Review of Electroanalytical-Based Approaches for the Determination of Benzodiazepines
Abstract
:1. Introduction
2. Electrochemical Behaviour of Benzodiazepines
3. Mercury-Based Working Electrodes
4. Mercury Free Electrodes
4.1. Metal Electrodes
4.2. Carbon Electrodes
4.3. Graphene-Modified Electrodes
4.4. Nanoparticles, Carbon Nanotubes, and Fullerene-Modified Electrodes
4.5. Screen-Printed Electrodes
5. Ion Selective Electrodes
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
 |
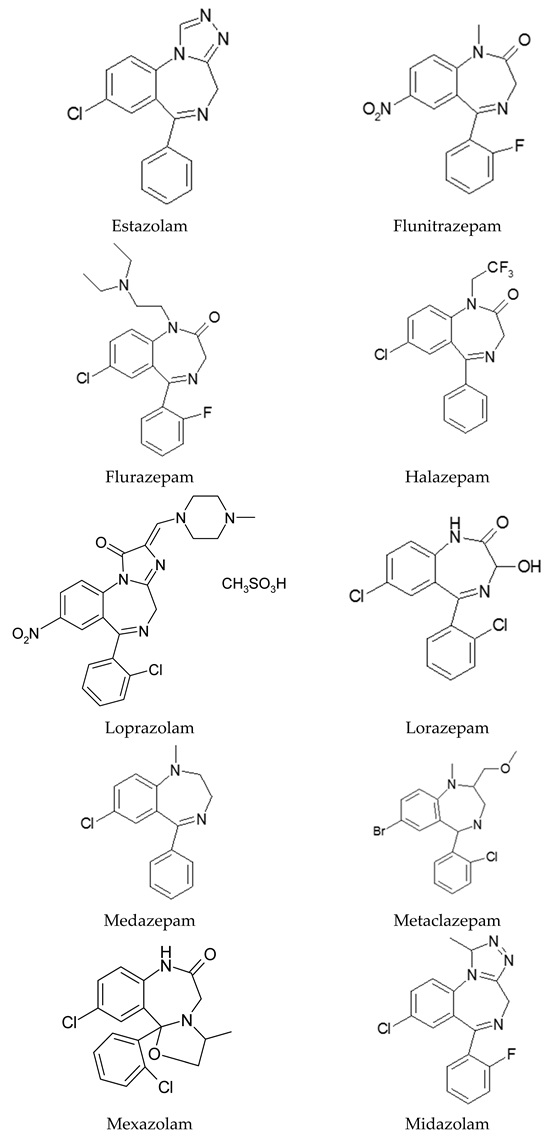 |
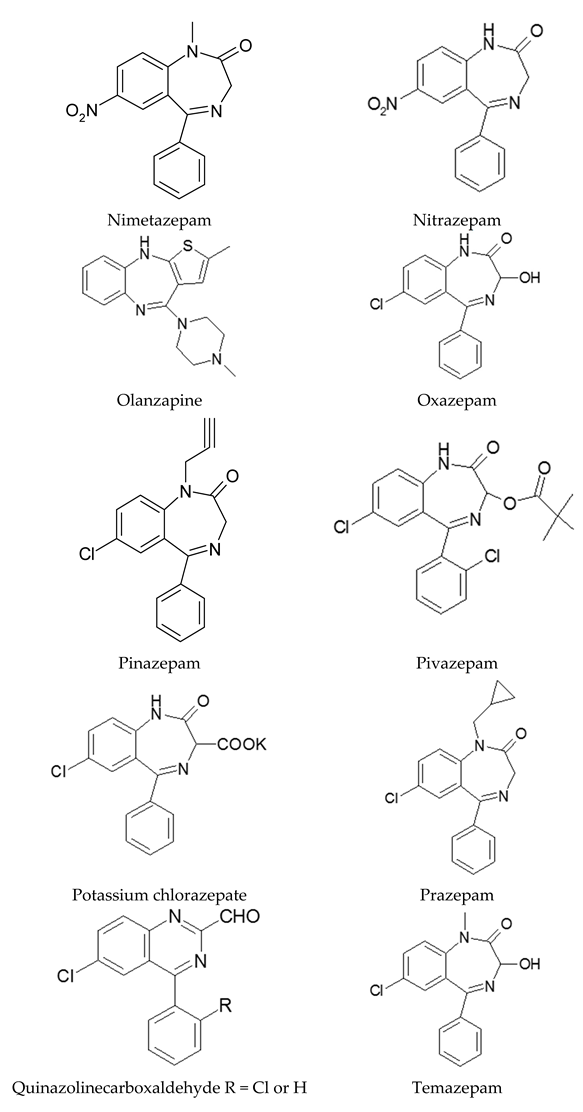 |
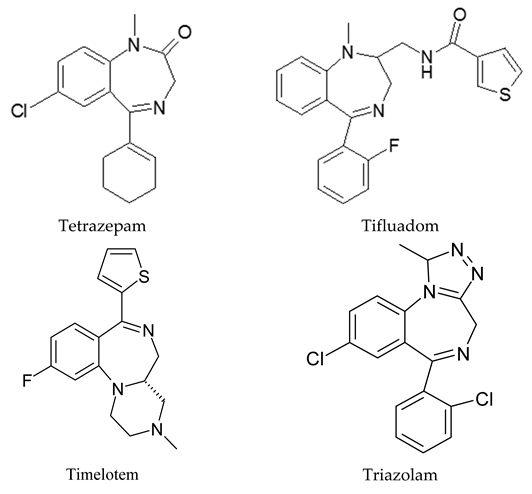 |
References
- Randall, L.O. Pharmacology of chlordiazepoxide (Librium). Dis. Nerv. Syst. 1961, 22 (Suppl. 7), 15. [Google Scholar]
- Riss, J.; Cloyd, J.; Gates, J.; Collins, S. Benzodiazepines in epilepsy: Pharmacology and pharmacokinetics. Acta Neurol. Scand. 2008, 118, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Mandrioli, R.; Mercolini, L.; Raggi, M.A. Metabolism of benzodiazepine and non-benzodiazepine anxiolytic–hypnotic drugs: An analytical point of view. Curr. Drug Metab. 2010, 11, 815–829. [Google Scholar] [CrossRef] [PubMed]
- U.S. Drug Enforcement Administration, Office of Diversion Control. National Forensic Laboratory Information System Special Report: Benzodiazepines Reported in NFLIS, 2009–2014; U.S. Drug Enforcement Administration: Springfield, VA, USA, 2016.
- Wesson, D.R.; Smith, D.E.; Ling, W.; Sabnani, S. Substance abuse: Sedative, hypnotic, or anxiolytic use disorders. In Psychiatry, 3rd ed.; Tasman, A., Kay, J., Lieberman, J.A., Eds.; JohnWiley & Sons: Chichester, UK, 2008; Volume 1, pp. 1186–1200. [Google Scholar]
- Donoghue, J.; Lader, M. Usage of benzodiazepines: A review. Int. J. Psychiatry Clin. Pract. 2010, 14, 78–87. [Google Scholar] [CrossRef]
- Westbury, J.; Jackson, S.; Gee, P.; Peterson, G. An Effective Approach to Disease Antipsychotic and Benzodiazepine Use in Nursing Homes: The RedUSe Project. Int. Psychogeriatr. 2010, 22, 26–36. [Google Scholar] [CrossRef]
- Madhusodanan, S.; Bogunovic, O. Safety of Benzodiazepines in the Geriatric Population. Expert Opin. Patient Saf. 2004, 3, 485–493. [Google Scholar] [CrossRef]
- Djezzar, S.; Questel, F.; Burin, E.; Dally, S. Chemical submission: Results of 4-year French inquiry. Int. J. Legal Med. 2009, 123, 213–219. [Google Scholar] [CrossRef]
- Scott-Ham, M.; Burton, F.C. Toxicological findings in cases of alleged drug-facilitated sexual assault in the United Kingdom over a 3-year period. J. Clin. Forensic Med. 2005, 12, 175–186. [Google Scholar] [CrossRef]
- Madea, B.; Mußhoff, F. Knock-Out Drugs: Their Prevalence, Modes of Action, and Means of Detection. Dtsch. Ärzteblatt Int. 2009, 106, 341–347. [Google Scholar]
- Papoutsis, I.; Khraiwesh, A.; Nikolaou, P.; Spiliopoulou, C.; Athanaselis, S. Benzodiazepines and driving pharmacological and legal aspects. Eur. J. Forensic Sci. 2016, 3, 26–34. [Google Scholar] [CrossRef]
- Dassanayake, T.L.; Michie, P.; Carter, G.; Jones, A.L. Effects of benzodiazepines, antidepressants and opioids on driving: A systematic review and meta-analysis of epidemiological and experimental evidence. Drug Saf. 2010, 34, 125–156. [Google Scholar] [CrossRef] [PubMed]
- Calisto, V.; Esteves, V.I. Psychiatric Pharmaceuticals in the Environment. Chemosphere 2009, 77, 1257–1274. [Google Scholar] [CrossRef] [PubMed]
- Collier, A.C. Pharmaceutical Contaminants in Potable Water: Potential Concerns for Pregnant Women and Children. EcoHealth. 2007, 4, 164–171. [Google Scholar] [CrossRef]
- Nunes, C.N.; Pauluk, L.E.; dos Anjos, V.E.; Lopes, M.C.; Quináia, S.P. New approach to the determination of contaminants of emerging concern in natural water: Study of alprazolam employing adsorptive cathodic stripping voltammetry. Anal. Bioanal. Chem. 2015, 407, 6171–6179. [Google Scholar] [CrossRef]
- Pascoe, D.; Karntanut, W.; Müller, C.T. Do Pharmaceuticals Affect Freshwater Invertebrates? A Study with the Cnidarian Hydra vulgaris. Chemosphere 2003, 51, 521–528. [Google Scholar] [CrossRef]
- Uddin, M.N.; Samanidou, V.F.; Papadoyannis, I.N. An Overview on Total Analytical Methods for the Detection of 1,4-Benzodiazepines. Pharm. Anal. Acta 2014, 5, 6. [Google Scholar]
- Szatkowska, P.; Koba, M.; Kośliński, P.; Wandas, J.; Bączek, T. Analytical methods for determination of benzodiazepines. A short review. Cent. Eur. J. Chem. 2014, 12, 994–1007. [Google Scholar] [CrossRef]
- Persona, K.; Madej, K.; Knihnicki, P.; Piekoszewski, W. Analytical methodologies for the determination of benzodiazepines in biological samples. J. Pharm. Biomed. Anal. 2015, 113, 239–264. [Google Scholar] [CrossRef]
- Salamone, S.J. (Ed.) Benzodiazepine and GHB, Detection and Pharmacology; Humana Press: Totowa, NJ, USA, 2002. [Google Scholar]
- Samanidou, V.F.; Uddin, M.N.; Papadoyannis, I.N. Benzodiazepines: Sample preparation and HPLC methods for their determination in biological samples. Bioanalysis 2009, 1, 755–784. [Google Scholar] [CrossRef]
- Smyth, W.F. Voltammetric Determination of Molecules of Biological Significance; Wiley: Chichester, UK, 1992. [Google Scholar]
- El-Maali, N.A. Voltammetric analysis of drugs. Bioelectrochemistry 2004, 64, 99–107. [Google Scholar] [CrossRef]
- Gupta, V.K.; Jain, R.; Radhapyari, K.; Jadon, N.; Agarwal, S. Voltammetric techniques for the assay of pharmaceuticals—A review. Anal. Biochem. 2011, 408, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, M.; Battista, H.-J.; Obendorf, D. Selective and sensitive assay for the determination of benzodiazepines by high-performance liquid chromatography with simultaneous ultraviolet and reductive electrochemical detection at the hanging mercury drop electrode. J. Chromatograph. A 2000, 897, 215–225. [Google Scholar] [CrossRef]
- Wilhelm, M.; Battista, H.-J.; Obendorf, D. HPLC with simultaneous UV and reductive electrochemical detection at the hanging mercury drop electrode: A highly sensitive and selective tool for the determination of benzodiazepines in forensic samples. J. Anal. Toxicol. 2001, 25, 250–257. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, L.M.; Correia, D.; Garcia, S.C.; de Bairros, A.V.; do Nascimento, P.C.; Bohrer, D. A new method for the simultaneous determination of 1,4-benzodiazepines and amfepramone as adulterants in phytotherapeutic formulations by voltammetry. Forensic Sci. Int. 2010, 202, 75–81. [Google Scholar] [CrossRef]
- Husain, S.; Prasad, P.R.; Swamy, N.S. Simultaneous quantitative determination of chloral hydrate and diazepam by differential pulse polarography. Indian J. Technol. 1991, 29, 362–363. [Google Scholar]
- Honeychurch, K.C.; Hart, J.P. Electrochemical detection of benzodiazepines, following liquid chromatography, for applications in pharmaceutical, biomedical and forensic investigations. Insci. J. Sens. 2014, 4, 1–18. [Google Scholar] [CrossRef]
- Özkan, S.A.; Uska, B.; Adoul-Enein, H.Y. Analysis of pharmaceuticals and biological fluids using modern electrochemical techniques. Crit. Rev. Anal. Chem. 2003, 33, 155–181. [Google Scholar] [CrossRef]
- Brooks, M.A. The electrochemical determination of 1,4-benzodiazepines in biological fluids. Bioelectrochem. Bioenerg. 1983, 10, 37–55. [Google Scholar] [CrossRef]
- Smyth, W.F. Polarography of Molecules of Biological Significance; Academic Press: London, UK, 1979. [Google Scholar]
- Smyth, M.R.; Smyth, W.F. Voltammetric Methods for the Determination of Foreign Organic Compounds of Biological Significance. A Review. Analyst 1978, 103, 529–567. [Google Scholar] [CrossRef]
- Brooks, M.A.; de Siliva, J.A.F. Determination of 1,4-benzodiazepines in biological fluids by differential pulse polarography. Talanta 1975, 22, 849–860. [Google Scholar] [CrossRef]
- Smyth, W.F.; Smyth, M.R. Trace analysis by polarography and voltammetry in pharmaceutical and environmental chemistry. TrAC 1982, 1, 215–218. [Google Scholar] [CrossRef]
- Clifford, J.M.; Smyth, W.F. The Determination of Some 1,4-Benzodiazepines and their Metabolites in Body Fluids. A Review. Analyst 1974, 99, 241–272. [Google Scholar] [CrossRef] [PubMed]
- Bond, A.M. Modern Polarographic Methods in Analytical Chemistry; Marcel Dekker: New York, NY, USA, 1980. [Google Scholar]
- Oelschlager, H. On the Polarographic Behavior Of Chlordiazepoxide (Librium). 2. Drug Analysis By Polarography or Oscillopolarography. Arch. Pharm. 1963, 296, 396–403. [Google Scholar]
- Ghoneim, M.M.; El-Desoky, H.S.; El-Ries, M.A.; Abd-Elaziz, A.M. Electrochemical determination of muscle relaxant drug tetrazepam in bulk form, pharmaceutical formulation, and human serum. Chem. Pap. Chem. Zvesti 2008, 62, 127–134. [Google Scholar] [CrossRef]
- Jain, R.; Mishra, R.; Dwivedi, A. Voltammetric behaviour of Diazepam in Solubilised Systems. J. Sci. Ind. Res. 2009, 69, 540–547. [Google Scholar]
- Samiec, P.; Navrátilová, Z. Voltammetric determination of Nordiazepam at meniscus modified silver solid amalgam electrode. Acta Chim. Slovaca 2014, 7, 105–108. [Google Scholar] [CrossRef] [Green Version]
- Barrett, J.; Smyth, W.F.; Hart, J.P. The polarographic and spectral behaviour of some 1,4-benzodiazepine metabolites: Application to differentiation of mixtures. J. Pharm. Pharmac. 1974, 26, 9–17. [Google Scholar] [CrossRef]
- Dos Santos, M.M.C.; Famila, V.; Simões Gonçalves, M.L. Square-wave voltammetric techniques for determination of psychoactive 1,4-benzodiazepine drugs. Anal. Bioanal. Chem. 2002, 374, 1074–1081. [Google Scholar] [CrossRef]
- Ellaithy, M.M.; Volke, J.; Manoušek, O. Differential pulse polarography of some benzodiazepine and their determination in urine. Talanta 1977, 24, 137–140. [Google Scholar] [CrossRef]
- Ribes, A.J.; Osteryoung, J. Determination of 8-Chloro-6-(2-fluoropheny1)-1-methyl-4H-imidazo[1,5-a][1,4]benzodiazepine by Adsorptive Stripping with Pulse Voltammetry. Anal. Chem. 1990, 62, 2632–2636. [Google Scholar] [CrossRef]
- Ribes, A.J.; Osteryoung, J. Kinetics of cathodic reduction of adsorbed material. The reduction of midazolam at mercury electrodes. J. Electroanal. Chem. 1990, 287, 125–147. [Google Scholar] [CrossRef]
- Kir, S.; Onar, A.N.; Temizer, A. Adsorptive stripping voltammetric determination of midazolam as a method for quality control. Anal. Chim. Acta 1990, 229, 145–147. [Google Scholar] [CrossRef]
- Vire, J.C.; Patriarche, G.J.; Hermosa, B.G. Polarographic behaviour and hydrolysis of midazolam and its metabolites. Anal. Chim. Acta 1987, 196, 205–212. [Google Scholar] [CrossRef]
- Hernandez, L.; Zapardiel, A.; Perez Lopez, J.A.; Bermejo, E. Determination of Camazepam and Bromazepam in Human Serum by Adsorptive Stripping Voltammetry. Analyst 1987, 112, 1149–1153. [Google Scholar] [CrossRef] [PubMed]
- Groves, J.A.; Smyth, W.F. Polarographic Study of Flurazepam and Its Major Metabolites. Analyst 1981, 106, 890–897. [Google Scholar] [CrossRef]
- Arcos, J.; Vire, J.-C.; El Jammal, A.; Patriarche, G.J.; Christian, G.D. Adsorptive voltammetry and hydrolysis kinetics of Loprazolam mesilate. Talanta 1990, 37, 661–666. [Google Scholar] [CrossRef]
- Acros, J.; El Jammal, A.; Vire, J.-C.; Patriarche, G.J.; Christian, G.D. Electrochemical reduction of Loprazolam mesilate in an aqueous medium. Electroanalysis 1990, 2, 279–287. [Google Scholar]
- Sreedhar, N.Y.; Reddy, S.J. Voltammetric determination of oxazepam and lorazepam. J. Indian Soc. 1993, 70, 553–558. [Google Scholar]
- Goldsmith, J.A.; Jenkins, H.A.; Grant, J.; Smyth, W.F. The polarographic behaviour of the 1,4-benzodiazepines, oxazepam and lorazepam and a method for quality control. Anal. Chim. Acta 1973, 66, 427–434. [Google Scholar] [CrossRef]
- Chelidze, T.R.; Khokhashvilli, M.O.; Gurgenidze, I.A.; Imnadze, N.É.; Dzhaparidze, D.I. Polarographic determination and electrochemical properties of Nozepam. Pharm. Chem. J. 2005, 39, 444–446. [Google Scholar] [CrossRef]
- Zapardiel, A.; Bermejo, E.; Lopez, J.A.P.; Hernandez, L.; Gil, E. Electrochemical determination of halazepam: Studies of the interaction with human serum albumin. Microchem. J. 1995, 52, 41–52. [Google Scholar] [CrossRef]
- Brooks, M.A.; Hackman, M.R. Trace Level Determination of 1,4-Benzodiazepines in Blood by Differential Pulse Polarography. Anal. Chem. 1975, 47, 2059–2062. [Google Scholar] [CrossRef] [PubMed]
- Bermejo, E.; Zapardiel, A.; Perez, J.A.; Huerta, A.; Hernandez, L. Voltammetric studies of a psychotropic drug with nitro groups. Determination of flunitrazepam in urine using HMDE. Talanta 1993, 40, 1649–1656. [Google Scholar] [CrossRef]
- Sreedhar, N.Y.; Rajendra, P.; Reddy, K.; Reddy, S.J. Electrochemical reduction of flunitrazepam and its determination in pharmaceutical dosage forms by differential pulse voltammetry. J. Indian Chem. Soc. 1997, 74, 477–479. [Google Scholar]
- Zapardiel, A.; Lopez, J.; Bermejo, E.; Hernandez, L. Voltammetric Studies of Psychotropic-Drugs with Nitro-Groups Determination of Clonazepam in Urine by Adsorptive Stripping Voltammetry. Anal. Lett. 1991, 24, 233–248. [Google Scholar] [CrossRef]
- Buckova, M.; Vanickova, M.; Labuda, J. Some analytical properties of the nafion-coated mercury electrodes. Chem. Pap. Chem. Zvesti 1996, 50, 279–282. [Google Scholar]
- Mishra, A.K.; Gode, K.D. Polarographic Assay of Nitrazepam Formulations. Analyst 1985, 110, 1105–1109. [Google Scholar] [CrossRef]
- Halvorsen, S.; Jacobsen, E. Electroreduction and polarographic determination of nitrazepam in serum. Anal. Chim. Acta 1972, 59, 127–136. [Google Scholar] [CrossRef]
- Richter, P.; Morales, A.; Lahsen, J. Voltammetric Study of 7-Nitro-1,4-benzodiazepin-2-ones and Their Acid Hydrolysis Products, 2-Amino-5-nitrobenzophenones. Analyst 1990, 115, 409–411. [Google Scholar] [CrossRef]
- Zapardiel, A.; Lopez, J.A.P.; Bermejo, E.; Hernandez, L.; Chicharro, M. Voltammetric studies on the interactions between Camazepam metabolic series and human serum albumin. Determination of oxazepam using adsorptive stripping voltammetry. Anal. Chim. Acta 1991, 244, 49–57. [Google Scholar] [CrossRef]
- Chan, H.K.; Fogg, A.G. Polarographic Determination of Temazepam in Soft Gelatin Capsule Formulations. Analyst 1981, 106, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Rivera, P.; Bermejo, E.; Zapardiel, A.; Antonio, J.; Hernandez, L. Electrochemical behaviour of the psychotropic drug Pivazepam in determination in pharmaceutical formulations and in urine. Electroanalysis 1991, 3, 399–404. [Google Scholar] [CrossRef]
- Smyth, W.F.; Smyth, M.R.; Grovest, J.A.; Tan, S.B. Polarographic Method for the Identification of 1,4-Benzodiazepines. Analyst 1978, 103, 497–508. [Google Scholar] [CrossRef]
- Smyth, W.F.; Leo, B. A spectral and polarographic study of potassium chlorazepate. Anal. Chim. Acta 1975, 76, 289–297. [Google Scholar] [CrossRef]
- Cailie, G.; Braun, J.; Mockle, J.A. Analyse spectrofluorometrique et polarographique de formes pharmaceutiques renfermant des derives de la 1-4 benzodiazepine. Can. J. Pharm. Sci. 1970, 5, 78–80. [Google Scholar]
- Thomas, L.; Vilchez, J.L.; Crovetto, G.; Thomas, J. Electrochemical Reduction of Diazepam. Proc. Indian Acad. Sci. (Chem. Sci.) 1987, 98, 221–228. [Google Scholar] [CrossRef]
- Garcia, M.G.; Garcia, A.; Gonzalez, I. Extraction and electrochemical quantification of the active ingredient (diazepam) in pharmaceutical products. Talanta 1993, 40, 1775–1779. [Google Scholar] [CrossRef]
- Guo, W.; Lin, H.; Liu, L.; Song, J. Polarographic determination of diazepam with its parallel catalytic wave in the presence of persulfate. J. Pharm. Biomed. Anal. 2004, 34, 1137–1144. [Google Scholar] [CrossRef]
- Iturbe, A.; Rodriguez, F.J.; Alonso, R.M.; Jimenez, R.M. Electroanalytical Study of a Benzodiazepinooxazole –Mexazolam. Fresenius J. Anal. Chem. 1993, 345, 451–455. [Google Scholar] [CrossRef]
- Hernández, L.; Hernández, P.; Lorenzo, E.; Gonzalez, C.; Gonzalez, I. Determination of Bentazepam by differential pulse polarography and adsorptive stripping voltammetry. Fresenius J. Anal. Chem. 1990, 336, 222–225. [Google Scholar] [CrossRef]
- El-Hefnaway, G.B.; El-Hallag, I.S.; Ghoneim, E.M.; Ghoneim, M.M. Voltammetric behaviour and quantification of the sedative-hypnotic drug chlordiazepoxide in bulk form, pharmaceutical formulation and human serum at a mercury electrode. J. Pharm. Biomed. Anal. 2004, 34, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Yarnitzky, C.; Smyth, W.F. Square wave polarographic and voltammetric analysis of selected electroreducible drugs. Int. J. Pharm. 1991, 75, 161–169. [Google Scholar] [CrossRef]
- Guo, W.; Lin, H.; Liu, L.-M.; Guo, Z.A.; Song, J.-F. Study and application of polarographic catalytic wave of chlordiazepoxide in the presence of persulfate. Chin. J. Chem. 2002, 20, 502–508. [Google Scholar] [CrossRef]
- Lorenzo, E.; Hernandez, L. Adsorptive stripping voltammetry of chlordiazepoxide at a hanging mercury drop electrode. Anal. Chim. Acta 1987, 201, 275–280. [Google Scholar] [CrossRef]
- Jacobsen, E.; Jacobsen, T.V. electrochemical reduction of chlordiazepoxide at mercury electrodes. Anal. Chim. Acta 1971, 55, 293–301. [Google Scholar] [CrossRef]
- Hackman, M.R.; Brooks, M.A.; de Silva, J.A.F. Determination of Chlordiazepoxide Hydrochloride (Librium) and Its Major Metabolites in Plasma by Differential Pulse Polarography. Anal. Chem. 1974, 46, 1075–1082. [Google Scholar] [CrossRef]
- Lund, W.; Opheim, L.-N. Automated polarographic analysis. Part III. Determination of chlordiazepoxide and diazepam in tablets. Anal. Chim. Acta 1977, 88, 275–279. [Google Scholar] [CrossRef]
- Ghasemi, J.; Niazi, A.; Ghorbani, R. Determination of Trace Amounts of Lorazepam by Adsorptive Cathodic Differential Pulse Stripping Method in Pharmaceutical Formulations and Biological Fluids. Anal. Lett. 2006, 39, 1159–1169. [Google Scholar] [CrossRef]
- Zapardiel, A.; Lopez, J.A.P.; Bermejo, E.; Hernandez, L.; Valenciano, M.J. Determination of lorazepam in human urine by adsorptive stripping voltammetry. Microchem. J. 1990, 41, 10–21. [Google Scholar] [CrossRef]
- Kobiela, A. Polarographic determination of Nimetazepam in serum and whole blood. Pharmazie 1977, 32, 693–694. [Google Scholar]
- Hernandez, L.; Zapardiel, A.; Lopez, J.A.P.; Bermejo, E. Voltammetric studies of Pinazepam and BrTDO accumulation at the HMDE surface. J. Electroanal. Chem. Interfacial 1988, 255, 85–95. [Google Scholar] [CrossRef]
- Hernandez, L.; Zapardiel, A.; Lopez, J.A.P.; Bermejo, E. Measurement of nanomolar levels of psychoactive drugs in urine by adsorptive stripping voltammetry. Talanta 1988, 35, 287–292. [Google Scholar] [CrossRef]
- Abu-Khurmah, M.; Oelschläger, H. Electroanalysis and mechanism of the reduction of the antipsychotic drug timelotem, 10-fluoro-3-methyl-7-(2-thienyl)-1,2,3,4a,5-hexahydro-pyrazino[1,2-a][1,4]benzodiazepine, at the dropping mercury electrode. Pharmazie 1998, 53, 32–38, (abstract only, in German). [Google Scholar]
- Volke, J.; Ellalthy, M.M.; Manošek, O. A spectrophotometric and polarographic investigation of three new cyclohexane-substituted benzodiazepines. Talanta 1978, 25, 209–213. [Google Scholar] [CrossRef]
- Rojas, R.M.A.; Hernandez, L.H. Polarographic study of 1-methyl-5-o-chlorophenyl-7-ethyl-1,2-dihydro-3H-thieno[2,3-e],[1,4]-diazepin-2-one (clotiazepam). Anal. Chim. Acta 1986, 186, 295–299. [Google Scholar] [CrossRef]
- Li, Q.-L.; Ji, G. Studies on the polarographic behaviour of estazolam. Talanta 1990, 27, 937–940. [Google Scholar] [CrossRef]
- Bermejo, E.; Zapardiel, A.; Hernandez, L. Electrochemical behaviour and degradation studies of Camazepam. Microchem. J. 1988, 38, 86–100. [Google Scholar] [CrossRef]
- Gonzalez, E.; Hernández, L. Study of the electrochemical behaviour of Metaclazepam in aqueous media and its determination in biological fluids. Fresenius J. Anal. Chem. 1991, 339, 187–192. [Google Scholar] [CrossRef]
- Alonso, R.M.; Jimenez, R.M.; Fogg, A.G. Differential-pulse Adsorptive Stripping Voltammetry of the Psychotropic Drugs Triazolam and Clotiazepam. Analyst 1988, 113, 27–30. [Google Scholar] [CrossRef]
- Kalvoda, R. Adsorptive stripping voltammetry of electroactive organic compounds. Anal. Chim. Acta 1984, 162, 197–205. [Google Scholar] [CrossRef]
- Channaa, H.; Surmann, P. Voltammetric analysis of N-containing drugs using the hanging galinstan drop electrode (HGDE). Pharmazie 2009, 6, 161–165. [Google Scholar]
- Tyszczuk, K. Determination of Diazepam, Temazepam and Oxazepam at the Lead Film Electrode by Adsorptive Cathodic Stripping Voltammetry. Electroanalysis 2010, 22, 1975–1984. [Google Scholar] [CrossRef]
- Norouzi, P.; Ganjali, M.R.; Emami Meibodi, A.S. A Novel Adsorptive Square Wave Voltammetric Method for Pico Molar Monitoring of Lorazepam at Gold Ultra Microelectrode in a Flow Injection System by Application of Fast Fourier Transform Analysis. Anal. Lett. 2008, 41, 1208–1224. [Google Scholar] [CrossRef]
- Doménech-Carbó, A.; Martini, M.; Machado de Carvalho, L.; Viana, C.; Doménech-Carbó, M.T.; Silva, M. Screening of pharmacologic adulterant classes in herbal formulations using voltammetry of microparticles. J. Pharm. Biomed. Anal. 2013, 74, 194–204. [Google Scholar] [CrossRef]
- Doménech-Carbó, A.; Martini, M.; Machado de Carvalho, L.; Viana, C.; Doménech-Carbó, M.T.; Silva, M. Standard additions-dilution method for absolute quantification in voltammetry of microparticles. Application for determining psychoactive 1,4-benzodiazepine and antidepressants drugs as adulterants in phytotherapeutic formulations. J. Pharm. Biomed. Anal. 2013, 80, 159–163. [Google Scholar]
- El-Sayed, G.O.; Yasin, S.A.; El Badawy, A.A. Adsorptive voltammetric determination of chlordiazepoxide in pure and dosage forms. J. Chem. Pharm. Res. 2009, 1, 225–232. [Google Scholar]
- Samiec, P.; Svorc, Ĺ.; Stanković, D.M.; Vojs, M.; Marton, M.; Navrátilová, Z. Mercury-free and modification-free electroanalytical approach towards bromazepam and alprazolam sensing: A facile and efficient assay for their quantification in pharmaceuticals using boron-doped diamond electrodes. Sens. Actuator B Chem. 2017, 245, 963–971. [Google Scholar] [CrossRef] [Green Version]
- Ashrafi, H.; Mobed, A.; Hasanzadeh, M.; Babaie, P.; Ansarin, K.; Jouyban, A. Monitoring of five benzodiazepines using a novel polymeric interface prepared by layer by layer strategy. Microchem. J. 2019, 146, 121–125. [Google Scholar] [CrossRef]
- Motaharian, A.; Hosseini, M.R.M. Electrochemical sensor based on a carbon paste electrode modified by graphene nanosheets and molecularly imprinted polymer nanoparticles for determination of a chlordiazepoxide drug. Anal. Methods 2016, 8, 6305–6312. [Google Scholar] [CrossRef]
- Rezaei, B.; Boroujeni, M.K.; Ensaf, A.A. A novel electrochemical nanocomposite imprinted sensor for the determination of lorazepam based on modified polypyrrole@sol-gel@gold nanoparticles/pencil graphite electrode. Electrochim. Acta 2014, 123, 332–339. [Google Scholar] [CrossRef]
- Panahia, Y.; Motaharian, A.; Reza Milani Hosseinic, M.; Mehrpour, O. High sensitive and selective nano-molecularly imprinted polymer based electrochemical sensor for midazolam drug detection in pharmaceutical formulation and human urine samples. Sens. Actuator B Chem. 2018, 273, 1579–1586. [Google Scholar] [CrossRef]
- Khoshroo, A.; Hosseinzadeh, L.; Sobhani-Nasab, A.; Rahimi-Nasrabadid, M.; Ehrlich, H. Development of electrochemical sensor for sensitive determination of oxazepam based on silver-platinum core–shell nanoparticles supported on graphene. J. Electroanal. Chem. 2018, 823, 61–66. [Google Scholar] [CrossRef]
- Fritea, L.; Bănică, F.; Costea, T.O.; Moldovand, L.; Iovan, C.; Cavalu, S. A gold nanoparticles—Graphene based electrochemical sensor for sensitive determination of nitrazepam. J. Electroanal. Chem. 2018, 830–831, 63–71. [Google Scholar] [CrossRef]
- Rahimi-Nasrabadi, M.; Khoshroo, A.; Mazloum-Ardakani, M. Electrochemical determination of diazepam in real samples based on fullerene-functionalized carbon nanotubes/ionic liquid nanocomposite. Sens. Actuator B Chem. 2017, 240, 125–131. [Google Scholar] [CrossRef]
- Ghalkhani, M.; Khaloo, S.S.; Mirzaie, R.A. Klonopin assay using modified electrode with multiwalled carbon nanotubes and poly melamine nanocomposite. Mater. Sci. Eng. C 2019, 99, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Ashrafia, H.; Hassanpour, S.; Saadati, A.; Hasanzadeh, M.; Ansarina, K.; Ayşil Ozkand, S.; Shadjou, N.; Jouyban, A. Sensitive detection and determination of benzodiazepines using silver nanoparticles-N-GQDs ink modified electrode: A new platform for modern pharmaceutical analysis. Microchem. J. 2019, 145, 1050–1057. [Google Scholar] [CrossRef]
- Senkowski, B.Z.; Levin, M.S.; Urbigkit, J.R.; Wollish, E.G. Polarography of Some Benzodiazepines. Anal. Chem. 1964, 36, 1991–1994. [Google Scholar] [CrossRef]
- McGuire, N.D.; Honeychurch, K.C.; Hart, J.P. The Electrochemical Behavior of Nitrazepam at a Screen-Printed Carbon Electrode and Its Determination in Beverages by Adsorptive Stripping Voltammetry. Electroanalysis 2009, 21, 2165–2170. [Google Scholar] [CrossRef]
- Honeychurch, K.C.; Brooks, J.; Hart, J.P. Development of a voltammetric assay, using screen-printed electrodes, for clonazepam and its application to beverage and serum samples. Talanta 2016, 147, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Honeychurch, K.C.; Brooks, J.; Hart, J.P. Voltammetric Behaviour and Determination of Clonazepam Using a Disposable Screen-Printed Sensor and its Determination in Serum and Wine. Presented at the Electrochem 2016, University of Leicester, Leicester, UK, 17–19 August 2016; Available online: https://uwe-repository.worktribe.com/output/919836/voltammetric-behaviour-and-determination-of-clonazepam-using-a-disposable-screen-printed-sensor-and-its-determination-in-serum-and-wine (accessed on 26 September 2019).
- Tseliou, F.; Pappas, P.; Spyrou, K.; Hrbac, J.; Prodromidis, M.I. Lab-on-a-screen-printed electrochemical cell for drop-volume voltammetric screening of flunitrazepam in untreated, undiluted alcoholic and soft drinks. Biosens. Bioelectron. 2019, 132, 136–142. [Google Scholar] [CrossRef]
- Garcia-Gutierrez, E.; Lledo-Fernandez, C. The Electroanalytical Sensing of Flunitrazepam (Rohypnol) and 7-amino Flunitrazepam in Oral Fluid, Urine and Alcoholic Beverages. Univ. J. Chem. 2013, 1, 121–127. [Google Scholar]
- Elisa Lozano-Chaves, M.; Palacios-Santander, J.M.; Cubillana-Aguilera, L.M.; Naranjo-Rodrıguez, I.; Hidalgo-Hidalgo-de-Cisneros, J.L. Modified carbon-paste electrodes as sensors for the determination of 1,4-benzodiazepines: Application to the determination of diazepam and oxazepam in biological fluids. Sens. Actuator B Chem. 2006, 115, 575–583. [Google Scholar] [CrossRef]
- Arenaza, M.J.; Gallo, B.; Berrueta, L.A.; Vicente, F. Electrooxidation and determination of the 1,4-benzodiazepine loprazolam at the carbon-paste electrode. Anal. Chim. Acta 1995, 305, 91–95. [Google Scholar] [CrossRef]
- Hernandez, L.; Hernandez, P.; Blanco, M.H.; Lorenzo, E. Determination of Flunitrazepam by Differential-pulse Voltammetry Using a Bentonite-modified Carbon Paste Electrode. Analyst 1988, 113, 1719–1722. [Google Scholar] [CrossRef]
- Raggi, M.A.; Casamenti, G.; Mandrioli, R.; Izzo, G.; Kenndler, E. Quantitation of olanzapine in tablets by HPLC, CZE, derivative spectrometry and linear voltammetry. J. Pharm. Biomed. 2000, 23, 973–981. [Google Scholar] [CrossRef]
- Hernández, P.; Lorenzo, E.; Cerrada, J.; Hernández, L. Preconcentration and determination of Bentazepam at C18-modified carbon paste electrode. Fresenius J. Anal. Chem. 1992, 342, 429–432. [Google Scholar] [CrossRef]
- Bıryol, İ.; Erk, N. Voltammetric, Spectrophotometric, and High Performance Liquid Chromatographic Analysis of Olanzapine. Anal. Lett. 2003, 36, 2497–2513. [Google Scholar] [CrossRef]
- Ruiz, E.; Blanco, M.H.; Abad, E.L.; Hernandez, L. Determination of Nitrazepam and Flunitrazepam by Flow Injection Analysis Using a Voltammetric Detector. Analyst 1987, 112, 697–699. [Google Scholar] [CrossRef]
- Latorre, C.; Blanco, M.H.; Abad, E.L.; Vicente, J.; Hernandez, L. Determination of Clonazepam by Flow Injection Analysis. Analyst 1988, 113, 317–319. [Google Scholar] [CrossRef]
- Nagappa, N.; Mimani, T.; Sheshadri, B.; Mayanna, S.; Mayanna, G. Cyclic voltammetric studies of diazepam using glassy carbon electrode-estimation of diazepam in pharmaceutical samples. Chem. Pharm. Bull. 1998, 46, 715–717. [Google Scholar] [CrossRef]
- Blaedel, W.J.; Hahn, Y. Investigation of diazepam by pulsed rotation voltammetry. Arch. Pharm. Res. 1979, 2, 111–114. [Google Scholar] [CrossRef]
- Smyth, W.F.; Ivaska, A.; Burmicz, J.S.; Davidson, I.E.; Vaneesorn, Y. Voltammetric on-line analysis of molecules of biological importance. Bioelectrochem. Bioenerg. 1981, 8, 459–467. [Google Scholar] [CrossRef]
- González, E.; Hernández, P.; Hernández, L. Cyclic voltammetry of tifluadom at a C18 modified carbon-paste electrode. Anal. Chim. Acta 1990, 228, 265–272. [Google Scholar] [CrossRef]
- Krishna, V.; Umamaheswara, B.R.; Rajuc, N. Spectral, electrochemical behaviour of Alprazolam and Voltammetric assaying of pharmaceutical formulation. J. Chem. Pharm. Res. 2015, 7, 766–771. [Google Scholar]
- Smyth, W.F.; Burmicz, J.S.; Ivaska, A. On-line electrochemical detection of oxidisable organic molecules of pharmaceutical importance. Analyst 1982, 107, 1019–1025. [Google Scholar] [CrossRef]
- Smyth, W.F.; lvaska, A. A Study of the Electrochemical Oxidation of Some 1,4-Benzodiazepines. Analyst 1985, 110, 1377–1379. [Google Scholar] [CrossRef]
- El-Shal, M.A. Electrochemical Studies for the Determination of Quetiapine Fumarate and Olanzapine Antipsychotic Drugs. Adv. Pharm. Bull. 2013, 3, 339–344. [Google Scholar]
- Salem, A.E.A.; Barsoum, B.N.; Saad, G.R.; Izake, E.L. Potentiometric determination of some 1,4-benzodiazepines in pharmaceutical preparations and biological samples. J. Electroanal. Chem. 2002, 536, 1–9. [Google Scholar] [CrossRef]
- Al Attas, A.S. Construction and analytical application of ion selective bromazepam sensor. Int. J. Electrochem. Sci. 2009, 4, 20–29. [Google Scholar]
- Salem, A.A.; Barsoum, B.N.; Izake, E.L. Potentiometric determination of diazepam, bromazepam and clonazepam using solid contact ion-selective electrodes. Anal. Chim. Acta 2003, 498, 79–91. [Google Scholar] [CrossRef]

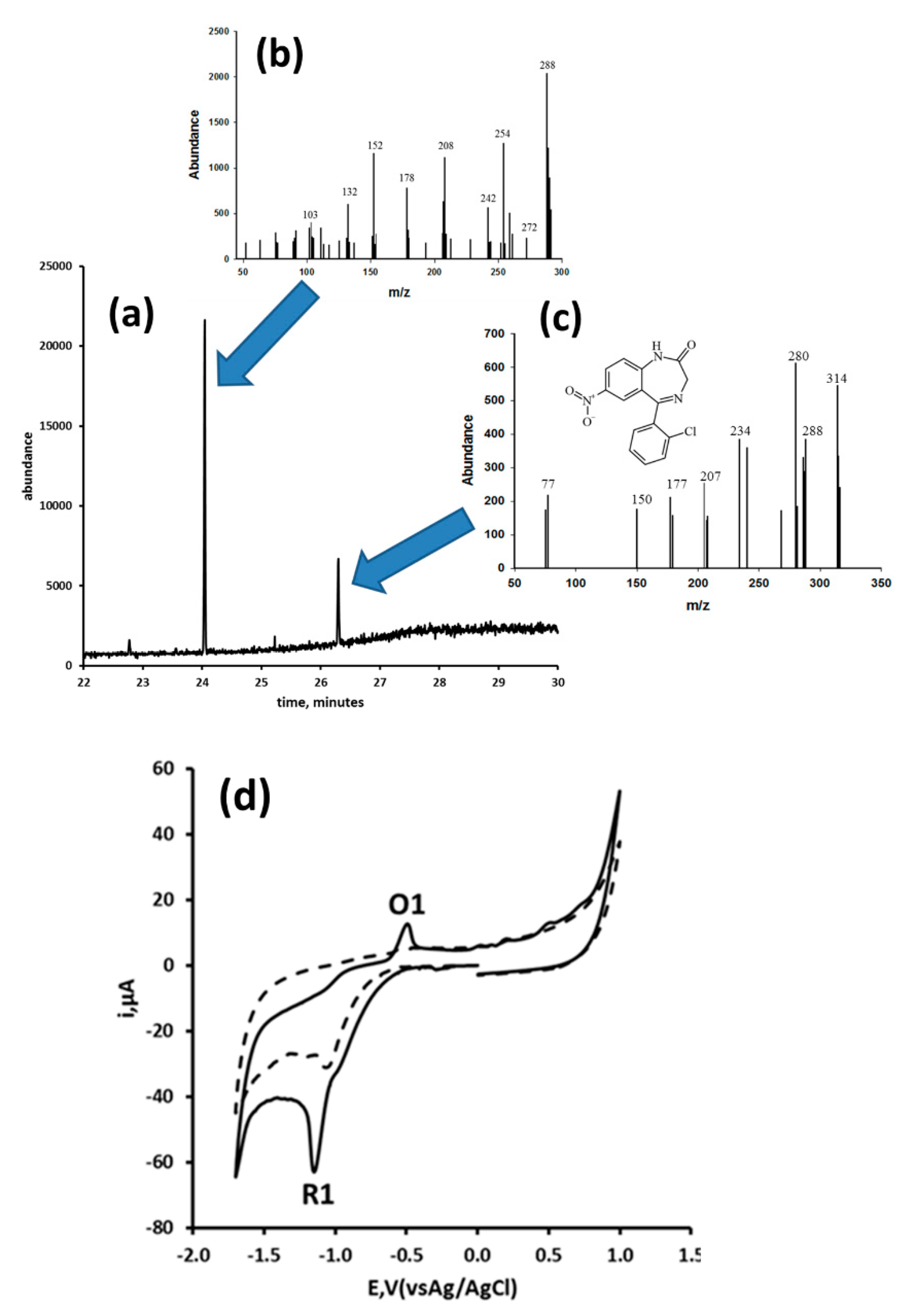
| Benzodiazepine | Electrode Material | Linear Range (M) | Detection Limit (M) | Electrolyte | Waveform and Technique | Sample (s) | Reference |
|---|---|---|---|---|---|---|---|
| 7-acetamido-nitrazepam, dimethyl-diazepam, chlordiazepoxide lactam, 7-amino-diazepam | Dropping mercury electrode | - | - | Britton-Robinson buffer, 0.04 M, plus 0.1 M NaOH to obtain desired pH | DPP | Mechanistic study | [43] |
| Bromazepam, clonazepam, diazepam, flurazepam, midazolam, and medazepam | Hanging mercury drop electrode | Clonazepam 1 × 10−7 to 1 × 10−5, bromazepam 1 × 10−7 to 1 × 10−5, midazolam 1 × 10−7 to 1 × 10−5, diazepam 1 × 10−7 to 1 × 10−5, SWCSV clonazepam 1 × 10−8 to 8 × 10−8¸ bromazepam 1 × 10−8 to 8 × 10−8, midazolam 1 × 10−8 to 8 × 10−8, diazepam 1 × 10−8 to 8 × 10–8, medazepam 1 × 10−8 to 8 × 10−8 | SWV, clonazepam 1.3 × 10−8, bromazepam 1.2 × 10−8, midazolam 1.6 × 10−8, diazepam 1.0 × 10−8, SWCSV clonazepam 2.9 × 10−9, bromazepam 2.6 × 10−9, midazolam 2.0 × 10−9, diazepam 2.0 × 10−9, medazepam 1.3 × 10−9, flurazepam 2.5 × 10−9 | 0.10 M KNO3, and Britton–Robinson pH 6.0 buffer | SWV and SWCSV | Tablets | [44] |
| Diazepam, oxazepam nitrazepam, and flurazepam | Dropping mercury electrode | 2 × 10−7 to 10 × 10−7 | 2 × 10−7 | 0.2 M acetate pH 4.8 buffer | DPP, DC polarography | Rabbit urine | [45] |
| Midazolam | Static mercury drop electrode | 3.6–36 µM | 100 pM | Acetate pH ca. 4.8 buffer | SWAdSV, NPV | - | [46] |
| Midazolam | Static mercury drop electrode | - | - | 0.1 M acetate pH 4.8 buffer | NPV | Mechanistic study | [47] |
| Midazolam | Hanging mercury drop electrode | 1.9 × 10−6 to 10−9 | Britton-Robinson pH 5.00 buffer | AdSV | Pharmaceutical formulations | [48] | |
| Midazolam and metabolites (α-hydroxy-midazolam, 3-hydroxy-midazolam and α,3-hydroxy-midazolam) | Static mercury drop electrode | 10−4 to 10−7 | 6 × 10−8 | Sulphuric acid 1.0 M | DPP | - | [49] |
| Camazepam and bromazepam | Hanging Hg drop electrode | Camazepam 1 × 10−9 to 9 × 10−9, bromazepam 1 × 10−8 × 10−8, 270 s at −0.60 V | Camazepam 20 ng/mL and bromazepam 200 ng/mL | DPAdSV | Human serum after isolation with Sep-Pak CI8 cartridges | [50] | |
| Flurazepam and metabolites | Dropping mercury electrode | Three orders of magnitude | 5 × 10−8 | Formate pH 4 buffer | DPP | 15 mg and 30 mg capsules | [51] |
| Loprazolam mesilate | Static mercury drop electrode | 2.5 × 10−10 to 8.0 × 10−9 | 2.5 × 10−10 | 0.04 M ammonium chloride pH 4.0 | SWAdCSV | - | [52] |
| Loprazolam mesilate | Dropping mercury electrode and a static mercury electrode | - | - | DC, AC, normal and inverse pulse polarography, CV, and controlled potential coulometry | Mechanistic study | [53] | |
| Oxazepam and lorazepam | Dropping and hanging mercury electrodes | 1.0 × 10−5 to 1.5 × 10−7 for oxazepam and 2.5 × 10−7 to 2.0 × 10−7 for lorazepam | 1.25 × 10−7 for oxazepam, 1.85 × 10−7 for lorazepam | Britton-Robinson buffer | DC and AC polarography, CV, DPP, rotating disk voltammetry | Oxazepam in serepax, serenal tablets, and serenid forete capsule; lorazepam in Larpose, activan, trapex tablets | [54] |
| Oxazepam, lorazepam, and quinazolinecarboxaldehyde | Dropping and hanging mercury electrodes | 10−5 to 10−3 in 10% DMSO/water, 10−5 to 10−4 in water | - | 0.1 M tetraethyl ammonium perchlorate in 90% DMSO/10% water | polarography | Serenid-D and Ativan tablets | [55] |
| Oxazepam | Forced drop mercury electrode | 1 × 10−5 to 1 × 10−2 | - | 0.1 M aqueous tetraethyl ammonium iodide | polarography | 10 mg tablets | [56] |
| Halazepam | Dropping and hanging mercury electrodes | 1 × 10−8 to 1.6 × 10−7 | 25 ng/mL | 0.04 M acetate pH 4.8 buffer | DPAdSV −0.5 V | Human serum and urine | [57] |
| Bromazepam and N-1-Desalkylflurazepam | Dropping mercury electrode | 5–500 ng/mL | 10–20 ng/mL | 1 M phosphate pH 4.0 and pH 7.0 buffers containing 0.005% methoxypolyethylene glycol 550 | DPP | Human blood | [58] |
| Flunitrazepam | Hanging mercury drop electrode | 0.6 × 10−8 to 3.1 × 10−7 | 9.0 × 10−10 | 0.02 M Britton-Robinson pH 10 buffer | Staircase voltammetry and DPAdSV | Urine, following extraction with diethyl ether | [59] |
| Flunitrazepam | Dropping Hg electrode | 1.5 × 10−5 to 2.5 × 10−7 | - | 0.2 M boric acid, 0.05 M citric acid, and 0.1 M trisodium orthophosphate pH 4 buffer, containing 0.2% Triton X-100 | DPP | Rohypnol tablets | [60] |
| Clonazepam | Hanging mercury drop electrode | Up to 550 ng/mL | 10 ng/mL | 0.025 M carbonate pH 10.0 buffer | DPAdSV | Urine, after extraction | [61] |
| Nitrazepam | Nafion-coated Hg film glassy carbon electrode | Up to 7 × 10−6 | - | Acetate pH 4.6 buffer | DPAdSV | - | [62] |
| Nitrazepam | Dropping mercury electrode | - | 0.27 µg/mL | McIlvaine pH 3.1 buffer | Polarography | Tablets: Nitravet, Nirven, Sedamon, Hypnotex, Restorem, and Capsule: Hypnotex | [63] |
| Nitrazepam | Hanging mercury drop | 0.5–80 µg/mL in serum by polarography | 0.5 µg/mL | Phosphate pH 6.9 buffer | Polarography, CV, chronopotentiometry, controlled coulometry | Serum (without dilution or pre-treatment) | [64] |
| Nitrazepam, flunitrazepam, and clonazepam as their 2-amino-5-nitrobenzophenones acid hydrolysis products | Glassy carbon electrode | - | - | Polarography, 0.11 M pyridine, and 0.12 M formic acid pH 4.5, CV formate buffer | CV, polarography | - | [65] |
| Camazepam, oxazepam, and temazepam | Hanging mercury electrode | Oxazepam 2.8 × 10−8 to 4.0 × 10−7 | Oxazepam 3.6 × 10–10 | 0.008 M Britton–Robinson pH 2.0 buffer | DPAdSV | Human serum albumin | [66] |
| Temazepam | Hanging mercury electrode | 10–100 µg/mL | Acetate pH 4.7 buffer solution containing 10% dimethylfonnamide | Polarography and DPP | Soft gelatine capsule formulations after enzyme dissolution | [67] | |
| Pivazepam | Hanging mercury electrode | 10−7 to 10−5 | Urine 15 ng/mL, following diethyl ether extraction | DPAdSV 0.1 M McIlvaine pH 4.0 buffer, 10% methanol, DPP 0.04 M Britton-Robinson pH 5 buffer | DPP, DPAdSV | Pharmaceutical tablet formulations and urine | [68] |
| Chlordiazepoxide, nitrazepam, clonazepam, flunitrazepam, oxazepam, lorazepam, diazepam, bromazepam, prazepam, flurazepam, -otassium chlorazepate, and medazepam | Dropping mercury electrode | - | - | Britton–Robinson pH 4.0 and pH 12.0 buffers | Polarography | Mechanistic studies, hydrolysis products investigated | [69] |
| chlorazepate | Dropping Hg electrode | 10−7 to 3 × 10−5 | ca. 10−7 | Britton-Robinson pH 4 buffer | Cathode ray polarography | Urine after adjustment to pH 9 and liquid-liquid extraction with ethyl acetate | [70] |
| Oxazepam | Dropping Hg electrode | - | - | 0.1 M HCl/20% methanol | Polarography | Unsuccessful for the direct determination of pharmaceutical formulation | [71] |
| Diazepam | Dropping mercury electrode | 2 × 10−6 to 10−3 | - | Britton-Robinson pH 1.81 buffer—0% methanol | DCP, CV, coulometry | Mechanism study | [72] |
| Diazepam | Dropping mercury electrode | Alternating current polarography 17.4–167 µM; DPP 4.8–96 µM | - | 2 M acetate pH 4.6 solution | DCP, DPP, and alternating current polarography | 10 mg tablets | [73] |
| Diazepam | Hanging mercury drop electrode | - | 1 µg/mL | 0.1 M KCl solution in 50% ethanol | DPP | Forensic investigations of Toddy (fermented sap or exudate of date, Palmyra, coconut, sago, etc.) | [29] |
| Diazepam | Hanging mercury drop electrode | 5.6 × 10−8 to 8.8 × 10−6 and 8.8 × 10−6 to 2.0 × 10−4 | 9.6 × 10−9 | 0.20 M pH 4.7 acetate buffer, −2.0 × 10−2 M K2S2O8 | Polarographic parallel catalytic wave of persulfate | Tablets | [74] |
| Mexazolam | Hanging mercury drop electrode | Up to 13 µg/mL | 80 ng/mL | DPP | [75] | ||
| Bentazepam | Hanging mercury drop electrode | 1.9–9.4 ng/mL by DPAdSV | 2.7 ng/mL in urine by DPAdSV; 3.1 × 10−9 M by DPP | DPP in 0.04 M Britton-Robinson pH 9 buffer; DPAdSV in 0.08 M phosphate pH 8.0 buffer | DPP, DPAdSV | Tiadipona tablets by DPP and in urine by DPAdSV | [76] |
| Chlordiazepoxide | Hanging mercury drop electrode | 5 × 10−9 to 2 × 10−7 in human serum by SWAdCSV | 4.4 × 10−10 and 6.6 × 10−10 for pharmaceutical and human serum, respectively, by SWAdCSV | Britton-Robinson pH 8 buffer | Polarography, CV, controlled potential coulometry and SWAdCSV | Bulk form, Librax tablets and human serum (after protein precipitation with ethanol and centrifugation) | [77] |
| Chlordiazepoxide | Static mercury drop electrode | - | - | 0.1 M acetate buffer | SWAdCSV | Synthetic solutions measured in the presence of chloramphenicol, metronidazole, oxytetracycline, cephalothin, and trimethoprim | [78] |
| Chlordiazepoxide | Hanging mercury drop electrode | Quasilinear over the ranges: 3.20 × 10−8 to 1.60 × 10−7; 1.60 × 10−7 to 1.44 × 10−6; 1.44 × 10−6 to 1.44 × 10−5 | 9 × 10−9 | 0.12 M NH3/NH4Cl pH 10.2/0.016 M K2S2O6 | Polarography | - | [79] |
| Chlordiazepoxide | Hanging mercury drop electrode | 0.8 to 11 × 10−8 | 0.9 × 10−9 | Britton-Robinson pH 6.8 buffer | DPAdCSV | Human serum, after solid phase extraction with Sep-Pak C18 cartridge | [80] |
| Chlordiazepoxide | Dropping Hg electrode | 2.5 × 10−6 to 10−3 | 2.5 × 10−6 | 0.1 M sulphuric acid | Polarography, CV, coulometry | Alpoxide and Prodixamon tablets, serum | [81] |
| Chlordiazepoxide and metabolites: N-desmethylchlordiazepoxide, and demoxepam | Dropping Hg electrode | 0.05–1.0 µg/mL | Chlordiazepoxide and its desmethyl metabolite approximately 0.05 µg/mL for plasma; demoxepam, 0.10 µg/mL | 0.1 N sulphuric acid | DPP | Plasma after 30 mg dose extracted with diethyl ether at pH 9 and analytes isolation by thin layer chromatography | [82] |
| Chlordiazepoxide and diazepam | Dropping Hg electrode | - | - | 0.1 M sulphuric acid | Automated polarography | Alpoxide and Vival tablets | [83] |
| Lorazepam | Hanging mercury drop electrode | 0.05–1.15 µg/mL | 0.019 µg/mL | Britton-Robinson pH 2 buffer | CAdSV | Urine, plasma, Chemidarou and Zahrav tablets | [84] |
| Lorazepam | Hanging mercury drop electrode | 15 ng/mL in urine | Britton-Robinson pH 2 buffer | DPAdSV | Human urine | [85] | |
| Nimetazepam | Dropping Hg electrode | 30–150 µg/mL | 30 µg/mL | Britton–Robinson pH 3.8 buffer, 50% methanol | Polarography, 0.0 to −0.7 V | Serum and whole blood after extraction with ethyl acetate/toluene 1:1 | [86] |
| Pinazepam and BrTDO | Hanging mercury drop electrode | Pinazepam 2.8 × 10−10, BrTDO 2.2 × 10−10 | 0.04 M Britton-Robinson buffers pH 4 and pH 5 | DPAdSV | Human serum | [87] | |
| Pinazepam, camazepam, bromazepam, and BrTDO | Hanging mercury drop electrode | Pinazepam 100–700 ng/mL, camazepam 100–1000 ng/mL, bromazepam 100–1000 ng/mL, and BrTDO 100–1000 ng/mL | Pinazepam 15 ng/mL, camazepam 10 ng/mL, bromazepam 20 ng/mL, and BrTDO 20 ng/mL | Britton–Robinson pH 4 buffer for Pinazepam and pH 5 for bromazepam and BrTDO; Acetate pH 5 buffer for camazepam | DPAdSV | Urine after solid-phase extraction with C18 Sep-pak | [88] |
| Timelotem | Dropping mercury electrode | - | 2.5 × 10−6 | Britton–Robinson buffers containing 10% methanol | - | - | [89] |
| Tetrazepam, nortetrazepam, and menitrazepam | Hanging and dropping mercury electrodes | 5 × 10−7 to 5 × 10−6 tetrazepam | 10−7 | 0.1 M sulphuric acid, containing 1% dimethylacetamide | Polarography and DPP | - | [90] |
| Clotiazepam | Dropping mercury electrode | 6.5 × 10−7 to 1.10 × 10−5 | - | 0.1 M sulphuric acid | Polarography and DPP | Distensan tablets | [91] |
| Estazolam | Dropping mercury electrode | 1 × 10−7 to 9.0 × 10−6 | 5 × 10−8 | 0.1 M NH3–NH4Cl pH 9.2 buffer | Polarography | - | [92] |
| Camazepam | Dropping mercury electrode | 1.3 × 10−6 to 1.0 × 10−5 by DPP | 1.7 × 10−8 by DPP | 0.2 M phosphate pH 6.0 buffer | Current sampled polarography, CV, and DPP | Urine, with and without solid phase extraction (Sep-Pak C18) and Albego dragees (sugar-coated) 10 and 20 mg tablets | [93] |
| Metaclazepam | DC tast, DPP and DPAdSV at dropping mercury, hanging mercury electrodes; CV at hanging mercury electrode; coulometry at mercury pool | 1.8 × 10−9 to 3.2 × 10−7 by DPAdSV | 4.4 × 10−10 by DPAdSV | Polarography: Britton-Robinson buffer; coulometry: 4% ethanol/Britton-Robinson buffer | DC tast and DPP, CV, microcoulometry, and DPAdSV | Human urine, 2 mL diluted with 1 mL of 0.04 M pH 9.2 borate buffer; extracted with 1 mL of diethyl ether Evaporated to dryness and reconstituted in ethanol; diluted with 25 mL 0.04 M Britton-Robinson buffer pH 9.1 | [94] |
| Triazolam and clotiazepam | Hanging mercury drop electrode | Up to 2.2 × 10−7 for both triazolam and clotiazepam | 6 × 10−10 for triazolam and clotiazepam with accumulation times of 4 and 10 min by DPAdSV | 10−2 M carbonate pH 10 buffer | DPAdSV | - | [95] |
| Diazepam and nitrazepam | Hanging mercury drop electrode | 1 × 10−9 to 1 × 10−6 | - | Acetate pH 4.6 buffer | DPAdSV | - | [96] |
| Benzodiazepine | Electrode Material | Linear Range (M) | Detection Limit (M) | Electrolyte | Waveform and Technique | Sample (s) | Reference |
|---|---|---|---|---|---|---|---|
| Diazepam, temazepam, and oxazepam | Modified carbon-paste electrodes | Diazepam 0.025–3.0 µg/mL, Temazepam 0.025–0.8 µg/mL, oxazepam 0.025–1.0 µg/mL | Diazepam 0.021 µg/mL, temazepam 0.021 µg/mL, oxazepam 0.012 µg/mL | Britton-Robinson buffer 0.1 M | DPV | Plasma and urine, following SPE | [119] |
| Loprazolam | Carbon-paste electrode | 1.5 × 10−7 | Britton-Robinson pH 6.3 buffer | DPV | Somnovit (1 mg) tablets | [120] | |
| Flunitrazepam | Bentonite-modified carbon paste | 0.2–4.0 µg/mL | 0.04 µg/mL | Accumulation media: pH 3.8, adjusted with either nitric acid or potassium hydroxide; measurement media: 0.5 M potassium nitrate pH 3.8 | DPV, adsorption at open circuit | Serum and urine | [121] |
| Olanzapine | Glassy carbon electrode | 6.2–50.0 µg/mL | 3 µg/mL | 0.25 M phosphate pH 2.5 buffer | LSV | Ziprexa tablets | [122] |
| Bentazepam | C18(Bonda pack, 50% w/w)-modified carbon-paste electrode | 0.5–2 µg/mL | 0.5 µg/mL | Accumulation media: 0.04 M Britton-Robinson pH 6 buffer; measurement: 0.3 M phosphate pH 2 buffer | DPAdSV, open circuit accumulation, measurement DPV scan 0 to +1.3 V | Human urine | [123] |
| Olanzapine | Glassy carbon electrode | DPV and OSWV 7.00 × 10−5 to 1.00 × 10−3 | DPV 6.79 × 10−6, OSWV 6.91 × 10−6 | Britton-Robinson pH 3.24 buffer | CV, DPV, and OSWV | 10 mg Zyprexa tablets | [124] |
| Nitrazepam and flunitrazepam | Glassy carbon electrode | 5.6–28.1 pg/mL and 6.3–31.3 pg/mL for nitrazepam and flunitrazepam, respectively | 1.8 µg/mL and 1.3 µg/mL for nitrazepam and flunitrazepam, respectively | 0.1 M phosphate pH 7.0 buffer, containing 10% v/v methanol | Flow injection analysis, 4.0 mL/min, −0.9 V | Tablet formulations of nitrazepam and flunitrazepam | [125] |
| Clonazepam | Glassy carbon electrode | 6.3–31.5 pg/mL | 3.3 µg/mL | 0.1 M phosphate buffer solution (pH 7.0) containing 10% v/v methanol | Flow injection analysis, 4.0 mL/min, −0.9 V | Tablet formulations and human urine | [126] |
| Diazepam | Glassy carbon electrode | 1 × 10−3 to 1 × 10−4 | - | HCl–KCl pH 1 buffer containing 10% ethanol | CV | Tablets, capsules, injection, and syrup formulations | [127] |
| Diazepam | Glassy carbon electrode | 0.158–9.48 µM | - | Solution of 0.1 M KCl and 0.01 M pH 4.0 potassium biphthalate buffer | Pulsed rotation voltammetry | - | [128] |
| Chlordiazepoxide, medazepam, nitrazepam, amino-nitrazepam, lorazepam, and flurazepam | Glassy carbon electrode | - | - | Britton–Robinson pH 4 buffer, containing 10% methanol | Differential pulse flow injection analysis +1.2 V (no oxidation signal seen for nitrazepam) | Tablets | [129] |
| Tifluadom | C18 µBondapak-modified carbon-paste electrode | 2.2 × 10−7 to 4.5 × 10−6 | 1.3 × 10−6 in urine, 1.3 × 10−7 in buffer | Britton-Robinson pH 6 buffer | CV | Urine | [130] |
| Alprazolam | Glassy carbon electrode and static Hg drop electrode | 2.5 × 10−7 to 3.25 × 10−5 | - | Britton-Robinson pH 9 buffer | Differential pulse polarography | - | [131] |
| Flurazepam, oxazepam, medazepam, lorazepam, flunitrazepam, prazepam, potassium chlorazepate, nitrazepam, and amino-nitrazepam | Wall-jet glassy carbon electrode | - | - | Britton-Robinson pH 4 buffer, containing 10% methanol | Differential pulse flow injection analysis +1.2 V | Tablets | [132] |
| Flurazepam, chlordiazepoxide, oxazepam, lorazepam, potassium chlorazepate, nitrazepam, flunitrazepam, prazepam, diazepamm and medazepam, and some of their metabolites | Wall-jet glassy carbon electrode | 5 × 10−5 to 2 × 10−4 | - | 0.01 M sodium hydroxide | Differential pulse flow injection analysis +1.2 V | - | [133] |
| Quetiapine fumarate and olanzapine | Glassy carbon electrode | Quetiapine 3 × 10−8 to 4 × 10−6, and quetiapine fumarate 2 × 10−8 to 5 × 10−6 | 1 × 10−8 for both | Britton-Robinson pH 2 buffer | Differential pulse voltammetry | Tablets (Zyprexa and Seroquel), urine, and serum | [134] |
| Benzodiazepine | Electrode Material | Linear Range (M) | Detection Limit (M) | Electrolyte | Sample (s) | Reference | |
|---|---|---|---|---|---|---|---|
| Diazepam, bromazepam, and clonazepam | PVC-based ion-selective electrodes | 2.5 × 10−6 to 10−4, 5 × 10−6 to 10−4, and 2.5 × 10−4 to 10−6 for diazepam, bromazepam, and clonazepam, respectively | 2, 0.307, and 0.9 µM, for diazepam, bromazepam, and clonazepam, respectively | Potentiometric, 10−4 M HCl | Ion-selective electrodes | Urine, Vallium, Rivotril, and Calmepam tablets | [135] |
| Bromazepam | Bromazepam-phosphotungestiate ion association complex in poly(vinyl chloride) membrane | 1 × 10−2 to 1 × 10−4 | 3 × 10−5 | Potentiometric | Ion-selective electrodes | Lextonil, Neoopt, and Calmepam tablets | [136] |
| Diazepam, bromazepam, and clonazepam | PVC solid contact ion-selective electrodes | 0.29–71.20, 0.79–31.62, and 0.32–31.57 µg/mL for diazepam, bromazepam, and clonazepam, respectively | 0.71, 0.79, and 0.79 µg/mL for diazepam, bromazepam, and clonazepam, respectively | Potentiometric, 10−4 M HCL | Ion-selective electrodes | Vallium, Rivotril, and Calmepam tablets | [137] |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Honeychurch, K.C. Review of Electroanalytical-Based Approaches for the Determination of Benzodiazepines. Biosensors 2019, 9, 130. https://doi.org/10.3390/bios9040130
Honeychurch KC. Review of Electroanalytical-Based Approaches for the Determination of Benzodiazepines. Biosensors. 2019; 9(4):130. https://doi.org/10.3390/bios9040130
Chicago/Turabian StyleHoneychurch, Kevin C. 2019. "Review of Electroanalytical-Based Approaches for the Determination of Benzodiazepines" Biosensors 9, no. 4: 130. https://doi.org/10.3390/bios9040130
APA StyleHoneychurch, K. C. (2019). Review of Electroanalytical-Based Approaches for the Determination of Benzodiazepines. Biosensors, 9(4), 130. https://doi.org/10.3390/bios9040130




