Current Trends in In Vitro Diagnostics Using Surface-Enhanced Raman Scattering in Translational Biomedical Research
Abstract
1. Introduction
2. History of Developing Immunoassays Based on SERS
3. Biomedical Diagnostics
4. Portable SERS-Based Diagnostic Systems
5. In Vitro Transcription (IVT) Process Monitoring
6. Conclusions
7. Future Perspective and Outlook
Funding
Conflicts of Interest
References
- Miao, W. Electrogenerated chemiluminescence and its biorelated applications. Chem. Rev. 2008, 108, 2506–2553. [Google Scholar] [CrossRef]
- Dequaire, M.; Degrand, C.; Limoges, B. An electrochemical metalloimmunoassay based on a colloidal gold label. Anal. Chem. 2000, 72, 5521–5528. [Google Scholar] [CrossRef]
- Henry, A.-I.; Sharma, B.; Cardinal, M.F.; Kurouski, D.; Van Duyne, R.P. Surface-enhanced Raman spectroscopy biosensing: In vivo diagnostics and multimodal imaging. Anal. Chem. 2016, 88, 6638–6647. [Google Scholar] [CrossRef]
- Lee, S.; Dang, H.; Moon, J.I.; Kim, K.; Joung, Y.; Park, S.; Yu, Q.; Chen, J.; Lu, M.; Chen, L.; et al. SERS-based microdevices for use as in vitro diagnostic biosensors. Chem. Soc. Rev. 2024, 53, 5394–5427. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, Q.; Joung, Y.; Jeon, C.S.; Pyun, S.H.; Joo, S.-W.; Chen, L.; Choo, J. Highly uniform self-assembly of gold nanoparticles by butanol-induced dehydration and its SERS applications in SARS-CoV-2 detection. Anal. Chem. 2023, 95, 12710–12718. [Google Scholar] [CrossRef]
- Park, S.; Jeon, C.S.; Choi, N.; Moon, J.I.; Lee, K.M.; Pyun, S.H.; Kang, T.; Choo, J. Sensitive and reproducible detection of SARS-CoV-2 using SERS-based microdroplet sensor. Chem. Eng. J. 2022, 446, 137085. [Google Scholar] [CrossRef]
- Kim, K.; Han, D.K.; Choi, N.; Kin, S.H.; Joung, Y.; Kim, K.; Ho, N.T.; Joo, S.-W.; Choo, J. SERS-based dual-flow lateral flow assay sensor for the ultrasensitive detection of thyroid-stimulating hormone. Anal. Chem. 2021, 93, 6673–6681. [Google Scholar] [CrossRef]
- Park, T.; Lee, S.; Seong, G.H.; Choo, J.; Lee, E.K.; Kim, Y.S.; Ji, W.H.; Hwang, S.Y.; Gweon, D.G.; Lee, S. Highly sensitive signal detection of duplex dye-labelled DNA oligonucleotides in a PDMS microfluidic chip: Confocal surface-enhanced Raman spectroscopic study. Lab Chip 2005, 5, 437–442. [Google Scholar] [CrossRef]
- Hwang, J.; Lee, S.; Choo, J. Application of a SERS-based lateral flow immunoassay strip for the rapid and sensitive detection of staphylococcal enterotoxin B. Nanoscale 2016, 8, 11418–11425. [Google Scholar] [CrossRef]
- Chen, H.; Das, A.; Bi, L.; Choi, N.; Moon, J.I.; Wu, Y.; Park, S.; Choo, J. Recent advances in surface-enhanced Raman scattering-based microdevices for point-of-care diagnosis of viruses and bacteria. Nanoscale 2020, 12, 21560–21570. [Google Scholar] [CrossRef]
- Langer, J.; Jimenez de Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguie, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and future of surface-enhanced Raman scattering. ACS Nano 2019, 14, 28–117. [Google Scholar] [CrossRef]
- Lee, S.H.; Hwang, J.; Kim, K.; Jeon, J.; Lee, S.; Juhui, K.; Lee, J.; Kang, M.; Chung, D.R.; Choo, J. Quantitative serodiagnosis of scrub typhus using SERS-based lateral flow assay platforms. Anal. Chem. 2019, 91, 12275–12282. [Google Scholar] [CrossRef]
- Wang, X.; Park, S.G.; Ko, J.; Xiao, X.; Giannini, V.; Maier, S.A.; Kim, D.H.; Choo, J. Sensitive and reproducible immunoassay of multiple mycotoxins using surface-enhanced Raman scattering mapping on three-dimensional plasmonic nanopillar arrays. Small 2018, 14, 1801623. [Google Scholar] [CrossRef]
- Gao, R.; Cheng, Z.; Wang, X.; Yu, L.; Guo, Z.; Zhao, G.; Choo, J. Simultaneous immunoassays of dual prostate cancer markers using a SERS-based microdroplet channel. Biosens. Bioelectron. 2018, 119, 126–133. [Google Scholar] [CrossRef]
- Fu, X.; Cheng, Z.; Yu, J.; Choo, P.; Chen, L.; Choo, J. A SERS-based lateral flow assay biosensor for highly sensitive detection of HIV-1 DNA. Biosens. Bioelectron. 2016, 78, 530–537. [Google Scholar] [CrossRef]
- Raman, C.V.; Krishnan, K.S. A New Type of Secondary Radiation. Nature 1928, 121, 501–502. [Google Scholar] [CrossRef]
- Landsberg, G.; Mandelstam, L. Eine neue Erscheinung bei der Lichtzerstreuung in Krystallen. Naturwissenschaften 1928, 16, 557–558. [Google Scholar]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman-Spectra of Pyridine Absorbed at a Silver Electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Talian, I.; Mogensen, K.B.; Orinak, A.; Kaniansky, D.; Hubner, J. Surface-Enhanced Raman Spectroscopy on Novel Black Silicon-Based Nanostructured Surfaces. J. Raman Spectrosc. 2009, 40, 982–986. [Google Scholar] [CrossRef]
- Yuan, H.K.; Fales, A.M.; Khoury, C.G.; Liu, J.; Vo-Dinh, T. Spectral Characterization and Intracellular Detection of Surface-Enhanced Raman Scattering (SERS)-Encoded Plasmonic Gold Nanostars. J. Raman Spectrosc. 2013, 44, 234–239. [Google Scholar] [CrossRef]
- Jana, D.; Mandal, A.; De, G. High Raman Enhancing Shape-Tunable Ag Nanoplates in Alumina: A Reliable and Efficient SERS Technique. ACS Appl. Mater. Interfaces 2012, 4, 3330–3334. [Google Scholar] [CrossRef]
- Ni, J.; Lipert, R.J.; Dawson, G.B.; Porter, M.D. Immunoassay Readout Method Using Extrinsic Raman Labels Adsorbed on Immunogold Colloids. Anal. Chem. 1999, 71, 4903–4908. [Google Scholar] [CrossRef]
- Li, J.-M.; Wei, C.; Ma, W.-F.; An, Q.; Guo, J.; Hu, J.; Wang, C.-C. Multiplexed SERS Detection of DNA Targets in a Sandwich-Hybridization Assay Using SERS-Encoded Core-Shell Nanospheres. J. Mater. Chem. 2012, 22, 12100–12106. [Google Scholar] [CrossRef]
- Zong, S.; Wang, Z.; Zhang, R.; Wang, C.; Xu, S.; Cui, Y. A Multiplex and Straightforward Aqueous Phase Immunoassay Protocol Through the Combination of SERS-Fluorescence Dual Mode Nanoprobes and Magnetic Nanobeads. Biosens. Bioelectron. 2013, 41, 745–751. [Google Scholar] [CrossRef]
- Zheng, F.; Cheng, Y.; Wang, J.; Lu, J.; Zhang, B.; Zhao, Y.; Gu, Z. Aptamer-Functionalized Barcode Particles for the Capture and Detection of Multiple Types of Circulating Tumor Cells. Adv. Mater. 2014, 26, 7333–7338. [Google Scholar] [CrossRef]
- Lee, M.; Lee, K.; Kim, K.H.; Oh, K.W.; Choo, J. SERS-Based Immunoassay Using a Gold Array-Embedded Gradient Microfluidic Chip. Lab Chip 2012, 12, 3720–3727. [Google Scholar] [CrossRef]
- Abbas, A.; Brimer, A.; Slocik, J.M.; Tian, L.M.; Naik, R.R.; Singamaneni, S. Multifunctional Analytical Platform on a Paper Strip: Separation, Preconcentration, and Subattomolar Detection. Anal. Chem. 2013, 85, 3977–3983. [Google Scholar] [CrossRef]
- Zhai, Q.; Zhang, X.; Li, J.; Wang, E. Molybdenum Carbide Nanotubes: A Novel Multifunctional Material for Label-Free Electrochemical Immunosensing. Nanoscale 2016, 8, 15303–15308. [Google Scholar] [CrossRef]
- Zhu, D.; Wang, Z.Y.; Zong, S.F.; Chen, H.; Chen, P.; Cui, Y.P. Wavenumber-Intensity Joint SERS Encoding Using Silver Nanoparticles for Tumor Cell Targeting. RSC Adv. 2014, 4, 60936–60942. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Zong, S.F.; Li, W.; Wang, C.L.; Xu, S.H.; Chen, H.; Cui, Y.P. SERS-Fluorescence Joint Spectral Encoding Using Organic-Metal-QD Hybrid Nanoparticles with A Huge Encoding Capacity for High-Throughput Biodetection: Putting Theory into Practice. J. Am. Chem. Soc. 2012, 134, 2993–3000. [Google Scholar] [CrossRef]
- Wu, L.; Wang, Z.Y.; Fan, K.Q.; Zong, S.F.; Cui, Y.P. A SERS-Assisted 3D Barcode Chip for High-Throughput Biosensing. Small 2015, 11, 2798–2806. [Google Scholar] [CrossRef] [PubMed]
- Kneipp, J.; Kneipp, H.; Kneipp, K. SERS—A Single-Molecule and Nanoscale Tool for Bioanalytics. Chem. Soc. Rev. 2008, 37, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.M.; Nie, S.M. Single-Molecule and Single-Nanoparticle SERS: From Fundamental Mechanisms to Biomedical Applications. Chem. Soc. Rev. 2008, 37, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Le Ru, E.C.; Etchegoin, P.G. Single-Molecule Surface-Enhanced Raman Spectroscopy. Annu. Rev. Phys. Chem. 2012, 63, 65–87. [Google Scholar] [CrossRef]
- Rodriguez-Lorenzo, L.; Fabris, L.; Alvarez-Puebla, R.A. Multiplex Optical Sensing with Surface-Enhanced Raman Scattering: A Critical Review. Anal. Chim. Acta 2012, 745, 10–23. [Google Scholar] [CrossRef]
- Cialla, D.; Pollok, S.; Steinbrucker, C.; Weber, K.; Popp, J. SERS-Based Detection of Biomolecules. Nanophotonics 2014, 3, 383–411. [Google Scholar] [CrossRef]
- Driskell, J.D.; Uhlenkamp, J.M.; Lipert, R.J.; Porter, M.D. Surface-enhanced Raman scattering immunoassays using a rotated capture substrate. Anal. Chem. 2007, 79, 4141–4148. [Google Scholar] [CrossRef]
- Song, C.; Wang, Z.; Zhang, R.; Yang, J.; Tan, X.; Cui, Y. Highly sensitive immunoassay based on Raman reporter-labeled immuno-Au aggregates and SERS-active immune substrate. Biosens. Bioelectron. 2009, 25, 826–831. [Google Scholar] [CrossRef]
- Song, C.; Wang, Z.; Yang, J.; Zhang, R.; Cui, Y. Preparation of 2-mercaptobenzothiazole-labeled immuno-Au aggregates for SERS-based immunoassay. Colloids Surf. B Biointerfaces 2010, 81, 285–288. [Google Scholar] [CrossRef]
- Sivashanmugan, K.; Liao, J.D.; Liu, B.H.; Yao, C.K. Focused-ion-beam-fabricated Au nanorods coupled with Ag nanoparticles used as surface-enhanced Raman scattering-active substrate for analyzing trace melamine constituents in solution. Anal. Chim. Acta 2013, 800, 56–64. [Google Scholar] [CrossRef]
- Kamińska, A.; Witkowska, E.; Winkler, K.; Dzięcielewski, I.; Weyher, J.L.; Waluk, J. Detection of Hepatitis B virus antigen from human blood: SERS immunoassay in a microfluidic system. Biosens. Bioelectron. 2015, 66, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Karn-orachai, K.; Sakamoto, K.; Laocharoensuk, R.; Bamrungsap, S.; Songsivilai, S.; Dharakul, T.; Miki, K. Extrinsic surface-enhanced Raman scattering detection of influenza A virus enhanced by two-dimensional gold@ silver core–shell nanoparticle arrays. RSC Adv. 2016, 6, 97791–97799. [Google Scholar] [CrossRef]
- Moon, J.; Yi, S.Y.; Hwang, A.; Eom, G.; Sim, J.; Jeong, J.; Lim, E.K.; Chung, B.H.; Kim, B.; Jung, J.; et al. Facile and sensitive detection of influenza viruses using SERS antibody probes. RSC Adv. 2016, 6, 84415–84419. [Google Scholar] [CrossRef]
- Kukushkin, V.I.; Ivanov, N.M.; Novoseltseva, A.A.; Gambaryan, A.S.; Yaminsky, I.V.; Kopylov, A.M.; Zavyalova, E.G. Highly sensitive detection of influenza virus with SERS aptasensor. PLoS ONE 2019, 14, e0216247. [Google Scholar] [CrossRef]
- Zengin, A.; Tamer, U.; Caykara, T. SERS detection of hepatitis B virus DNA in a temperature-responsive sandwich-hybridization assay. J. Raman Spectrosc. 2017, 48, 668–672. [Google Scholar] [CrossRef]
- Lu, Y.; Lin, Y.; Zheng, Z.; Tang, X.; Lin, J.; Liu, X.; Liu, M.; Chen, G.; Qiu, S.; Zhou, T.; et al. Label free hepatitis B detection based on serum derivative surface enhanced Raman spectroscopy combined with multivariate analysis. Biomed. Opt. Express 2018, 9, 4755–4766. [Google Scholar] [CrossRef]
- Zhang, M.; Li, X.; Pan, J.; Zhang, Y.; Zhang, L.; Wang, C.; Yan, X.; Liu, X.; Lu, G. Ultrasensitive detection of SARS-CoV-2 spike protein in untreated saliva using SERS-based biosensor. Biosens. Bioelectron. 2021, 190, 113421. [Google Scholar] [CrossRef]
- Shanmukh, S.; Jones, L.; Driskell, J.; Zhao, Y.; Dluhy, R.; Tripp, R.A. Rapid and sensitive detection of respiratory virus molecular signatures using a silver nanorod array SERS substrate. Nano Lett. 2006, 6, 2630–2636. [Google Scholar] [CrossRef]
- Driskell, J.D.; Kwarta, K.M.; Lipert, R.J.; Porter, M.D.; Neill, J.D.; Ridpath, J.F. Low-level detection of viral pathogens by a surface-enhanced Raman scattering based immunoassay. Anal. Chem. 2005, 77, 6147–6154. [Google Scholar] [CrossRef]
- Lin, C.C.; Yang, Y.M.; Chen, Y.F.; Yang, T.S.; Chang, H.C. A new protein A assay based on Raman reporter labeled immunogold nanoparticles. Biosens. Bioelectron. 2008, 24, 178–183. [Google Scholar] [CrossRef]
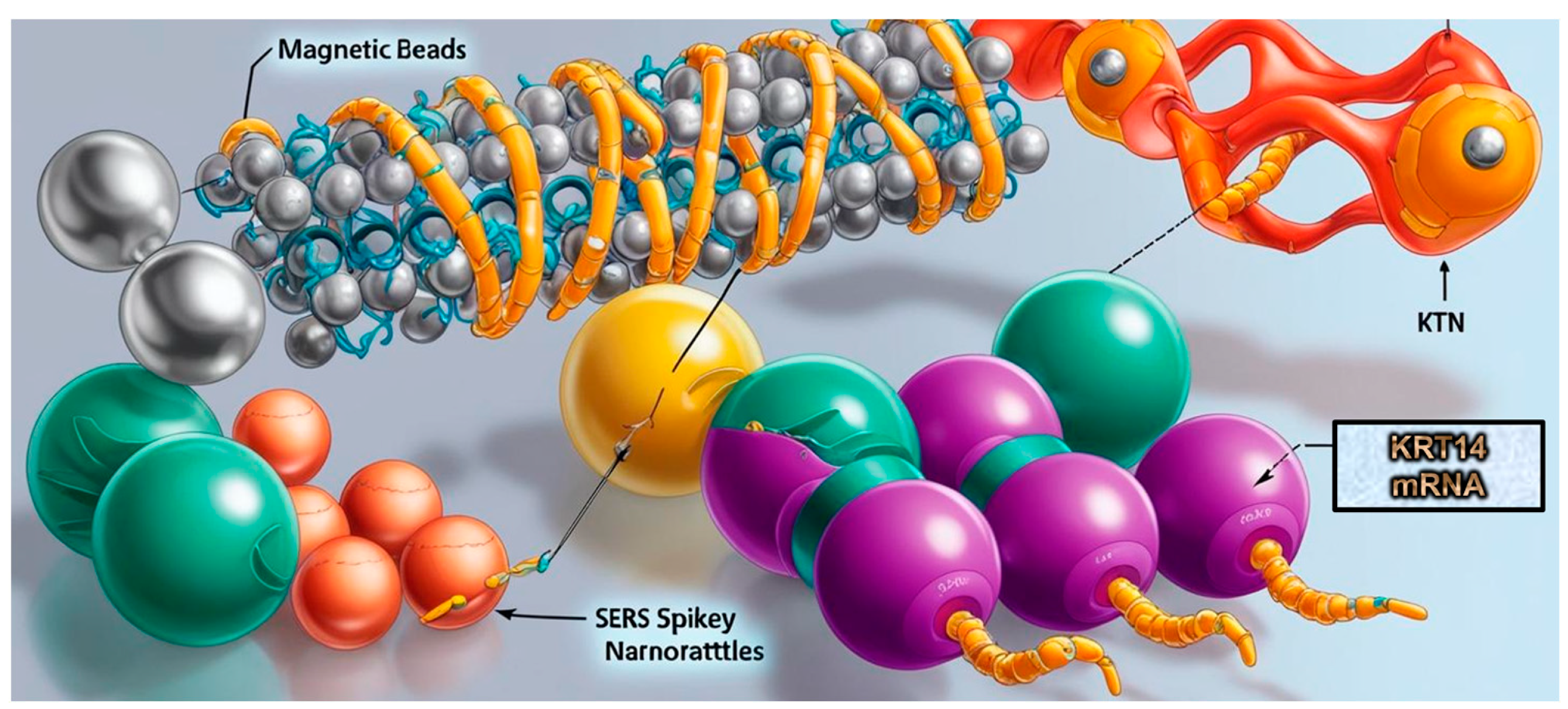
- Li, J.Q.; Atta, S.; Zhao, Y.; Hoang, K.; Canning, A.; Strobbia, P.; Canick, J.E.; Cho, J.H.; Rocke, D.J.; Lee, W.T.; et al. Plasmonics-enhanced spikey nanorattle-based biosensor for direct SERS detection of mRNA cancer biomarkers. Anal. Bioanal. Chem. 2024, 416, 7347–7355. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.; Ajmal, M.; Ashraf, G.; Muhammad, N.; Aziz, A.; Iftikhar, T.; Wang, J.; Liu, H. The role of biosensors in coronavirus disease-2019 outbreak. Curr. Opin. Electrochem. 2020, 23, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Abid, S.A.; Muneer, A.A.; Al-Kadmy, I.M.; Sattar, A.A.; Beshbishy, A.M.; Batiha, G.E.S.; Hetta, H.F. Biosensors as a future diagnostic approach for COVID-19. Life Sci. 2021, 273, 119117. [Google Scholar] [CrossRef] [PubMed]
- Awada, C.; Abdullah, M.M.B.; Traboulsi, H.; Dab, C.; Alshoaibi, A. SARS-CoV-2 receptor binding domain as a stable-potential target for SARS-CoV-2 detection by surface—Enhanced Raman spectroscopy. Sensors 2021, 21, 4617. [Google Scholar] [CrossRef]
- Wei, J.; Zhao, Z.; Luo, F.; Lan, K.; Chen, R.; Qin, G. Sensitive and quantitative detection of SARS-CoV-2 antibodies from vaccinated serum by MoS2-field effect transistor. 2D Mater. 2021, 9, 015030. [Google Scholar] [CrossRef]
- Varghese, R.; Salvi, S.; Sood, P.; Karsiya, J.; Kumar, D. Carbon nanotubes in COVID-19: A critical review and prospects. Colloid Interface Sci. Commun. 2022, 46, 100544. [Google Scholar] [CrossRef]
- Leong, S.X.; Leong, Y.X.; Tan, E.X.; Sim, H.Y.F.; Koh, C.S.L.; Lee, Y.H.; Chong, C.; Ng, L.S.; Chen, J.R.T.; Pang, D.W.C.; et al. Noninvasive and point-of-care surface-enhanced Raman scattering (SERS)-based breathalyzer for mass screening of coronavirus disease 2019 (COVID-19) under 5 min. ACS Nano 2022, 16, 2629–2639. [Google Scholar] [CrossRef]
- Sanchez, J.E.; Jaramillo, S.A.; Settles, E.; Velazquez Salazar, J.J.; Lehr, A.; Gonzalez, J.; Aranda, C.R.; Navarro-Contreras, H.R.; Raniere, M.O.; Harvey, M.; et al. Detection of SARS-CoV-2 and its S and N proteins using surface enhanced Raman spectroscopy. RSC Adv. 2021, 11, 25788–25794. [Google Scholar] [CrossRef]
- Shan, B.; Broza, Y.Y.; Li, W.; Wang, Y.; Wu, S.; Liu, Z.; Wang, J.; Gui, S.; Wang, L.; Zhang, Z.; et al. Multiplexed nanomaterial-based sensor array for detection of COVID-19 in exhaled breath. ACS Nano 2020, 14, 12125–12132. [Google Scholar] [CrossRef]
- Carlomagno, C.; Bertazioli, D.; Gualerzi, A.; Picciolini, S.; Banfi, P.I.; Lax, A.; Messina, E.; Navarro, J.; Bianchi, L.; Caronni, A.; et al. COVID-19 salivary Raman fingerprint: Innovative approach for the detection of current and past SARS-CoV-2 infections. Sci. Rep. 2021, 11, 4943. [Google Scholar] [CrossRef]
- Sur, U.K.; Santra, C. Spectroscopy: A versatile sensing tool for cost-effective and rapid detection of novel coronavirus (COVID-19). Emergent Mater. 2022, 5, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, X.; Ma, R.; Deng, S.; Wang, X.; Wang, X.; Zhang, X.; Huang, X.; Liu, Y.; Li, G.; et al. Ultra-fast and onsite interrogation of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in waters via surface enhanced Raman scattering (SERS). Water Res. 2021, 200, 117243. [Google Scholar] [CrossRef]
- Kim, W.; Kim, S.; Han, J.; Kim, T.G.; Bang, A.; Choi, H.W.; Min, G.E.; Shin, J.-H.; Moon, S.W.; Choi, S. An excitation wavelength-optimized, stable SERS biosensing nanoplatform for analyzing adenoviral and AstraZeneca COVID-19 vaccination efficacy status using tear samples of vaccinated individuals. Biosens. Bioelectron. 2022, 204, 114079. [Google Scholar] [CrossRef] [PubMed]
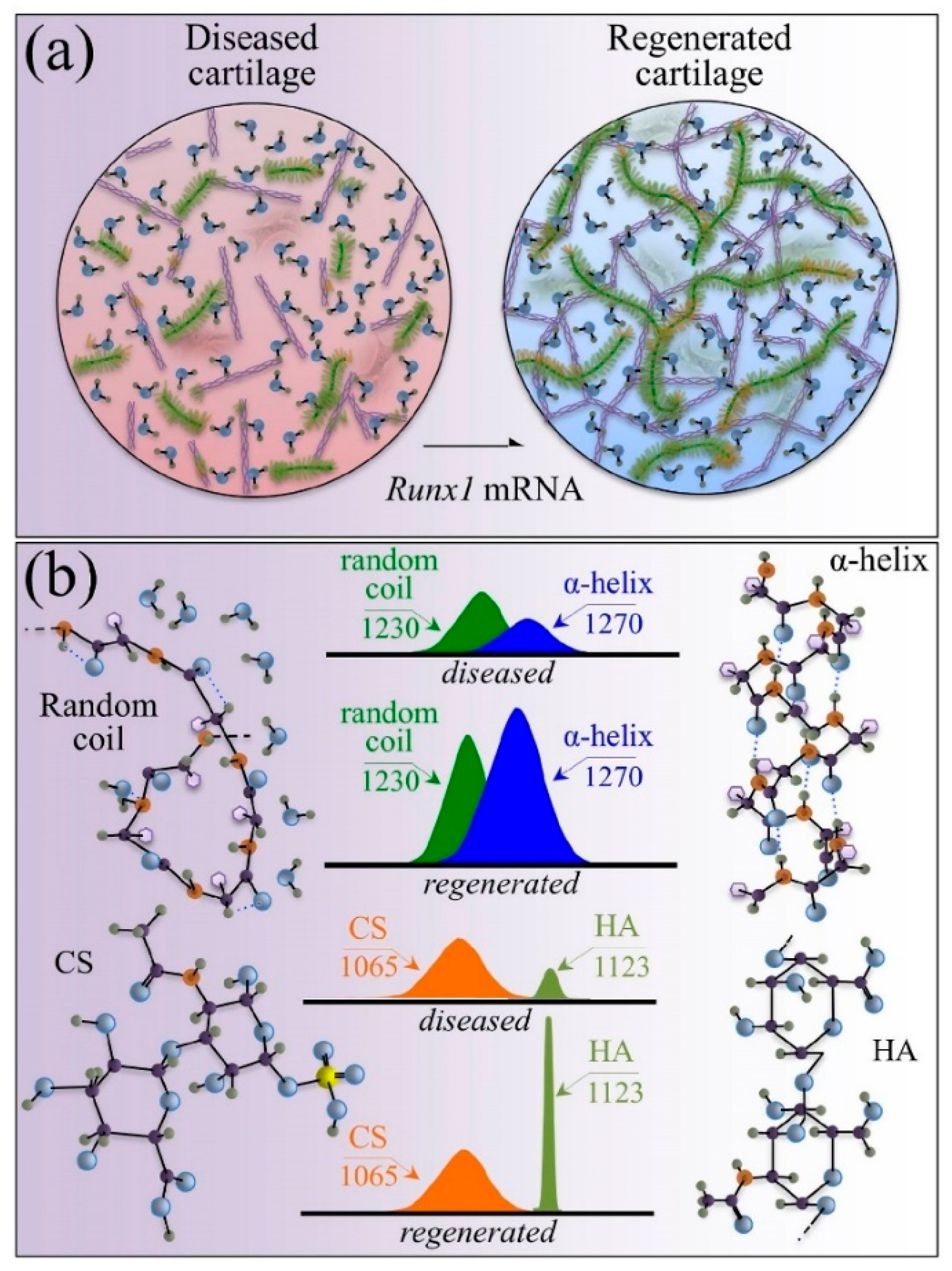
- Pezzotti, G.; Zhu, W.; Terai, Y.; Marin, E.; Boschetto, F.; Kawamoto, K.; Itaka, K. Raman spectroscopic insight into osteoarthritic cartilage regeneration by mRNA therapeutics encoding cartilage-anabolic transcription factor Runx1. Mater. Today Bio 2022, 13, 100210. [Google Scholar] [CrossRef]
- Huang, W.E.; Griffiths, R.I.; Thompson, I.P.; Bailey, M.J.; Whiteley, A.S. Raman Microscopic Analysis of Single Microbial Cells. Anal. Chem. 2004, 76, 4452–4458. [Google Scholar] [CrossRef]
- Xie, C.; Mace, J.; Dinno, M.A. Identification of Single Bacterial Cells in Aqueous Solution Using Confocal Laser Tweezers Raman Spectroscopy. Anal. Chem. 2005, 77, 4390–4397. [Google Scholar] [CrossRef]
- Saikia, D.; Jadhav, P.; Hole, A.R.; Krishna, C.M.; Singh, S.P. Growth Kinetics Monitoring of Gram-Negative Pathogenic Microbes Using Raman Spectroscopy. Appl. Spectrosc. 2022, 76, 1263–1271. [Google Scholar] [CrossRef]
- Nanda, S.S.; Yi, D.K.; Kim, K. Study of antibacterial mechanism of graphene oxide using Raman spectroscopy. Sci. Rep. 2016, 6, 28443. [Google Scholar] [CrossRef]
- Mukherjee, R.; Verma, T.; Nandi, D.; Umapathy, S. Understanding the Effects of Culture Conditions in Bacterial Growth: A Biochem ical Perspective Using Raman Microscopy. J. Biophotonics 2020, 13, e201900233. [Google Scholar] [CrossRef]
- Nanda, S.S.; Kim, B.J.; Kim, K.W.; Nasir, T.; Park, J.; Yun, K.; Hembram, K.P.S.S.; Papaefthymiou, G.C.; Choi, J.-Y.; Yi, D.K. A new device concept for bacterial sensing by Raman spectroscopy and voltage-gated monolayer graphene. Nanoscale 2019, 11, 8528–8537. [Google Scholar] [CrossRef]
- Hlaing, M.M.; Dunn, M.; Stoddart, P.R.; McArthur, S.L. Raman Spectroscopic Identification of Single Bacterial Cells at Different Stages of Their Lifecycle. Vib. Spectrosc. 2016, 86, 81–89. [Google Scholar] [CrossRef]
- Dukes, P.V.; Strobbia, P.; Ngo, H.T.; Odion, R.A.; Rocke, D.; Lee, W.T.; Vo-Dinh, T. Plasmonic assay for amplification-free cancer biomarkers detection in clinical tissue samples. Anal. Chim. Acta 2020, 1139, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Zavaleta, C.L.; Garai, E.; Liu, J.T.; Sensarn, S.; Mandella, M.J.; Van de Sompel, D.; Friedland, S.; Van Dam, J.; Contag, C.H.; Gambhir, S.S. A Raman-based endoscopic strategy for multiplexed molecular imaging. Proc. Natl. Acad. Sci. USA 2013, 110, E2288–E2297. [Google Scholar] [CrossRef] [PubMed]
- Shu, J.; Qiu, Z.; Lin, Z.; Cai, G.; Yang, H.; Tang, D. Semiautomated support photoelectrochemical immunosensing platform for portable and high-throughput immunoassay based on Au nanocrystal decorated specific crystal facets BiVO4 photoanode. Anal. Chem. 2016, 88, 12539–12546. [Google Scholar] [CrossRef]
- Jaeckle, E.; Brauchle, E.; Nottrodt, N.; Wehner, M.; Lensing, R.; Gillner, A.; Schenke-Layland, K.; Bach, M.; Burger-Kentischer, A. Towards automation in biologics production via Raman micro-spectroscopy, laser-induced forward cell transfer and surface-enhanced Raman spectroscopy. J. Biotechnol. 2020, 323, 313–321. [Google Scholar] [CrossRef]
- Schwarz, H.; Mäkinen, M.E.; Castan, A.; Chotteau, V. Monitoring of amino acids and antibody N-glycosylation in high cell density perfusion culture based on Raman spectroscopy. Biochem. Eng. J. 2022, 182, 108426. [Google Scholar] [CrossRef]
- Graf, A.; Lemke, J.; Schulze, M.; Söldner, R.; Rebner, K.; Hoehse, M.; Matuszczyk, J. A novel approach for non-invasive continuous in-line control of perfusion cell cultivations by Raman spectroscopy. Front. Bioeng. Biotechnol. 2022, 10, 719614. [Google Scholar] [CrossRef]
- Larger-Scale, S.T.B. Raman Spectrometric PAT Models. BioProcess Int. 2022, 20, 9. [Google Scholar]
- Feidl, F.; Garbellini, S.; Luna, M.F.; Vogg, S.; Souquet, J.; Broly, H.; Morbidelli, M.; Butté, A. Combining mechanistic modeling and Raman spectroscopy for monitoring antibody chromatographic purification. Processes 2019, 7, 683. [Google Scholar] [CrossRef]
- Hauptmann, A.; Hoelzl, G.; Mueller, M.; Bechtold-Peters, K.; Loerting, T. Raman marker bands for secondary structure changes of frozen therapeutic monoclonal antibody formulations during thawing. J. Pharm. Sci. 2023, 112, 51–60. [Google Scholar] [CrossRef]
- Wei, B.; Woon, N.; Dai, L.; Fish, R.; Tai, M.; Handagama, W.; Yin, A.; Sun, J.; Maier, A.; McDaniel, D.; et al. Multi-attribute Raman spectroscopy (MARS) for monitoring product quality attributes in formulated monoclonal antibody therapeutics. MAbs 2022, 14, 2007564. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Mallela, K.M.; Deorkar, N.; Brophy, G. Manufacturing challenges and rational formulation development for AAV viral vectors. J. Pharm. Sci. 2021, 110, 2609–2624. [Google Scholar] [CrossRef] [PubMed]
- Guardalini, L.G.O.; da Silva Cavalcante, P.E.; Leme, J.; de Mello, R.G.; Bernardino, T.C.; Astray, R.M.; Barbosa, E.; da Silveira, S.R.; Ho, P.L.; Tonso, A.; et al. Biochemical monitoring throughout all stages of rabies virus-like particles production by Raman spectroscopy using global models. J. Biotechnol. 2023, 363, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Matuszczyk, J.C.; Zijlstra, G.; Ede, D.; Ghaffari, N.; Yuh, J.; Brivio, V. Raman spectroscopy provides valuable process insights for cell-derived and cellular products. Curr. Opin. Biotechnol. 2023, 81, 102937. [Google Scholar] [CrossRef]
- Morder, C.J.; Scarpitti, B.T.; Balss, K.M.; Schultz, Z.D. Determination of lentiviral titer by surface enhanced Raman scattering. Anal. Methods 2022, 14, 1387–1395. [Google Scholar] [CrossRef]
- Lothert, K.; Eilts, F.; Wolff, M.W. Quantification methods for viruses and virus-like particles applied in biopharmaceutical production processes. Expert Rev. Vaccines 2022, 21, 1029–1044. [Google Scholar] [CrossRef]
- Chen, M.; McReynolds, N.; Campbell, E.C.; Mazilu, M.; Barbosa, J.; Dholakia, K.; Powis, S.J. The use of wavelength modulated Raman spectroscopy in label-free identification of T lymphocyte subsets, natural killer cells and dendritic cells. PLoS ONE 2015, 10, e0125158. [Google Scholar] [CrossRef]
- Hobro, A.J.; Kumagai, Y.; Akira, S.; Smith, N.I. Raman spectroscopy as a tool for label-free lymphocyte cell line discrimination. Analyst 2016, 141, 3756–3764. [Google Scholar] [CrossRef]
- Ichimura, T.; Chiu, L.D.; Fujita, K.; Machiyama, H.; Yamaguchi, T.; Watanabe, T.M.; Fujita, H. Non-label immune cell state prediction using Raman spectroscopy. Sci. Rep. 2016, 6, 37562. [Google Scholar] [CrossRef]
- Pistiki, A.; Ramoji, A.; Ryabchykov, O.; Thomas-Rüddel, D.; Press, A.T.; Makarewicz, O.; Giamarellos-Bourboulis, E.J.; Bauer, M.; Bocklitz, T.; Popp, J.; et al. Biochemical analysis of leukocytes after in vitro and in vivo activation with bacterial and fungal pathogens using Raman spectroscopy. Int. J. Mol. Sci. 2021, 22, 10481. [Google Scholar] [CrossRef]
- Pavillon, N.; Hobro, A.J.; Akira, S.; Smith, N.I. Noninvasive detection of macrophage activation with single-cell resolution through machine learning. Proc. Natl. Acad. Sci. USA 2018, 115, E2676–E2685. [Google Scholar] [CrossRef] [PubMed]
- Gavgiotaki, E.; Filippidis, G.; Zerva, I.; Kenanakis, G.; Archontakis, E.; Agelaki, S.; Georgoulias, V.; Athanassakis, I. Detection of the T cell activation state using nonlinear optical microscopy. J. Biophotonics 2019, 12, e201800277. [Google Scholar] [CrossRef] [PubMed]
- Ramoji, A.; Thomas-Rüddel, D.; Ryabchykov, O.; Bauer, M.; Arend, N.; Giamarellos-Bourboulis, E.J.; Eugen-Olsen, J.; Kiehntopf, M.; Bocklitz, T.; Popp, J.; et al. Leukocyte activation profile assessed by Raman spectroscopy helps diagnosing infection and sepsis. Crit. Care Explor. 2021, 3, e0394. [Google Scholar] [CrossRef] [PubMed]
- Agsalda-Garcia, M.; Shieh, T.; Souza, R.; Kamada, N.; Loi, N.; Oda, R.; Acosta-Maeda, T.; Choi, S.Y.; Lim, E.; Misra, A.; et al. Raman-enhanced spectroscopy (RESpect) probe for childhood non-Hodgkin lymphoma. SciMedicine J. 2020, 2, 1. [Google Scholar] [CrossRef]
- Ghita, A.; Pascut, F.C.; Sottile, V.; Denning, C.; Notingher, I. Applications of Raman micro-spectroscopy to stem cell technology: Label-free molecular discrimination and monitoring cell differentiation. EPJ Tech. Instrum. 2015, 2, 6. [Google Scholar] [CrossRef]
- Germond, A.; Panina, Y.; Shiga, M.; Niioka, H.; Watanabe, T.M. Following embryonic stem cells, their differentiated progeny, and cell-state changes during iPS reprogramming by Raman spectroscopy. Anal. Chem. 2020, 92, 14915–14923. [Google Scholar] [CrossRef]
- Hsu, C.C.; Xu, J.; Brinkhof, B.; Wang, H.; Cui, Z.; Huang, W.E.; Ye, H. A single-cell Raman-based platform to identify developmental stages of human pluripotent stem cell-derived neurons. Proc. Natl. Acad. Sci. USA 2020, 117, 18412–18423. [Google Scholar] [CrossRef]
- Kukolj, T.; Lazarević, J.; Borojević, A.; Ralević, U.; Vujić, D.; Jauković, A.; Lazarević, N.; Bugarski, D. A single-cell Raman spectroscopy analysis of bone marrow mesenchymal stem/stromal cells to identify inter-individual diversity. Int. J. Mol. Sci. 2022, 23, 4915. [Google Scholar] [CrossRef]
- Skok, J.; Megušar, P.; Vodopivec, T.; Pregeljc, D.; Mencin, N.; Korenč, M.; Krušič, A.; Celjar, A.M.; Pavlin, N.; Krušič, J.; et al. Gram-scale mRNA production using a 250-mL single-use bioreactor. Chem. Ing. Tech. 2022, 94, 1928–1935. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nanda, S.S.; Park, D.-G.; Yi, D.K. Current Trends in In Vitro Diagnostics Using Surface-Enhanced Raman Scattering in Translational Biomedical Research. Biosensors 2025, 15, 265. https://doi.org/10.3390/bios15050265
Nanda SS, Park D-G, Yi DK. Current Trends in In Vitro Diagnostics Using Surface-Enhanced Raman Scattering in Translational Biomedical Research. Biosensors. 2025; 15(5):265. https://doi.org/10.3390/bios15050265
Chicago/Turabian StyleNanda, Sitansu Sekhar, Dae-Gyeom Park, and Dong Kee Yi. 2025. "Current Trends in In Vitro Diagnostics Using Surface-Enhanced Raman Scattering in Translational Biomedical Research" Biosensors 15, no. 5: 265. https://doi.org/10.3390/bios15050265
APA StyleNanda, S. S., Park, D.-G., & Yi, D. K. (2025). Current Trends in In Vitro Diagnostics Using Surface-Enhanced Raman Scattering in Translational Biomedical Research. Biosensors, 15(5), 265. https://doi.org/10.3390/bios15050265






