Simultaneous Trace Analysis of Lead and Cadmium in Drinking Water, Milk, and Honey Samples Through Modified Screen-Printed Electrode
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Apparatus
2.3. Preparation of Modified Electrodes
2.4. Electrochemical Analysis Procedure with N-rGO@ppy/GCE
2.5. Sample Pretreatment
2.6. Real Sample Analysis with Modified Commercial SPE (N-rGO@ppy/SPE)
3. Results and Discussion
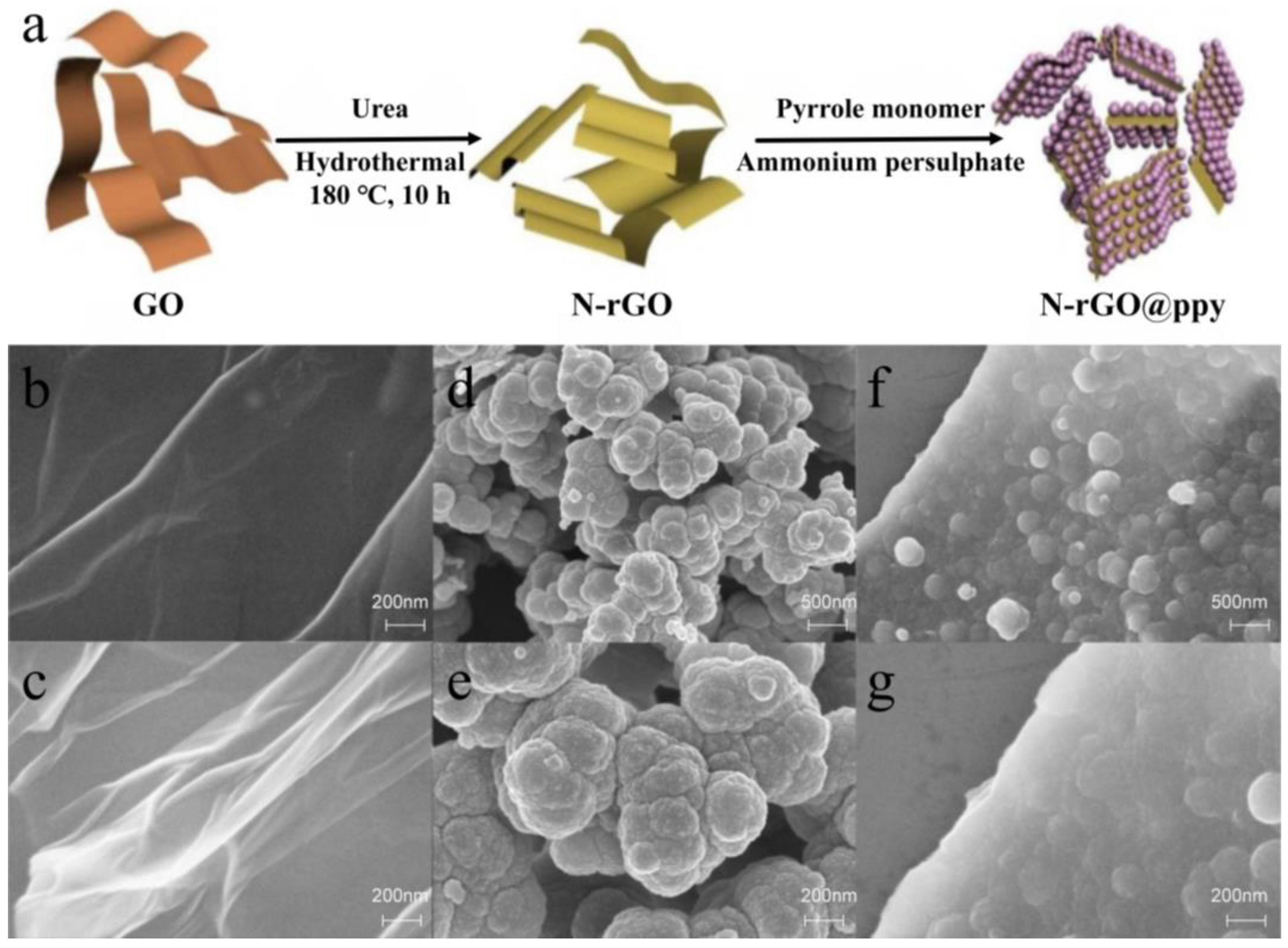
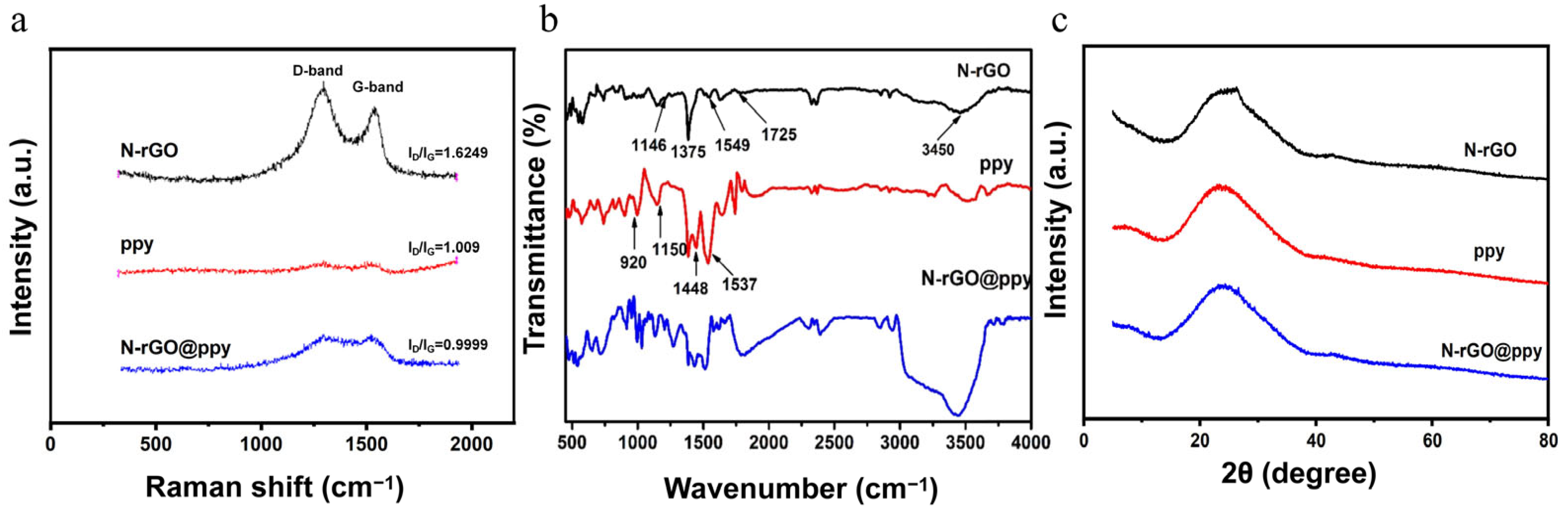
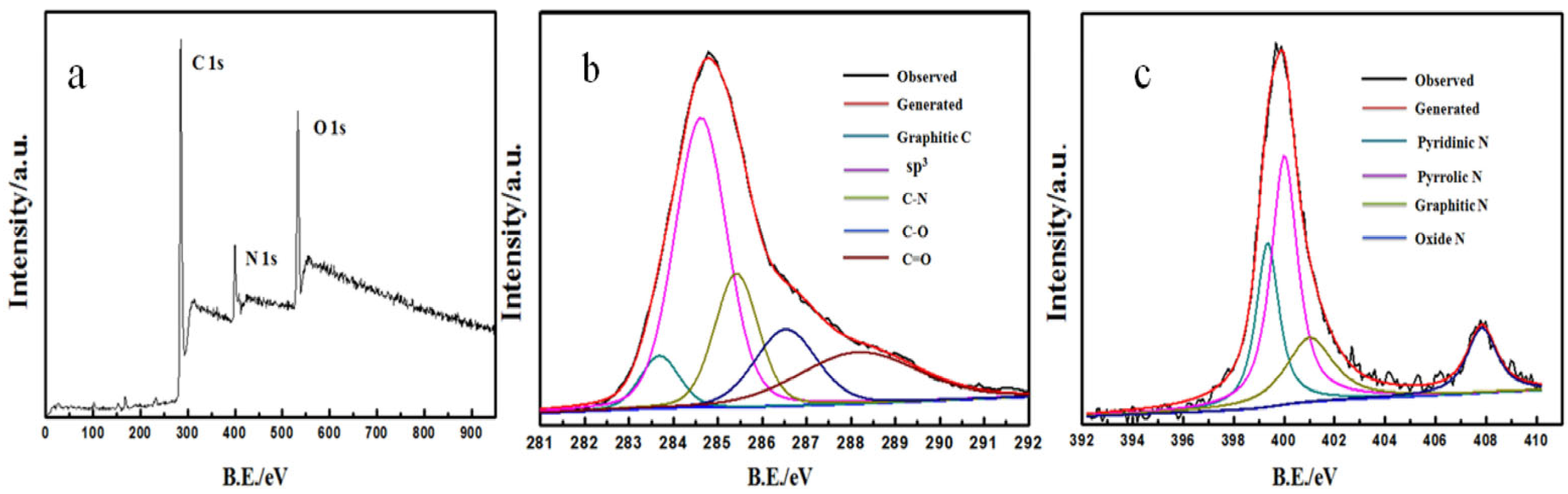
3.1. Characterization of N-rGO@ppy
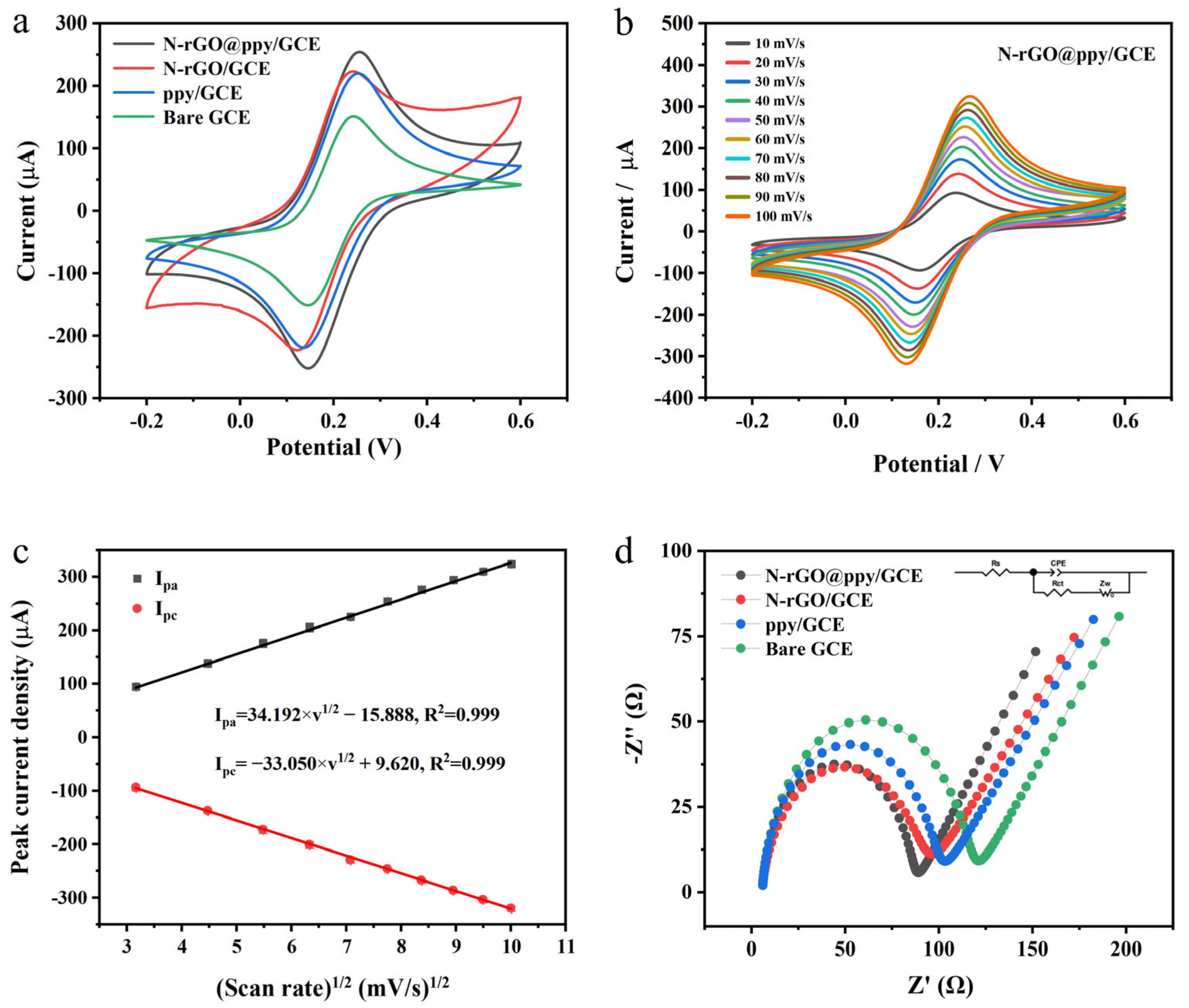
3.2. Electrochemical Behavior of GCEs
3.3. Optimization of Electrochemical Detection Parameters for N-rGO@ppy/GCE
3.4. Analytical Performance of GCEs for Simultaneous Detection of Cd2+ and Pb2+
3.5. Interference Resistance, Stability, and Repeatability of N-rGO@ppy/GCE
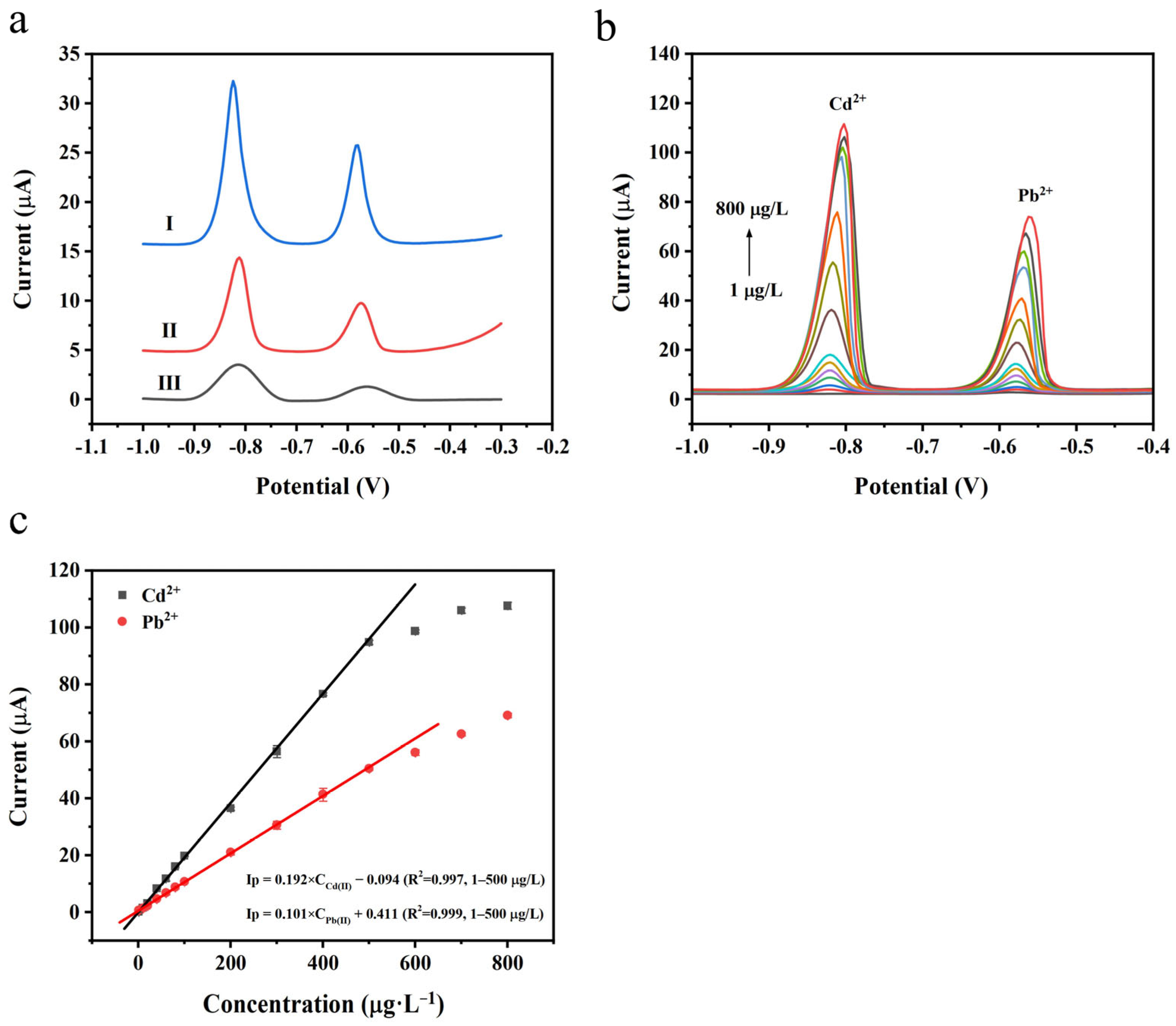
3.6. Practical Application of N-rGO@ppy/SPE
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kobielska, P.A.; Howarth, A.J.; Farha, O.K.; Nayak, S. Metal–organic frameworks for heavy metal removal from water. Coord. Chem. Rev. 2018, 358, 92–107. [Google Scholar] [CrossRef]
- Ali, Z.; Ullah, R.; Tuzen, M.; Ullah, S.; Rahim, A.; Saleh, T.A. Colorimetric sensing of heavy metals on metal doped metal oxide nanocomposites: A review. Trends Environ. Anal. Chem. 2023, 37, e00187. [Google Scholar] [CrossRef]
- Zhou, J.; Pan, K.; Qu, G.; Ji, W.; Ning, P.; Tang, H.; Xie, R. Rgo/mwcnts-cooh 3d hybrid network as a high-performance electrochemical sensing platform of screen-printed carbon electrodes with an ultra-wide detection range of cd (ii) and pb (ii). Chem. Eng. J. 2022, 449, 137853. [Google Scholar] [CrossRef]
- Tóth, G.; Hermann, T.; Da Silva, M.R.; Montanarella, L. Heavy metals in agricultural soils of the european union with implications for food safety. Environ. Int. 2016, 88, 299–309. [Google Scholar] [CrossRef]
- Liu, T.P.; Liu, J.X.; Mao, X.F.; Jiang, X.M.; Zhao, Y.B.; Qian, Y.Z. Rapid and portable detection of hg and cd in grain samples based on novel catalytic pyrolysis composite trap coupled with miniature atomic absorption spectrometry. Foods 2023, 12, 1778. [Google Scholar] [CrossRef]
- Liu, T.; Liu, J.; Mao, X.; Huang, X.; Qian, Y. Novel platinum-nickel composite trap for simultaneous and direct determination of mercury and cadmium in soil and its mechanism study. Anal. Chem. 2022, 95, 594–601. [Google Scholar] [CrossRef]
- Chen, T.; He, M.; Chen, B.; Hu, B. Thiol-functionalized mof modified 3d printed monolithic microextraction array for analysis of trace cd and pb in human urine. Talanta 2025, 281, 126859. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Wang, C.C.; Fan, M.L.; Cai, W.S.; Shao, X.G. Quantitative analysis of heave mental ion based on portable nir spectrometer. Guang Pu Xue Yu Guang Pu Fen Xi = Guang Pu 2016, 36, 4100–4104. [Google Scholar]
- Cui, L.; Wu, J.; Ju, H. Electrochemical sensing of heavy metal ions with inorganic, organic and bio-materials. Biosens. Bioelectron. 2015, 63, 276–286. [Google Scholar] [CrossRef]
- Rudnitskaya, A.; Legin, A.; Seleznev, B.; Kirsanov, D.; Vlasov, Y. Detection of ultra-low activities of heavy metal ions by an array of potentiometric chemical sensors. Microchim. Acta 2008, 163, 71–80. [Google Scholar] [CrossRef]
- Asadian, E.; Ghalkhani, M.; Shahrokhian, S. Electrochemical sensing based on carbon nanoparticles: A review. Sens. Actuators B Chem. 2019, 293, 183–209. [Google Scholar] [CrossRef]
- Fort, C.I.; Sanou, A.; Coulibaly, M.; Yao, K.B.; Turdean, G.L. Green modified electrode for sensitive simultaneous heavy metal ions electrodetection. Sens. Actuators B Chem. 2024, 418, 136326. [Google Scholar] [CrossRef]
- Bansod, B.; Kumar, T.; Thakur, R.; Rana, S.; Singh, I. A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms. Biosens. Bioelectron. 2017, 94, 443–455. [Google Scholar] [CrossRef]
- Rotariu, L.; Lagarde, F.; Jaffrezic-Renault, N.; Bala, C. Electrochemical biosensors for fast detection of food contaminants trends and perspective. TrAC Trends Anal. Chem. 2016, 79, 80–87. [Google Scholar] [CrossRef]
- Li, B.; Xie, X.; Meng, T.; Guo, X.; Li, Q.; Yang, Y.; Jin, H.; Jin, C.; Meng, X.; Pang, H. Recent advance of nanomaterials modified electrochemical sensors in the detection of heavy metal ions in food and water. Food Chem. 2024, 440, 138213. [Google Scholar] [CrossRef]
- Jovanovski, V.; Hocevar, S.B.; Ogorevc, B. Bismuth electrodes in contemporary electroanalysis. Curr. Opin. Electrochem. 2017, 3, 114–122. [Google Scholar] [CrossRef]
- Waheed, A.; Mansha, M.; Ullah, N. Nanomaterials-based electrochemical detection of heavy metals in water: Current status, challenges and future direction. TrAC Trends Anal. Chem. 2018, 105, 37–51. [Google Scholar] [CrossRef]
- Jain, R.; Jadon, N.; Pawaiya, A. Polypyrrole based next generation electrochemical sensors and biosensors: A review. TrAC Trends Anal. Chem. 2017, 97, 363–373. [Google Scholar] [CrossRef]
- Wang, N.; Zhao, W.; Shen, Z.; Sun, S.; Dai, H.; Ma, H.; Lin, M. Sensitive and selective detection of pb (ii) and cu (ii) using a metal-organic framework/polypyrrole nanocomposite functionalized electrode. Sens. Actuators B Chem. 2020, 304, 127286. [Google Scholar] [CrossRef]
- Li, X.; Zhou, H.; Wu, W.; Wei, S.; Xu, Y.; Kuang, Y. Studies of heavy metal ion adsorption on chitosan/sulfydryl-functionalized graphene oxide composites. J. Colloid Interface Sci. 2015, 448, 389–397. [Google Scholar] [CrossRef]
- Chen, D.; Feng, H.; Li, J. Graphene oxide: Preparation, functionalization, and electrochemical applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Zhang, S.; Huang, T.; Cui, F.; Xing, B. Effects of ph and electrolytes on the sheet-to-sheet aggregation mode of graphene oxide in aqueous solutions. Environ. Sci. Nano 2020, 7, 984–995. [Google Scholar] [CrossRef]
- Yao, Y.; Wu, H.; Ping, J. Simultaneous determination of Cd(ii) and Pb(ii) ions in honey and milk samples using a single-walled carbon nanohorns modified screen-printed electrochemical sensor. Food Chem. 2019, 274, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Priya, T.; Dhanalakshmi, N.; Thennarasu, S.; Pulikkutty, S.; Karthikeyan, V.; Thinakaran, N. Synchronous detection of cadmium and lead in honey, cocos nucifera and egg white samples using multiwalled carbon nanotube/hyaluronic acid/amino acids nanocomposites. Food Chem. 2020, 317, 126430. [Google Scholar] [CrossRef]
- Chouhan, A.; Mungse, H.P.; Khatri, O.P. Surface chemistry of graphene and graphene oxide: A versatile route for their dispersion and tribological applications. Adv. Colloid Interface Sci. 2020, 283, 102215. [Google Scholar] [CrossRef]
- Ji, Q.; Hu, C.; Liu, H.; Qu, J. Development of nitrogen-doped carbon for selective metal ion capture. Chem. Eng. J. 2018, 350, 608–615. [Google Scholar] [CrossRef]
- Yildiz, G.; Bolton-Warberg, M.; Awaja, F. Graphene and graphene oxide for bio-sensing: General properties and the effects of graphene ripples. Acta Biomater. 2021, 131, 62–79. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, X.; Chen, Y.; Yu, P.; Wang, C.; Ma, Y. Enhanced capacitance and rate capability of graphene/polypyrrole composite as electrode material for supercapacitors. J. Power Sources 2011, 196, 5990–5996. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Zhang, Y.; Wu, J.; Li, S.; Li, L. A disposable electrochemical sensor for lead ion detection based on in situ polymerization of conductive polypyrrole coating. J. Electron. Mater. 2023, 52, 1819–1828. [Google Scholar] [CrossRef]
- Peshoria, S.; Narula, A.K. One-pot synthesis of porphyrin@ polypyrrole hybrid and its application as an electrochemical sensor. Mater. Sci. Eng. B 2018, 229, 53–58. [Google Scholar] [CrossRef]
- Abdillah, O.B.; Rus, Y.B.; Ulfa, M.; Iskandar, F. Recent progress on reduced graphene oxide and polypyrrole composites for high performance supercapacitors: A review. J. Energy Storage 2023, 74, 109300. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, C.; Yue, B.; Gambhir, S.; Too, C.O.; Wallace, G.G. Electrochemically synthesized polypyrrole/graphene composite film for lithium batteries. Adv. Energy Mater. 2012, 2, 266–272. [Google Scholar] [CrossRef]
- Ammar, M.; Galy, N.; Rouzaud, J.; Toulhoat, N.; Vaudey, C.; Simon, P.; Moncoffre, N. Characterizing various types of defects in nuclear graphite using raman scattering: Heat treatment, ion irradiation and polishing. Carbon 2015, 95, 364–373. [Google Scholar] [CrossRef]
- Ma, R.; Ren, X.; Xia, B.Y.; Zhou, Y.; Sun, C.; Liu, Q.; Liu, J.; Wang, J. Novel synthesis of n-doped graphene as an efficient electrocatalyst towards oxygen reduction. Nano Res. 2016, 9, 808–819. [Google Scholar] [CrossRef]
- Si, Y.; Samulski, E.T. Synthesis of water soluble graphene. Nano Lett. 2008, 8, 1679–1682. [Google Scholar] [CrossRef]
- Lin, Z.; Waller, G.; Liu, Y.; Liu, M.; Wong, C.P. Facile synthesis of nitrogen-doped graphene via pyrolysis of graphene oxide and urea, and its electrocatalytic activity toward the oxygen-reduction reaction. Adv. Energy Mater. 2012, 2, 884–888. [Google Scholar] [CrossRef]
- Menon, V.P.; Lei, J.; Martin, C.R. Investigation of molecular and supermolecular structure in template-synthesized polypyrrole tubules and fibrils. Chem. Mater. 1996, 8, 2382–2390. [Google Scholar] [CrossRef]
- Zarei, M.; Samimi, A.; Khorram, M.; Abdi, M.M.; Golestaneh, S.I. Fabrication and characterization of conductive polypyrrole/chitosan/collagen electrospun nanofiber scaffold for tissue engineering application. Int. J. Biol. Macromol. 2021, 168, 175–186. [Google Scholar] [CrossRef]
- Balamurugan, J.; Thanh, T.D.; Heo, S.-B.; Kim, N.H.; Lee, J.H. Novel route to synthesis of n-doped graphene/cu–ni oxide composite for high electrochemical performance. Carbon 2015, 94, 962–970. [Google Scholar] [CrossRef]
- Fan, Z.; Zhu, J.; Sun, X.; Cheng, Z.; Liu, Y.; Wang, Y. High density of free-standing holey graphene/ppy films for superior volumetric capacitance of supercapacitors. Acs Appl. Mater. Interfaces 2017, 9, 21763–21772. [Google Scholar] [CrossRef]
- Hu, C.; Dai, L. Doping of carbon materials for metal-free electrocatalysis. Adv. Mater. 2019, 31, 1804672. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Paul, R.; Dai, Q.; Dai, L. Carbon-based metal-free electrocatalysts: From oxygen reduction to multifunctional electrocatalysis. Chem. Soc. Rev. 2021, 50, 11785–11843. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Jo, M.R.; Kang, M.; Huh, Y.S.; Jung, H.; Kang, Y.-M. Rapid and controllable synthesis of nitrogen doped reduced graphene oxide using microwave-assisted hydrothermal reaction for high power-density supercapacitors. Carbon 2014, 73, 106–113. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, L.; Ong, C.N.; Xie, J. Nitrogen-doped graphene nanosheets as reactive water purification membranes. Nano Res. 2016, 9, 1983–1993. [Google Scholar] [CrossRef]
- Shin, C.-H.; Ted, H.Y.; Lee, H.-Y.; Lee, B.-J.; Kwon, S.; Goddard, W.A., III; Yu, J.-S. Ru-loaded pyrrolic-n-doped extensively graphitized porous carbon for high performance electrochemical hydrogen evolution. Appl. Catal. B Environ. 2023, 334, 122829. [Google Scholar] [CrossRef]
- Tajik, S.; Beitollahi, H.; Jang, H.W.; Shokouhimehr, M. A screen printed electrode modified with Fe3O4@ polypyrrole-pt core-shell nanoparticles for electrochemical detection of 6-mercaptopurine and 6-thioguanine. Talanta 2021, 232, 122379. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, S. Electrochemical sensors based on nitrogen-doped reduced graphene oxide for the simultaneous detection of ascorbic acid, dopamine and uric acid. J. Alloys Compd. 2020, 842, 155873. [Google Scholar] [CrossRef]
- Ren, X.; Zhao, Q.; McCulloch, W.D.; Wu, Y. Mos 2 as a long-life host material for potassium ion intercalation. Nano Res. 2017, 10, 1313–1321. [Google Scholar] [CrossRef]
- Augustyn, V.; Come, J.; Lowe, M.A.; Kim, J.W.; Taberna, P.-L.; Tolbert, S.H.; Abruña, H.D.; Simon, P.; Dunn, B. High-rate electrochemical energy storage through li+ intercalation pseudocapacitance. Nat. Mater. 2013, 12, 518–522. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.; Lu, Y.; Wang, W.; Peng, T.; Zhang, Y.; Guo, Y.; Wang, Y.; Huo, K.; Kim, J.-K. Cable-like double-carbon layers for fast ion and electron transport: An example of cnt@ nct@ mno2 3d nanostructure for high-performance supercapacitors. Carbon 2019, 143, 335–342. [Google Scholar] [CrossRef]
- Chen, X.; Oh, W.-D.; Hu, Z.-T.; Sun, Y.-M.; Webster, R.D.; Li, S.-Z.; Lim, T.-T. Enhancing sulfacetamide degradation by peroxymonosulfate activation with n-doped graphene produced through delicately-controlled nitrogen functionalization via tweaking thermal annealing processes. Appl. Catal. B Environ. 2018, 225, 243–257. [Google Scholar] [CrossRef]
- Kang, W.; Pei, X.; Rusinek, C.A.; Bange, A.; Haynes, E.N.; Heineman, W.R.; Papautsky, I. Determination of lead with a copper-based electrochemical sensor. Anal. Chem. 2017, 89, 3345–3352. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Wang, N.; Wang, D.; Ma, H.; Lin, M. An electrochemical sensor based on phytic acid functionalized polypyrrole/graphene oxide nanocomposites for simultaneous determination of Cd (II) and Pb (II). Chem. Eng. J. 2016, 299, 150–155. [Google Scholar] [CrossRef]
- Xiao, L.; Xu, H.; Zhou, S.; Song, T.; Wang, H.; Li, S.; Gan, W.; Yuan, Q. Simultaneous detection of Cd (II) and Pb (II) by differential pulse anodic stripping voltammetry at a nitrogen-doped microporous carbon/nafion/bismuth-film electrode. Electrochim. Acta 2014, 143, 143–151. [Google Scholar] [CrossRef]
- Ping, J.; Wang, Y.; Wu, J.; Ying, Y. Development of an electrochemically reduced graphene oxide modified disposable bismuth film electrode and its application for stripping analysis of heavy metals in milk. Food Chem. 2014, 151, 65–71. [Google Scholar] [CrossRef]
- Baghayeri, M.; Alinezhad, H.; Fayazi, M.; Tarahomi, M.; Ghanei-Motlagh, R.; Maleki, B. A novel electrochemical sensor based on a glassy carbon electrode modified with dendrimer functionalized magnetic graphene oxide for simultaneous determination of trace Pb (II) and Cd (II). Electrochim. Acta 2019, 312, 80–88. [Google Scholar] [CrossRef]
- Baghayeri, M.; Amiri, A.; Maleki, B.; Alizadeh, Z.; Reiser, O. A simple approach for simultaneous detection of cadmium (ii) and lead (ii) based on glutathione coated magnetic nanoparticles as a highly selective electrochemical probe. Sens. Actuators B Chem. 2018, 273, 1442–1450. [Google Scholar] [CrossRef]
- Cui, L.; Wu, J.; Ju, H. Synthesis of bismuth-nanoparticle-enriched nanoporous carbon on graphene for efficient electrochemical analysis of heavy-metal ions. Chem.—A Eur. J. 2015, 21, 11525–11530. [Google Scholar] [CrossRef]
- Wang, Y.; Nie, Z.; Li, X.; Wang, R.; Zhao, Y.; Wang, H. Ppy-functionalized NiFe2O4 nanocomposites toward highly selective Pb2+ electrochemical sensing. ACS Sustain. Chem. Eng. 2022, 10, 6082–6093. [Google Scholar] [CrossRef]
- Guo, X.; Cui, R.; Huang, H.; Li, Y.; Liu, B.; Wang, J.; Zhao, D.; Dong, J.; Sun, B. Insights into the role of pyrrole doped in three-dimensional graphene aerogels for electrochemical sensing Cd (ii). J. Electroanal. Chem. 2020, 871, 114323. [Google Scholar] [CrossRef]
- Dai, H.; Qin, J.; Han, Z.; Xue, Q. A phytic acid (pa)-doped polypyrrole (ppy)/molybdenum disulfide (mos2) nanocomposite-modified electrode for simultaneous electrochemical analysis of Pb2+ and Cd2+ in water. Polym. Bull. 2024, 81, 6891–6903. [Google Scholar] [CrossRef]
- Raju, C.V.; Cho, C.H.; Rani, G.M.; Manju, V.; Umapathi, R.; Huh, Y.S.; Park, J.P. Emerging insights into the use of carbon-based nanomaterials for the electrochemical detection of heavy metal ions. Coord. Chem. Rev. 2023, 476, 214920. [Google Scholar] [CrossRef]
- Yu, J.; Lin, M.; Tan, Q.; Li, J. High-value utilization of graphite electrodes in spent lithium-ion batteries: From 3D waste graphite to 2D graphene oxide. J. Hazard. Mater. 2021, 401, 123715. [Google Scholar] [CrossRef] [PubMed]
- Arduini, F.; Calvo, J.Q.; Palleschi, G.; Moscone, D.; Amine, A. Bismuth-modified electrodes for lead detection. TrAC Trends Anal. Chem. 2010, 29, 1295–1304. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, Q.; Yang, B.; Xu, Q.; Xu, Q.; Hu, X. Electrochemical Sensor Construction Based on Nafion/Calcium Lignosulphonate Functionalized Porous Graphene Nanocomposite and Its Application for Simultaneous Detection of Trace Pb2+ and Cd2+. Sens. Actuators B Chem. 2018, 259, 540–551. [Google Scholar] [CrossRef]
- Pizarro, J.; Segura, R.; Tapia, D.; Navarro, F.; Fuenzalida, F.; Aguirre, M.J. Inexpensive and green electrochemical sensor for the determination of Cd(II) and Pb(II) by square wave anodic stripping voltammetry in bivalve mollusks. Food Chem. 2020, 321, 126682. [Google Scholar] [CrossRef]
- Huang, R.; Lv, J.; Chen, J.; Zhu, Y.; Zhu, J.; Wågberg, T.; Hu, G. Three-dimensional porous high boron-nitrogen-doped carbon for the ultrasensitive electrochemical detection of trace heavy metals in food samples. J. Hazard. Mater. 2023, 442, 130020. [Google Scholar] [CrossRef]
- Bashir, A.; Malik, L.A.; Ahad, S.; Manzoor, T.; Bhat, M.A.; Dar, G.; Pandith, A.H. Removal of heavy metal ions from aqueous system by ion-exchange and biosorption methods. Environ. Chem. Lett. 2019, 17, 729–754. [Google Scholar] [CrossRef]

| Electrode | Technique | Metal Ions | Linear Ranges (μg L−1) | Detection Limits (μg L−1) | References |
|---|---|---|---|---|---|
| PA/PPy/GO/GCE | DPASV | Cd2+ | 5–150 | 2.13 | [53] |
| Pb2+ | 5–150 | 0.41 | |||
| Nafion/Bi/NMC/GCE | DPASV | Cd2+ | 2–100 | 1.5 | [54] |
| Pb2+ | 0.5–100 | 0.05 | |||
| ERGNO/BiF/SPE | SWASV | Cd2+ | 1–60 | 0.5 | [55] |
| Pb2+ | 1–60 | 0.8 | |||
| GO-Fe3O4-PAMAM/GCE | SWASV | Cd2+ | 0.2–140 | 0.07 | [56] |
| Pb2+ | 0.4–120 | 0.13 | |||
| GSH@Fe3O4/MGCE | SWASV | Cd2+ | 0.5–100 | 0.171 | [57] |
| Pb2+ | 0.5–100 | 0.182 | |||
| BiNPs@NPCGSc/GCE | SWASV | Cd2+ | 9–90 | 0.5 | [58] |
| Pb2+ | 12–124 | 0.7 | |||
| NiFe2O4/PPy/GCE | SWASV | Pb2+ | 21–435 | 0.8 | [59] |
| 3DGO-Py10/GCE | SWASV | Cd2+ | 5–400 | 3.6 | [60] |
| PA-doped PPy/MoS2/GCE | DPASV | Cd2+ | 10–300 | 2.03 | [61] |
| Pb2+ | 10–300 | 1.78 | |||
| N-rGO@ppy/GCE | SWASV | Cd2+ | 1–500 | 0.029 | This work |
| Pb2+ | 1–500 | 0.080 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Peng, X.; Xiao, Z.; Ge, Y.; Tao, B.; Shou, Z.; Feng, Y.; Yuan, J.; Xiao, L. Simultaneous Trace Analysis of Lead and Cadmium in Drinking Water, Milk, and Honey Samples Through Modified Screen-Printed Electrode. Biosensors 2025, 15, 267. https://doi.org/10.3390/bios15050267
Wang F, Peng X, Xiao Z, Ge Y, Tao B, Shou Z, Feng Y, Yuan J, Xiao L. Simultaneous Trace Analysis of Lead and Cadmium in Drinking Water, Milk, and Honey Samples Through Modified Screen-Printed Electrode. Biosensors. 2025; 15(5):267. https://doi.org/10.3390/bios15050267
Chicago/Turabian StyleWang, Fei, Xiao Peng, Ziqian Xiao, Ying Ge, Bilin Tao, Zhaoyong Shou, Yifei Feng, Jing Yuan, and Liang Xiao. 2025. "Simultaneous Trace Analysis of Lead and Cadmium in Drinking Water, Milk, and Honey Samples Through Modified Screen-Printed Electrode" Biosensors 15, no. 5: 267. https://doi.org/10.3390/bios15050267
APA StyleWang, F., Peng, X., Xiao, Z., Ge, Y., Tao, B., Shou, Z., Feng, Y., Yuan, J., & Xiao, L. (2025). Simultaneous Trace Analysis of Lead and Cadmium in Drinking Water, Milk, and Honey Samples Through Modified Screen-Printed Electrode. Biosensors, 15(5), 267. https://doi.org/10.3390/bios15050267





