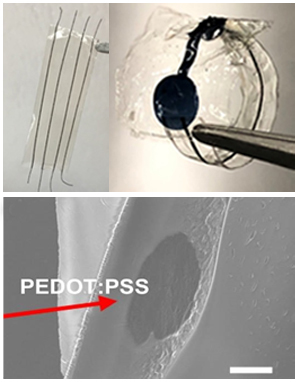
Silk Fibroin-Sheathed Conducting Polymer Wires as Organic Connectors for Biosensors
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Photocrosslinkable Silk Fibroin Proteins
2.3. Preparation of PEDOT:PSS Spinning Solution and Fibers
2.4. Fabrication of Sheathed Single or Multiple PEDOT:PSS Fibers
2.5. Electrical Characterization of the Sheathed PEDOT:PSS Wires
2.6. Fabrication of a Flexible Biosensor with the Integration of Coated PEDOT:PSS Fibers
2.7. Electrochemical Detection of Ascorbic Acid
3. Results and Discussion
3.1. Wet Spinning of Pure PEDOT:PSS Fibers
3.2. Properties of PEDOT:PSS Conducting Fibers
3.3. The Stability of PEDOT:PSS Fibers in Water
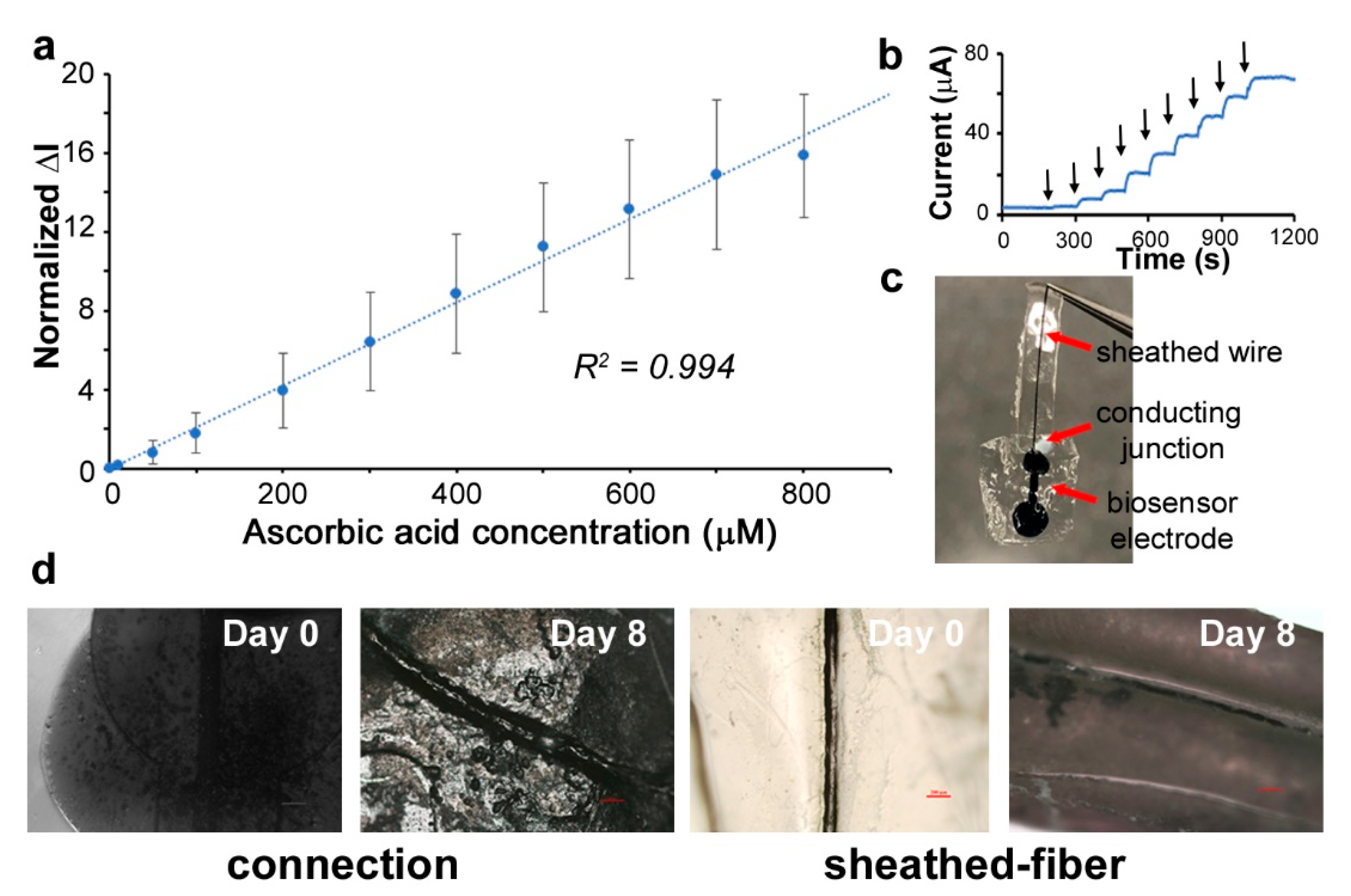
3.4. Sheathing of Conductive PEDOT:PSS Fibers to Form Insulated Organic Wires
3.5. Detection of Ascorbic Acid Using the Assembled Biodegradable Biosensor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, C.; Wang, C.; Huang, Z.; Xu, S. Materials and Structures toward Soft Electronics. Adv. Mater. 2018, 30, e1801368. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.; Lee, H.; Ghaffari, R.; Hyeon, T.; Kim, D.-H. Recent Advances in Flexible and Stretchable Bio-Electronic Devices Integrated with Nanomaterials. Adv. Mater. 2016, 28, 4203–4218. [Google Scholar] [CrossRef]
- Geddes, L.A.; Roeder, R. Criteria for the Selection of Materials for Implanted Electrodes. Ann. Biomed. Eng. 2003, 31, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.; Dong, Z.; Lee, N.; Liu, Y.; Xue, D.; Guo, X.; Kuhlmann, J.; Doepke, A.; Halsall, H.B.; Heineman, W.; et al. Revolutionizing biodegradable metals. Mater. Today 2009, 12, 22–32. [Google Scholar] [CrossRef]
- Green, R.; Abidian, M.R. Conducting Polymers for Neural Prosthetic and Neural Interface Applications. Adv. Mater. 2015, 27, 7620–7637. [Google Scholar] [CrossRef]
- Berggren, M.; Richter-Dahlfors, A. Organic bioelectronics. Adv. Mater. 2007, 19, 3201–3213. [Google Scholar] [CrossRef]
- Someya, T.; Bao, Z.; Malliaras, G.G. The rise of plastic bioelectronics. Nature 2016, 540, 379. [Google Scholar] [CrossRef]
- Lund, A.; Van Der Velden, N.M.; Persson, N.-K.; Hamedi, M.M.; Müller, C. Electrically conducting fibres for e-textiles: An open playground for conjugated polymers and carbon nanomaterials. Mater. Sci. Eng. R Rep. 2018, 126, 1–29. [Google Scholar] [CrossRef]
- Mirabedini, A.; Foroughi, J.; Farajikhah, S.; Wallace, G.G. Correction: Developments in conducting polymer fibres: From established spinning methods toward advanced applications. RSC Adv. 2016, 6, 108152. [Google Scholar] [CrossRef]
- Wallace, G.G.; Campbell, T.E.; Innis, P.C. Putting function into fashion: Organic conducting polymer fibres and textiles. Fibers Polym. 2007, 8, 135–142. [Google Scholar] [CrossRef]
- Gualandi, I.; Marzocchi, M.; Achilli, A.; Cavedale, D.; Bonfiglio, A.; Fraboni, B. Textile Organic Electrochemical Transistors as a Platform for Wearable Biosensors. Sci. Rep. 2016, 6, 33637. [Google Scholar] [CrossRef] [PubMed]
- Stoppa, M.; Chiolerio, A. Wearable Electronics and Smart Textiles: A Critical Review. Sensors 2014, 14, 11957–11992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Macosko, C.W.; White, H.S. Electrochemical Processing of Conducting Polymer Fibers. Science 1993, 259, 957–960. [Google Scholar] [CrossRef]
- Wang, W.; Li, W.; Zhang, R. The Preparation of Conducting Polymer Micro-and Nanofibers by Electrospinning. J. Polym. Mater. 2010, 27, 293. [Google Scholar]
- Lu, X.; Zhang, W.; Wang, C.; Wen, T.-C.; Wei, Y. One-dimensional conducting polymer nanocomposites: Synthesis, properties and applications. Prog. Polym. Sci. 2011, 36, 671–712. [Google Scholar] [CrossRef]
- Okuzaki, H.; Ishihara, M. Spinning and Characterization of Conducting Microfibers. Macromol. Rapid Commun. 2003, 24, 261–264. [Google Scholar] [CrossRef]
- Kim, N.; Kee, S.; Lee, S.H.; Lee, B.H.; Kahng, Y.H.; Jo, Y.R.; Kim, B.J.; Lee, K. Highly conductive PEDOT: PSS nanofibrils induced by solution-processed crystallization. Adv. Mater. 2014, 26, 2268–2272. [Google Scholar] [CrossRef]
- Xia, Y.; Ouyang, J. Significant Different Conductivities of the Two Grades of Poly(3,4-ethylenedioxythiophene):Poly(styrenesulfonate), Clevios P and Clevios PH1000, Arising from Different Molecular Weights. ACS Appl. Mater. Interfaces 2012, 4, 4131–4140. [Google Scholar] [CrossRef]
- Jalili, R.; Razal, J.M.; Wallace, G.G. Wet-spinning of PEDOT:PSS/Functionalized-SWNTs Composite: A Facile Route toward Production of Strong and Highly Conducting Multifunctional Fibers. Sci. Rep. 2013, 3, 3438. [Google Scholar] [CrossRef]
- Zhou, J.; Lubineau, G. Improving Electrical Conductivity in Polycarbonate Nanocomposites Using Highly Conductive PEDOT/PSS Coated MWCNTs. ACS Appl. Mater. Interfaces 2013, 5, 6189–6200. [Google Scholar] [CrossRef]
- Seyedin, M.Z.; Razal, J.M.; Innis, P.C.; Wallace, G.G. Strain-Responsive Polyurethane/PEDOT:PSS Elastomeric Composite Fibers with High Electrical Conductivity. Adv. Funct. Mater. 2014, 24, 2957–2966. [Google Scholar] [CrossRef] [Green Version]
- Kayser, L.V.; Lipomi, D.J. Stretchable Conductive Polymers and Composites Based on PEDOT and PEDOT:PSS. Adv. Mater. 2019, 31, e1806133. [Google Scholar] [CrossRef] [PubMed]
- Green, R.A.; Lovell, N.H.; Wallace, G.G.; Poole-Warren, L.A. Conducting polymers for neural interfaces: Challenges in developing an effective long-term implant. Biomaterials 2008, 29, 3393–3399. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Lee, J.; Kang, B.; Mead, J. Preparation of Core-Sheath Nanofibers from Conducting Polymer Blends. Macromol. Rapid Commun. 2005, 26, 1127–1132. [Google Scholar] [CrossRef]
- Aussawasathien, D.; He, P.; Dai, L. Polymer Nanofibers and Polymer Sheathed Carbon Nanotubes for Sensors. Polym. Nanofibers 2006, 918, 246–268. [Google Scholar]
- Xie, J.; MacEwan, M.R.; Willerth, S.M.; Li, X.; Moran, D.W.; Sakiyama-Elbert, S.E.; Xia, Y. Conductive Core-Sheath Nanofibers and Their Potential Application in Neural Tissue Engineering. Adv. Funct. Mater. 2009, 19, 2312–2318. [Google Scholar] [CrossRef] [Green Version]
- Jun, Y.; Kang, E.; Chae, S.; Lee, S.-H. Microfluidic spinning of micro- and nano-scale fibers for tissue engineering. Lab Chip 2014, 14, 2145–2160. [Google Scholar] [CrossRef]
- Vepari, C.; Kaplan, D.L. Silk as a biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef]
- Tao, H.; Kaplan, D.L.; Omenetto, F.G. Silk Materials—A Road to Sustainable High Technology. Adv. Mater. 2012, 24, 2824–2837. [Google Scholar] [CrossRef]
- Liu, Y.; He, K.; Chen, G.; Leow, W.R.; Chen, X. Nature-Inspired Structural Materials for Flexible Electronic Devices. Chem. Rev. 2017, 117, 12893–12941. [Google Scholar] [CrossRef]
- Tsukada, S.; Nakashima, H.; Torimitsu, K. Conductive Polymer Combined Silk Fiber Bundle for Bioelectrical Signal Recording. PLoS ONE 2012, 7, e33689. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.; Hamedi, M.; Karlsson, R.; Jansson, R.; Marcilla, R.; Hedhammar, M.; Inganäs, O. Woven electrochemical transistors on silk fibers. Adv. Mater. 2011, 23, 898–901. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.K.; Farghaly, A.A.; Wang, C.; Collinson, M.M.; Kundu, S.C.; Yadavalli, V.K. Conducting polymer-silk biocomposites for flexible and biodegradable electrochemical sensors. Biosens. Bioelectron. 2016, 81, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, D.N.; Preda, R.C.; Yucel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials Fabrication from Bombyx mori Silk Fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef] [PubMed]
- Kurland, N.E.; Dey, T.; Kundu, S.C.; Yadavalli, V.K. Precise Patterning of Silk Microstructures Using Photolithography. Adv. Mater. 2013, 25, 6207–6212. [Google Scholar] [CrossRef] [PubMed]
- Kurland, N.E.; Dey, T.; Wang, C.; Kundu, S.C.; Yadavalli, V.K. Silk Protein Lithography as a Route to Fabricate Sericin Microarchitectures. Adv. Mater. 2014, 26, 4431–4437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Seyedin, S.; Qin, S.; Lynch, P.A.; Wang, Z.; Yang, W.; Wang, X.; Razal, J.M. Fast and scalable wet-spinning of highly conductive PEDOT:PSS fibers enables versatile applications. J. Mater. Chem. A 2019, 7, 6401–6410. [Google Scholar] [CrossRef]
- Pal, R.K.; Farghaly, A.A.; Collinson, M.M.; Kundu, S.C.; Yadavalli, V.K. Photolithographic Micropatterning of Conducting Polymers on Flexible Silk Matrices. Adv. Mater. 2016, 28, 1406–1412. [Google Scholar] [CrossRef]
- Ryan, J.D.; Mengistie, D.A.; Gabrielsson, R.; Lund, A.; Müller, C. Machine-Washable PEDOT:PSS Dyed Silk Yarns for Electronic Textiles. ACS Appl. Mater. Interfaces 2017, 9, 9045–9050. [Google Scholar] [CrossRef]
- Yao, B.; Wang, H.; Zhou, Q.; Wu, M.; Zhang, M.; Li, C.; Shi, G. Ultrahigh-Conductivity Polymer Hydrogels with Arbitrary Structures. Adv. Mater. 2017, 29, 1700974. [Google Scholar] [CrossRef]
- Gershoff, S.N. Vitamin C (Ascorbic Acid): New Roles, New Requirements? Nutr. Rev. 1993, 51, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Schellhorn, H.E. New developments and novel therapeutic perspectives for vitamin C. J. Nutr. 2007, 137, 2171–2184. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Yadavalli, V.K. Flexible Biosensors for the Impedimetric Detection of Protein Targets Using Silk-Conductive Polymer Biocomposites. ACS Sens. 2019, 4, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Lei, T.; Guan, M.; Liu, J.; Lin, H.-C.; Pfattner, R.; Shaw, L.; McGuire, A.F.; Huang, T.-C.; Shao, L.; Cheng, K.-T.; et al. Biocompatible and totally disintegrable semiconducting polymer for ultrathin and ultralightweight transient electronics. Proc. Natl. Acad. Sci. USA 2017, 114, 5107–5112. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| PEDOT:PSS Concentration | Gauge | Avg. Diameter (μm) | Im/Vf (A/V) | Conductivity (S/cm) |
|---|---|---|---|---|
| 2.5% | 32 G | 22.34 | 2.21 × 10−4 | 214 |
| 27 G | 23.60 | 2. 49 × 10−4 | 216 | |
| 21 G | 38.36 | 6.87 × 10−4 | 226 | |
| 5% | 32G | 11.53 | 2.49 × 10−4 | 716 |
| 27G | 18.82 | 4.48 × 10−4 | 483 | |
| 21G | 29.79 | 1.02 × 10−3 | 439 | |
| 10% | 27G | 49.10 | 7.13 × 10−4 | 144 |
| Day | Avg. Diameter (μm) | Im/Vf (A/V) (One Cycle) | Conductivity (One Cycle) (S/cm) | Im/Vf (A/V) (2–100 Cycles) | Conductivity Stability (2–100 Cycles) (S/cm) |
|---|---|---|---|---|---|
| 1 | 48.79 | 6.57 × 10−4 | 133 | 6.57 × 10−4 | 133 |
| 7 | 49.18 | 6.48 × 10−4 | 130 | 6.85 × 10−4–5.40 × 10−4 | 137–128 |
| 14 | 48.81 | 5.93 × 10−4 | 120 | 6.35 × 10−4–5.53 × 10−4 | 128–112 |
| 21 | 49.12 | 5.54 × 10−4 | 111 | 5.96 × 10−4–5.06 × 10−4 | 119–101 |
| 28 | 48.91 | 5.36 × 10−4 | 108 | 5.68 × 10−4–4.73 × 10−4 | 114–95 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Xu, M.; Yadavalli, V.K. Silk Fibroin-Sheathed Conducting Polymer Wires as Organic Connectors for Biosensors. Biosensors 2019, 9, 103. https://doi.org/10.3390/bios9030103
Jiang Y, Xu M, Yadavalli VK. Silk Fibroin-Sheathed Conducting Polymer Wires as Organic Connectors for Biosensors. Biosensors. 2019; 9(3):103. https://doi.org/10.3390/bios9030103
Chicago/Turabian StyleJiang, Yanke, Meng Xu, and Vamsi K Yadavalli. 2019. "Silk Fibroin-Sheathed Conducting Polymer Wires as Organic Connectors for Biosensors" Biosensors 9, no. 3: 103. https://doi.org/10.3390/bios9030103
APA StyleJiang, Y., Xu, M., & Yadavalli, V. K. (2019). Silk Fibroin-Sheathed Conducting Polymer Wires as Organic Connectors for Biosensors. Biosensors, 9(3), 103. https://doi.org/10.3390/bios9030103