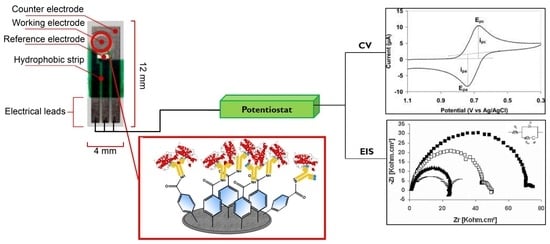
Diazonium-Modified Screen-Printed Electrodes for Immunosensing Growth Hormone in Blood Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Electrode Preparation and Modification
2.3. Electrochemistry
2.4. Surface Plasmon Resonance (SPR)
2.5. X-Ray Photoelectron Spectroscopy (XPS)
2.6. Contact Angle Goniometry
2.7. ELISA-Based Detection
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gooding, J.J. Advances in interfacial design for electrochemical biosensors and sensors: Aryl diazonium salts for modifying carbon and metal electrodes. Electroanalysis 2007, 20, 573–582. [Google Scholar] [CrossRef]
- Gooding, J.J.; Ciampi, S. The molecular level modification of surfaces: From self-assembled monolayers to complex molecular assemblies. Chem. Soc. Rev. 2011, 40, 2704–2718. [Google Scholar] [CrossRef]
- Liu, G.; Luais, E.; Gooding, J.J. The fabrication of stable gold nanoparticle-modified interfaces for electrochemistry. Langmuir 2011, 27, 4176–4183. [Google Scholar] [CrossRef]
- Liu, G.; Gooding, J.J. An interface comprising molecular wires and poly(ethylene glycol) spacer units self-assembled on carbon electrodes for studies of protein electrochemistry. Langmuir 2006, 22, 7421–7430. [Google Scholar] [CrossRef]
- Eissa, S.; Tlili, C.; L’Hocine, L.; Zourob, M. Electrochemical immunosensor for the milk allergen β-lactoglobulin based on electrografting of organic film on graphene modified screen-printed carbon electrodes. Biosens. Bioelectron. 2012, 38, 308–313. [Google Scholar] [CrossRef]
- Eissa, S.; Zourob, M. A Graphene-based electrochemical competitive immunosensor for the sensitive detection of okadaic acid in shellfish. Nanoscale 2012, 4, 7593–7599. [Google Scholar] [CrossRef]
- Eissa, S.; L-Hocine, L.; Siaj, M.; Zourob, M. A graphene-based label-free voltammetric immunosensor for sensitive detection of the egg allergen ovalbumin. Analyst 2013, 138, 4378–4384. [Google Scholar] [CrossRef]
- Won, Y.H.; Jang, H.S.; Kim, S.M.; Stach, E.; Ganesana, J.; Andreescu, S.; Stanciu, L.A. Biomagnetic glasses: Preparation, characterization, and biosensor applications. Langmuir 2010, 26, 4320–4326. [Google Scholar] [CrossRef]
- Kolliopoulos, A.V.; Metters, J.P.; Banks, C.E. Electroanalytical sensing of selenium(IV) utilising screen printed graphite macro electrodes. Anal. Methods 2013, 5, 851–856. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, Y.; Kang, A.; Wang, W.; Ho, S.V.; Gao, J.; Ma, G.; Su, Z. A novel sustained-release formulation of recombinant human growth hormone and its pharmacokinetic, pharmacodynamic and safety profiles. Mol. Pharm. 2012, 9, 2039–2048. [Google Scholar] [CrossRef]
- Kwon, O.S.; Ahn, S.R.; Park, S.J.; Song, H.S.; Lee, S.H.; Lee, J.S.; Hong, J.Y.; Lee, J.S.; You, S.A.; Yoon, H.; et al. Ultrasensitive and selective recognition of peptide hormone using close-packed arrays of hPTHR-conjugated polymer nanoparticles. ACS Nano 2012, 6, 5549–5558. [Google Scholar] [CrossRef]
- Bains, R.K.; Wells, S.E.; Flavell, D.M.; Fairhall, K.M.; Storm, M.; Le Tissier, P.; Robinson, I.C. Visceral obesity without insulin resistance in late-onset obesity rats. Endocrinology 2004, 145, 2666–2679. [Google Scholar] [CrossRef]
- Higham, C.E.; Trainer, P.J. Growth hormone excess and the development of growth hormone receptor antagonists. Exp. Physiol. 2008, 93, 1157–1169. [Google Scholar] [CrossRef]
- Hecht, M.L.; Tsai, Y.H.; Liu, X.; Wolfrum, C.; Seeberger, P.H. Synthetic inositol phosphoglycans related to GPI lack insulin-mimetic activity. ACS Chem. Biol. 2010, 5, 1075–1086. [Google Scholar] [CrossRef]
- Wei, H.; Li, H.; Mao, S.; Lin, J.M. Cell signaling analysis by mass spectrometry under coculture conditions on an integrated microfluidic device. Anal. Chem. 2011, 83, 9306–9313. [Google Scholar] [CrossRef]
- Wu, X.; Schultz, P.G. Synthesis at the interface of chemistry and biology. J. Am. Chem. Soc. 2009, 131, 12497–12515. [Google Scholar] [CrossRef]
- Mukhopadhyay, R. Deciphering cellular conversations. Anal. Chem. 2007, 79, 3963–3965. [Google Scholar] [CrossRef]
- Thomas, A.; Delahaut, P.; Krug, O.; Schänzer, W.; Thevis, M. Metabolism of growth hormone releasing peptides. Anal. Chem. 2012, 84, 10252–10259. [Google Scholar] [CrossRef]
- Holt, R.I.G.; Soenksen, P.H. Growth hormone, IGF-I and insulin and their abuse in sport. J. Pharmacol. 2008, 154, 542–556. [Google Scholar] [CrossRef] [Green Version]
- Nelson, A.E.; Ho, K.K. A robust test for growth hormone doping-present status and future prospects. Asian J. Androl. 2008, 10, 416–425. [Google Scholar] [CrossRef]
- Powrie, J.K.; Bassett, E.E.; Rosen, T.; Jørgensen, J.O.; Napoli, R.; Sacca, L.; Christiansen, J.S.; Bengtsson, B.A.; Sönksen, P.H. Detection of growth hormone abuse in sport. Growth Horm. IGF Res. 2007, 17, 220–226. [Google Scholar] [CrossRef]
- Cung, L.; Baxter, R.C. Detection of growth hormone responsive proteins using SELDI-TOF mass spectrometry. Growth Horm. IGF Res. 2009, 19, 383–387. [Google Scholar]
- Yang, S.Y.; Yang, J.A.; Kim, E.S.; Jeon, G.; Oh, J.E.; Choi, W.Y.; Hahn, S.K.; Kim, J.K. Single-file diffusion of protein drugs through cylindrical nanochannels. ACS Nano 2010, 4, 3817–3822. [Google Scholar] [CrossRef]
- Dong, W.; Shang, S.; Li, J.; Tan, Z.; Dean, T.; Maeda, A.; Gardella, T.J.; Danishefsky, S.J. Engineering of therapeutic polypeptides through chemical synthesis: Early lessons from human parathyroid hormone and analogues. J. Am. Chem. Soc. 2012, 134, 15122–15129. [Google Scholar] [CrossRef]
- Kausaite-Minkstimiene, A.; Ramanaviciene, A.; Kirlyte, J.; Ramanavicius, A. Comparative study of random and oriented antibody immobilization techniques on the binding capacity of immunosensor. Anal. Chem. 2010, 82, 6401–6408. [Google Scholar] [CrossRef]
- Seth, J.; Ellis, A.; Al-Sadie, R. Serum growth hormone measurements in clinical practice: An audit of performance from the UK national external quality assessment scheme. Horm. Res. 1993, 51, 13–19. [Google Scholar] [CrossRef]
- Bauman, G.; Stolar, M.W.; Buchanan, T.A. The metabolic clearance, distribution, and degradation of dimeric and monomeric growth hormone (GH): Implications for the pattern of circulating GH forms. Endocrinology 1986, 119, 1497–1501. [Google Scholar] [CrossRef]
- Chatelain, P.; Bouillat, B.; Coehn, R.; Sassolas, G.; Souberbielle, J.C.; Ruitton, A.; Joly, M.O.; Job, J.C. Assay of growth hormone levels in human plasma using commercial kits: Analysis of some factors influencing the results. Acta Paediatr. 1990, 370, 56–61. [Google Scholar] [CrossRef]
- Reiter, E.O.; Morris, A.H.; Macgillivray, M.H.; Weber, D. Variable estimates of serum growth hormone concentrations by different radioassay systems. J. Clin. Endocrinol. Metab. 1988, 66, 68–71. [Google Scholar] [CrossRef]
- Jan, T.; Shaw, M.A.; Baumann, G. Effects of growth hormone-binding proteins on serum growth hormone measurements. J. Clin. Endocrinol. Metab. 1991, 72, 387–391. [Google Scholar] [CrossRef]
- Tasca, F.; Harreither, W.; Ludwig, R.; Gooding, J.J.; Gorton, L. Cellobiose dehydrogenase aryl diazonium modified single walled carbon nanotubes: Enhanced direct electron transfer through a positively charged surface. Anal. Chem. 2011, 83, 3042–3049. [Google Scholar] [CrossRef]
- Liu, J.; Wang, R.; Cui, L.; Tang, J.; Liu, Z.; Kong, Q.; Yang, W.; Gooding, J.J. Using molecular level modification to tune the conductivity of praphene papers. J. Phys. Chem. C 2012, 116, 17939–17946. [Google Scholar] [CrossRef]
- Boland, S.; Barriere, F.; Leech, D. Designing stable redox-active surfaces: Chemical attachment of an osmium complex to glassy carbon electrodes prefunctionalized by electrochemical reduction of an in situ-generated aryl diazonium cation. Langmuir 2008, 24, 6351–6358. [Google Scholar] [CrossRef]
- Li, N.; Brahmendra, A.; Veloso, A.J.; Prashar, A.; Cheng, X.R.; Hung, V.W.S.; Guyard, C.; Terebiznik, M.; Kerman, K. Disposable immunochips for the detection of legionella pneumophila using electrochemical impedance spectroscopy. Anal. Chem. 2012, 84, 3485–3488. [Google Scholar] [CrossRef]
- Li, Q.; Li, N.; Le Tisser, P.; Grattan, D.; Kerman, K. Miniaturized electrochemical immunosensors for the detection of growth hormone. Electroanalysis 2012, 24, 1272–1276. [Google Scholar] [CrossRef]
- Pei, Y.; Travas-Sejdic, J.; Williams, D.E. Water structure change-induced expansion and collapse of zwitterionic polymers surface-grafted onto carbon black. Langmuir 2012, 28, 8072–8083. [Google Scholar] [CrossRef]
- Liu, G.Z.; Liu, J.Q.; Bocking, T.; Eggers, P.K.; Gooding, J.J. The modification of glassy carbon and gold electrodes with aryl diazonium salt: The impact of the electrode substrate on the rate of heterogeneous electron transfer. Chem. Phys. 2005, 319, 136–146. [Google Scholar] [CrossRef]
- Ozhikandathil, J.; Packirisamy, M. Detection of recombinant growth hormone by evanescent cascaded waveguide coupler on silica-on-silicon. J. Biophotonics 2013, 6, 457–467. [Google Scholar] [CrossRef]
- Sadabadi, H.; Badilescu, S.; Packirisamy, M.; Wüthrich, R. Integration of gold nanoparticles in PDMS microfluidics for lab-on-a-chip plasmonic biosensing of growth hormones. Biosens. Bioelectron. 2013, 44, 77–84. [Google Scholar] [CrossRef]
- Vance, S.; Zeidan, E.; Henrich, V.C.; Sandros, M.G. Comparative analysis of human growth hormone in serum using SPRi, nano-SPRi and ELISA assays. J. Vis. Exp. 2016, 7, e53508. [Google Scholar] [CrossRef]
- Gonzalez-Guerrero, A.B.; Maldonado, J.; Dante, S.; Grajales, D.; Lechuga, L.M. Direct and label-free detection of the human growth hormone in urine by an ultrasensitive bimodal waveguide biosensor. J. Biophotonics 2017, 10, 61–67. [Google Scholar] [CrossRef]
- Sadabadi, H.; Packirisamy, M. Nano-integrated suspended polymeric microfluidics (SPMF) platform for ultra-sensitive biomolecular recognition of bovine growth hormones. Sci. Rep. 2017, 7, 10969. [Google Scholar] [CrossRef]
- Trevino, J.; Calle, A.; Rodriguez-Frade, J.M.; Mellado, M.; Lechuga, L.M. Determination of human growth hormone in human serum samples by surface plasmon resonance immunoassay. Talanta 2009, 78, 1011–1016. [Google Scholar] [CrossRef]
- Trevino, J.; Calle, A.; Rodriguez-Frade, J.M.; Mellado, M.; Lechuga, L.M. Surface plasmon resonance immunoassay analysis of pituatry hormones in urine and serum samples. Clin. Chim. Acta 2009, 403, 56–62. [Google Scholar] [CrossRef]
- Rezaei, B.; Khayamian, T.; Majidi, N.; Rahmani, H. Immobilization of specific monoclonal antibody on Au nanoparticles for hGH detection by electrochemical impedance spectroscopy. Biosens. Bioelectron. 2009, 25, 395–399. [Google Scholar] [CrossRef]
- Ramanaviciene, A.; German, N.; Kausaite-Minkstimiene, A.; Voronovic, J.; Kirlyte, J.; Ramanavicius, A. Comparative study of surface plasmon resonance, electrochemical and electroassisted chemiluminescence methods based immunosensor for the determination of antibodies against human growth hormone. Biosens. Bioelectron. 2012, 36, 48–55. [Google Scholar] [CrossRef]
- Serafin, V.; Ubeda, N.; Agui, L.; Yanez-Sedeno, P.; Pingarron, J.M. Ultrasensitive determination of human groth hormone (hGH) with a disposable electrochemical magneto-immunosensor. Anal. Bioanal. Chem. 2012, 403, 939–946. [Google Scholar] [CrossRef]
- de Juan-Franco, E.; Caruz, A.; Pedrajas, J.R.; Lechuga, L.M. Site-directed antibody immobilization using a protein A-gold binding domain fusion protein for enhanced SPR immunosensing. Analyst 2013, 138, 2023–2031. [Google Scholar] [CrossRef]
- German, N.; Kausaite-Minkstimiene, A.; Kirlyte, J.; Makaraviciute, A.; Ramanavicius, A.; Mikoliunaite, L.; Ramanaviciene, A. Determination of antibodies against human growth hormone using a direct immunoassay format and different electrochemical methods. Analyst 2013, 138, 1427–1433. [Google Scholar] [CrossRef]
- de Juan-Franco, E.; Radriguez-Frade, J.M.; Mellado, M.; Lechuga, L.M. Implementation of a SPR immunosensor for the simultaneous detection of the 22K and 20K hGH isoforms in human serum samples. Talanta 2013, 114, 268–275. [Google Scholar] [CrossRef]
- Ozhikandathil, J.; Badilescu, S.; Packirisamy, M. Plasmonic gold decorated MWCNT nanocomposite for localized plasmon resonance sensing. Sci. Rep. 2015, 5, 13181. [Google Scholar] [CrossRef]
- Crespi, F. Central [CNS] and peripheral [Gastric tissue] selective monitoring of somatostatin (SRIF) with micro-sensor and voltammetry in rats: Influence of growth factors (GH, EGF). Biosensors 2017, 7, 53. [Google Scholar] [CrossRef]





| Biosensor Surface | Detection Technique | Limit of Detection | Reference |
|---|---|---|---|
| Silica-on-silicon (SOS) with a cascaded waveguide coupler | Evanescent wave-based fluoroimmunoassay | 25 ng/mL | [38] |
| Gold nanoparticles synthesized in a poly(dimethylsiloxane) (PDMS) microfluidic chip | LSPR-based immunoassay | 3.7 ng/mL | [39] |
| Anti-hGH coated with near-infrared quantum dots | SPRi (SPR imaging)- & Nano-SPRi-based immunoassay | 0.03 ng/mL–100 ng/mL | [40] |
| Anti-hGH-modified interferometer | Bimodal waveguide interferometry-based immunoassay | 10 pg/mL | [41] |
| Nano-integrated suspended polymeric microfluidics (SPMF) platform | Microcantilever-based immunoassay | 2 ng/mL | [42] |
| Anti-hGH modified gold surfaces | SPR-based immunoassay | 6 ng/mL | [43] |
| Anti-hGH modified gold surfaces | SPR-based immunoassay | 1-6 ng/mL | [44] |
| Gold nanoparticles immobilized on gold electrodes using 1,6-hexanedithiol | EIS-based immunoassay | 0.64 pg/mL | [45] |
| Sandwich-based immunoassay using horseradish peroxidase (HRP)-labeled secondary antibody | SPR, pulsed amperometry (PA), electrochemically-assisted chemiluminescence (ECL), CV | 0.051 nM by SPR 0.027 nM by PA 0.061 nM by ECL 0.056 nM by CV | [46] |
| Tosyl-activated magnetic microparticles on screen-printed gold electrodes | Square-wave voltammetry (SWV) of 4-aminophenyl phosphate as the substrate of alkaline phosphatase | 0.005 ng/mL | [47] |
| Protein A-gold binding domain fusion protein | SPR-based immunoassay | 90 ng/mL | [48] |
| Anti-hGH immobilized on gold surfaces | PA & CV | 75 nM by PA 108 nM by CV | [49] |
| Oriented anti-hGH-modified gold surfaces using biotin-streptavidin | SPR-based immunoassay | 0.9 ng/mL for 22K and 20K hGH isoforms | [50] |
| Plasmonic gold decorated multi-walled carbon nanotube nanocomposite | LSPR-based immunoassay | 1 ng/mL | [51] |
| Carbon fiber microelectrode | Differential pulse voltammetry for in vivo and ex vivo measurements using rats | 2 µg/µL | [52] |
| Anti-GH modified SPCE | EIS-based immunoassay | 5 pg/mL | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Chow, A.M.; Ganesh, H.V.S.; Ratnam, M.; Brown, I.R.; Kerman, K. Diazonium-Modified Screen-Printed Electrodes for Immunosensing Growth Hormone in Blood Samples. Biosensors 2019, 9, 88. https://doi.org/10.3390/bios9030088
Li N, Chow AM, Ganesh HVS, Ratnam M, Brown IR, Kerman K. Diazonium-Modified Screen-Printed Electrodes for Immunosensing Growth Hormone in Blood Samples. Biosensors. 2019; 9(3):88. https://doi.org/10.3390/bios9030088
Chicago/Turabian StyleLi, Nan, Ari M. Chow, Hashwin V. S. Ganesh, Melanie Ratnam, Ian R. Brown, and Kagan Kerman. 2019. "Diazonium-Modified Screen-Printed Electrodes for Immunosensing Growth Hormone in Blood Samples" Biosensors 9, no. 3: 88. https://doi.org/10.3390/bios9030088
APA StyleLi, N., Chow, A. M., Ganesh, H. V. S., Ratnam, M., Brown, I. R., & Kerman, K. (2019). Diazonium-Modified Screen-Printed Electrodes for Immunosensing Growth Hormone in Blood Samples. Biosensors, 9(3), 88. https://doi.org/10.3390/bios9030088