Electrical Conductivity | Young’s Modulus | Biocompatibility |
|---|---|---|---|---|---|
| Poly(3,4-ethylenedioxythiophene): poly(styrene sulfonate) | PEDOT:PSS | p | 0.1–1 to 4380 S/cm [86,87] | Hydrogels: 2–10 MPa [84]; films: 0.9 ± 0.2 to 2.9 ± 0.5 GPa at 55% and 23% relative humidity [94] | High biocompatibility in printing inks after collagen coating [88] or in scaffolds [89] |
| Polypyrrole | PPy | p | 7.5 × 103 to 10 × 103 S/cm [95,96] | 180 MPa to 4.98 GPa [97,98] | Partial and concentration-dependent toxicity [99]. Structure [100], coating, and surface modification [101] can improve the biocompatibility |
| Polyaniline | PANI | n, p | 10−10 to 10 S/cm for standard doped PANI [102]; 30 to 200 S/cm for PANI with strong protonic acid doping [95,103] | 2.9–3.1 ± 0.2 GPa [104] | Significant cytotoxicity due to residual ammonium persulfate and low-molecular-weight polar substances [105] |
| Poly(3-hexylthiophene-2,5-diyl) | P3HT | p | 10−4 to 224 S/cm [106] | 260 ± 27 MPa [107] | Stable in physiological media but limited biocompatibility. Can be improved by introducing cell adhesion functional groups through protein-based coating and by manipulating the surface wettability [108,109] |
| Poly(2-(3,3′-bis(2-(2-(2-methoxyethoxy)-ethoxy)ethoxy)-[2,2′-bithiophen]-5-yl)thieno[3,2-b] thiophene) | p(g2T-TT) | p | 1 to 616.7 S/cm [110,111] | N/A | No toxicity when exposed to tissues [112] |
| Poly((ethoxy)ethyl2-(2-(2-methoxy ethoxy)ethoxy)acetate)-naphthalene-1,4,5,8 tetracarboxylic-diimide-co-3,3ʹ-bis(2-(2-(2-methoxy ethoxy)ethoxy)ethoxy)-(bithiophene)) | p(gNDI-T2) | n | 0.1085 S/cm; shows the highest capacitance of the channel per unit volume (397 F.cm−3) and the smallest electronic carrier mobility (0.00031 cm2/V/s) among other p-type polymers [113] | N/A | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coquart, P.; El Haddad, A.; Koutsouras, D.A.; Bolander, J. Organic Bioelectronics in Microphysiological Systems: Bridging the Gap Between Biological Systems and Electronic Technologies. Biosensors 2025, 15, 253. https://doi.org/10.3390/bios15040253
Coquart P, El Haddad A, Koutsouras DA, Bolander J. Organic Bioelectronics in Microphysiological Systems: Bridging the Gap Between Biological Systems and Electronic Technologies. Biosensors. 2025; 15(4):253. https://doi.org/10.3390/bios15040253
Chicago/Turabian StyleCoquart, Pauline, Andrea El Haddad, Dimitrios A. Koutsouras, and Johanna Bolander. 2025. "Organic Bioelectronics in Microphysiological Systems: Bridging the Gap Between Biological Systems and Electronic Technologies" Biosensors 15, no. 4: 253. https://doi.org/10.3390/bios15040253
APA StyleCoquart, P., El Haddad, A., Koutsouras, D. A., & Bolander, J. (2025). Organic Bioelectronics in Microphysiological Systems: Bridging the Gap Between Biological Systems and Electronic Technologies. Biosensors, 15(4), 253. https://doi.org/10.3390/bios15040253






