Surveillance for Antibiotic-Resistant E. coli in the Salish Sea Ecosystem
Abstract
:1. Introduction
2. Results
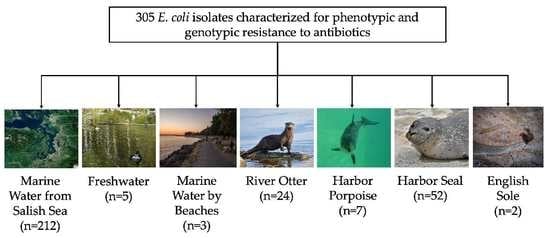
2.1. E. coli Isolates and Antibiotic Resistance
2.2. MLSTs and ExPEC Strains
2.3. Comparison of Susceptibility Rates
2.4. Phylogenetic Trees for ST10 and ST73
2.5. Virulence Factors in Nonsusceptible E. coli
3. Discussion
4. Materials and Methods
4.1. Study Setting
4.2. E. coli Collection and Isolation
4.2.1. Freshwater, Marine Water by Beaches, and Marine Water Samples
4.2.2. English Sole Samples
4.2.3. River Otter Samples
4.2.4. Marine Mammal Samples
4.3. fumC Typing
4.4. Antimicrobial-Susceptibility Testing
4.4.1. Phenotypic Characterization
4.4.2. Genotypic Characterization
4.5. Comparison of Susceptibility Rates
4.6. Phylogenetic Trees
4.7. Mapping
4.8. Antimicrobial Resistance (AMR) Genes and Virulence Factor Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bengtsson-Palme, J.; Kristiansson, E.; Larsson, D.G.J. Environmental factors influencing the development and spread of antibiotic resistance. FEMS Microbiol. Rev. 2018, 42. [Google Scholar] [CrossRef]
- Cristóvão, F.; Alonso, C.A.; Igrejas, G.; Sousa, M.; Silva, V.; Pereira, J.E.; Lozano, C.; Cortés-Cortés, G.; Torres, C.; Poeta, P. Clonal diversity of extended-spectrum Beta-Lactamase producing Escherichia coli isolates in fecal samples of wild animals. FEMS Microbiol. Lett. 2017, 364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raverty, S.A.; Rhodes, L.D.; Zabek, E.; Eshghi, A.; Cameron, C.E.; Hanson, M.B.; Schroeder, J.P. Respiratory microbiome of endangered Southern Resident Killer Whales and microbiota of surrounding sea surface microlayer in the Eastern North Pacific. Sci. Rep. 2017, 7, 394. [Google Scholar] [CrossRef] [PubMed]
- Frazzon, A.P.G. Antibiotic-resistant bacteria in free-living marine species. Vet. Rec. 2016, 179, 648–649. [Google Scholar] [CrossRef] [PubMed]
- Casas, C.; Anderson, E.C.; Ojo, K.K.; Keith, I.; Whelan, D.; Rainnie, D.; Roberts, M.C. Characterization of PRAS1-like plasmids from atypical north American psychrophilic Aeromonas salmonicida. FEMS Microbiol. Lett. 2005, 242, 59–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, M.C.; No, D.; Kuchmiy, E.; Miranda, C.D. Tetracycline resistance gene Tet(39) identified in three new genera of bacteria isolated in 1999 from Chilean salmon farms. J. Antimicrob. Chemother. 2015, 70, 619–621. [Google Scholar] [CrossRef] [Green Version]
- Protocols used for MLST of Escherichia coli and Shigella spp.—EnteroBase Documentation. Available online: https://enterobase.readthedocs.io/en/latest/mlst/mlst-legacy-info-ecoli.html (accessed on 30 March 2021).
- Sarowska, J.; Futoma-Koloch, B.; Jama-Kmiecik, A.; Frej-Madrzak, M.; Ksiazczyk, M.; Bugla-Ploskonska, G.; Choroszy-Krol, I. Virulence factors, prevalence and potential transmission of extraintestinal pathogenic Escherichia coli isolated from different sources: Recent Reports. Gut Pathog. 2019, 11, 10. [Google Scholar] [CrossRef] [Green Version]
- Lorenzo, P.; Adriana, A.; Jessica, S.; Carles, B.; Marinella, F.; Marta, L.; Luis, B.J.; Pierre, S. Antibiotic resistance in urban and hospital wastewaters and their impact on a receiving freshwater ecosystem. Chemosphere 2018, 206, 70–82. [Google Scholar] [CrossRef]
- Watkinson, A.J.; Micalizzi, G.B.; Graham, G.M.; Bates, J.B.; Costanzo, S.D. Antibiotic-resistant Escherichia coli in wastewaters, surface waters, and oysters from an urban riverine system. Appl. Environ. Microbiol. 2007, 73, 5667–5670. [Google Scholar] [CrossRef] [Green Version]
- Pitout, J.D.D. Extraintestinal pathogenic Escherichia coli: A combination of virulence with antibiotic resistance. Front. Microbiol. 2012, 3. [Google Scholar] [CrossRef] [Green Version]
- Valat, C.; Drapeau, A.; Beurlet, S.; Bachy, V.; Boulouis, H.-J.; Pin, R.; Cazeau, G.; Madec, J.-Y.; Haenni, M. Pathogenic Escherichia coli in dogs reveals the predominance of ST372 and the human-associated ST73 extra-intestinal lineages. Front. Microbiol. 2020, 11, 580. [Google Scholar] [CrossRef]
- Manges, A.R.; Geum, H.M.; Guo, A.; Edens, T.J.; Fibke, C.D.; Pitout, J.D.D. Global extraintestinal pathogenic Escherichia coli (ExPEC) lineages. Clin. Microbiol. Rev. 2019, 32. [Google Scholar] [CrossRef] [PubMed]
- Melendez, D.; Roberts, M.C.; Greninger, A.L.; Weissman, S.; No, D.; Rabinowitz, P.; Wasser, S. Whole-genome analysis of extraintestinal pathogenic Escherichia coli (ExPEC) MDR ST73 and ST127 isolated from endangered Southern Resident Killer Whales (Orcinus Orca). J. Antimicrob. Chemother. 2019, 74, 2176–2180. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.A.; Weagant, S.D.; Feng, P. Glutamate decarboxylase genes as a prescreening marker for detection of pathogenic Escherichia coli groups. Appl. Environ. Microbiol. 2001, 67, 3110–3114. [Google Scholar] [CrossRef] [Green Version]
- Davis, M.A.; Besser, T.E.; Orfe, L.H.; Baker, K.N.K.; Lanier, A.S.; Broschat, S.L.; New, D.; Call, D.R. Genotypic-phenotypic discrepancies between antibiotic resistance characteristics of Escherichia coli isolates from calves in management settings with high and low antibiotic use. Appl. Environ. Microbiol. 2011, 77, 3293–3299. [Google Scholar] [CrossRef] [Green Version]
- Mazurek, J.; Pusz, P.; Bok, E.; Stosik, M.; Baldy-Chudzik, K. The phenotypic and genotypic characteristics of antibiotic resistance in Escherichia coli populations isolated from farm animals with different exposure to antimicrobial agents. Pol. J. Microbiol. 2013, 62, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.R.; Gast, R.J.; Fujioka, R.S.; Solo-Gabriele, H.M.; Meschke, J.S.; Amaral-Zettler, L.A.; del Castillo, E.; Polz, M.F.; Collier, T.K.; Strom, M.S.; et al. The Coastal environment and human health: Microbial indicators, pathogens, sentinels and reservoirs. Environ. Health 2008, 7, S3. [Google Scholar] [CrossRef] [Green Version]
- Rozen, Y.; Belkin, S. Survival of enteric bacteria in seawater. FEMS Microbiol. Rev. 2001, 25, 513–529. [Google Scholar] [CrossRef]
- Krogh, J.; Lyons, S.; Lowe, C.J. Pharmaceuticals and personal care products in municipal wastewater and the marine receiving environment near Victoria, Canada. Front. Mar. Sci. 2017, 4, 415. [Google Scholar] [CrossRef] [Green Version]
- Krepakevich, A.; Pospelova, V. Tracing the influence of sewage discharge on coastal bays of Southern Vancouver Island (BC, Canada) using sedimentary records of phytoplankton. Cont. Shelf Res. 2010, 30, 1924–1940. [Google Scholar] [CrossRef]
- Census. Available online: https://data.census.gov/cedsci/profile?g=0400000US53 (accessed on 3 May 2021).
- Port, J.A.; Wallace, J.C.; Griffith, W.C.; Faustman, E.M. Metagenomic profiling of microbial composition and antibiotic resistance determinants in Puget Sound. PLoS ONE 2012, 7, e48000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meador, J.P.; Yeh, A.; Young, G.; Gallagher, E.P. Contaminants of emerging concern in a large temperate estuary. Environ. Pollution 2016, 213, 254–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weissman, S.J.; Johnson, J.R.; Tchesnokova, V.; Billig, M.; Dykhuizen, D.; Riddell, K.; Rogers, P.; Qin, X.; Butler-Wu, S.; Cookson, B.T.; et al. High-resolution two-locus clonal typing of extraintestinal pathogenic Escherichia Coli. Appl. Environ. Microbiol. 2012, 78, 1353–1360. [Google Scholar] [CrossRef] [Green Version]
- Rice, E.W.; Eaton, A.D.; Baird, R.B.; Clesceri, L.S. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Norman, S.A.; Lambourn, D.M.; Huggins, J.L.; Gaydos, J.K.; Dubpernell, S.; Berta, S.; Olson, J.K.; Souze, V.; Evans, A.; Carlson, B.; et al. Antibiotic resistance of bacteria in two marine mammal species, harbor seals and harbor porpoises, living in an urban marine ecosystem, the Salish Sea, Washington State, USA. Oceans 2021, 2, 86–104. [Google Scholar] [CrossRef]
- Weinstein, M.P. Clinical and Laboratory Standards Institute Performance Standards for Antimicrobial Susceptibility Testing: Supplement M100, 31st ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2021; ISBN 978-1-68440-066-9. [Google Scholar]
- Escherichia coli (ID 283914)-BioProject-NCBI. Available online: https://www.ncbi.nlm.nih.gov//bioproject/283914 (accessed on 25 February 2021).
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seemann, T. Tseemann/Snippy. 2021. Available online: https://github.com/tseemann/snippy (accessed on 11 March 2021).
- Seemann, T. Tseemann/Snp-Dists. 2021. Available online: https://github.com/tseemann/snp-dists (accessed on 11 March 2021).
- Larsson, A. AliView: A fast and lightweight alignment viewer and editor for large datasets. Bioinformatics 2014, 30, 3276–3278. [Google Scholar] [CrossRef] [PubMed]
- PHYLIP. Available online: https://evolution.genetics.washington.edu/phylip.html (accessed on 11 March 2021).
- FigTree. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 11 March 2021).
- ResFinder. Available online: https://cge.cbs.dtu.dk (accessed on 11 March 2021).
- VirulenceFinder. Available online: https://cge.cbs.dtu.dk/services/VirulenceFinder/ (accessed on 11 March 2021).



| Sample Source | Isolates Characterized | Intermediate | Resistant | Susceptible |
|---|---|---|---|---|
| Marine Water (Total) | 212 | 7 (3.3%) | 7 (3.3%) | 198 (93.4%) |
| North Puget Sound | 49 | 3 (6.1%) | 4 (8.2%) | 42 (85.7%) |
| Central Puget Sound | 55 | 0 (0%) | 2 (3.6%) | 53 (96.4%) |
| South Puget Sound | 56 | 3 (5.4%) | 0 (0%) | 53 (94.6%) |
| Strait of Juan de Fuca | 52 | 1 (1.9%) | 1 (1.9%) | 50 (96.2%) |
| Freshwater | 5 | 1 (20%) | 3 (60.0%) | 1 (20.0%) |
| Marine water by beaches | 3 | 0 (0%) | 0 (0%) | 3 (100%) |
| Harbor Seal (Total) | 52 | 6 (11.5%) | 8 (15.4%) | 38 (73.1%) |
| Dead Seal | 35 | 6 (17.1%) | 3 (8.6%) | 26 (74.3%) |
| Live Seal | 17 | 0 (0%) | 5 (29.4%) | 12 (70.6%) |
| Harbor Porpoise | 7 | 2 (28.6%) | 0 (0%) | 5 (71.4%) |
| River Otter | 24 | 4 (16.7%) | 13 (54.2%) | 7 (29.2%) |
| Sole | 2 | 0 (0%) | 0 (0%) | 2 (100%) |
| Total | 305 | 20 (6.6%) | 31 (10.2%) | 254 (83.3%) |
| Isolate ID | Source | MLST | Resistance Phenotype | Resistance Phenotype by Antibiotic | Resistant Genes by WGS a | Virulence Factors a |
|---|---|---|---|---|---|---|
| 353985-001-1210 | South Puget Sound | 2 | Intermediate | Imipenem (Intermediate) | None | ast, chuA, lpfA |
| 339942-001-501 | North Puget Sound | 10 | Resistant | Minocycline, SXT b | qnrB19, sulIII, dfrA12, floR, tet(A) | gad, terC |
| HAM6D | River Otter | 10 | Resistant | Ampicillin, SXT, Tetracycline | aph(6)-Id, blaTEM-1B, tet(B) | astA, cia, gad, terC, traT |
| CWG3I | River Otter | 10 | Resistant | Cefotaxime (Intermediate), Tetracycline, Minocycline (Intermediate), Sulfisoxazole (Intermediate) | tet(B) | gad, kpsE, kpsM II, terC |
| 344914-013-1036 | Central Puget Sound | 58 | Resistant | Doxycycline, Minocycline (Intermediate) | tet(B), aph(3″)-Ib, aph(6)-Id | cia, cvaC, etsC, fyuA, gad, hlyF, iroN, iss, iucC, iutA, lpfA, chF, ompT, terC, traT |
| 339942-002-506 | North Puget Sound | 58 | Resistant | Aztreonam, Cefotaxime, Doxycycline, SXT, Ciprofloxacin (Intermediate) | sulIII, dfrA12, tet(A), floR, blaCTX-M-15, qnrS1, qnrB19 | gad, hlyF, lpfA, terC |
| HAM5E | River Otter | 69 | Resistant | Ampicillin, SXT, Tetracycline, Minocycline, Sulfisoxazole | aadA5, aph(3″)-Ib, aph(6)-Id, blaTEM-1B, catA1, qnrB19, qnrB82, sulII, tet(B), dfrA17 | air, chuA, eilA, fyuA, gad, hra, iha, irp2, iucC, lutA, kpsE, kpsM II_K52, lpfA, ompT, papA, fsiA(F16), papC, sat, senB, traT |
| SSW080719 (AN0077) | Dead Seal | 117 | Resistant | Doxycycline | tet(B), sulII, aph(6)-Id, aph(3″)-Ib, aph(3′)-Ia | astA, chuA, etsC, fyuA, hlyF, hra, iroN, irp2, iss, lucC, ompT, pic, traT, vat |
| SSW082919 (AN0092) | Dead Seal | 117 | Resistant | Doxycycline | tet(B), sulII, aph(6)-Id, aph(3″)-Ib, aph(3′)-Ia | astA, chuA, etsC, fyuA, hlyF, hra, iroN, irp2, iss, lucC, ompT, pic, traT, vat |
| WDFW2019-154 (AN0107) | Dead Seal | 131 | Resistant | Amoxicillin, Gentamicin, SXT | aac(3)-Iid, aadA2, dfrA12, sulI, mph(A), blaTEM-1B | afaA, afaC, afaD, afaE, chuA, fyuA, gad, iha, irp2, iss, iucC, iutA, kpsE, kpsM II_K5, ompT, sat, senB, traT, yfcV |
| 343170-001-909 | North Puget Sound | 131 | Intermediate | Ciprofloxacin (Intermediate), Ticarcillin/Clavulanic Acid (Intermediate) | blaTEM-1B, gyrA (S83L) | afaA, afaD, chuA, fyuA, gad, kpsE, kpsM II_K5, ompT, senB, traT, yfcV |
| GRNRA2B | River Otter | 131 | Resistant | Ampicillin, Imipenem (Intermediate), Kanamycin (Intermediate), Sulfisoxazole (Intermediate) | blaTEM-1C | chuA, gad, ibeA, irp2, iss, kpsM II, papA_F48, sitA, yfcV |
| WDFW2019-107 (AN0070) | Dead Seal | 162 | Intermediate | Florfenicol (Intermediate), Chloramphenicol (Intermediate) | None | gad, lpfA, terC, traT |
| CWG7G | River Otter | 162 | Resistant | Sulfisoxazole, Cefotaxime (Intermediate), Amikacin (Intermediate), Kanamycin (Intermediate) | None | gad, hlyF, iss, iucC, iutA, lpfA, terC |
| CWG7H | River Otter | 162 | Resistant | Ampicillin (Intermediate), Amikacin (Intermediate), Kanamycin (Intermediate), Sulfisoxazole | None | gad, hlyF, lucC, lutA, lpfA, terC |
| 342381-006-850 | Strait of Juan de Fuca | 206 | Resistant | Aztreonam, Cefotaxime, Ceftazidime | None | astA, gad, traT |
| PCB4Cef | Fresh Water | 297 | Resistant | Ampicillin, Amoxicillin/Clavulanic Acid, Ceftriaxone, Aztreonam, Ceftazidime, Ticarcillin/Clavulanic Acid (Intermediate) | blaCMY-2 | cib, gad, lpfA, mchB |
| SKMMR2020-01-025 Gut #1 | Live Seal | 345 | Resistant | SXT | dfrA5 | cia, cvaC, etsC, gad, hlyF, iroN, iss, lpfA, ompT, sitA |
| GRNRA3B | River Otter | 362 | Intermediate | Cefotaxime (Intermediate), Sulfisoxazole (Intermediate) | None | chuA, iss, kpsE, kpsM II_K5 |
| GRNRA4A | River Otter | 362 | Intermediate | Cefotaxime (Intermediate), Imipenem (Intermediate), Meropenem (Intermediate), Amikacin (Intermediate), Kanamycin (Intermediate), Sulfisoxazole (Intermediate) | qnrB19 | chuA, iss, kpsE, kpsM II_K5 |
| GRNRA4B | River Otter | 362 | Resistant | Sulfisoxazole, Cefotaxime (Intermediate), Imipenem (Intermediate), Meropenem (Intermediate), Kanamycin (Intermediate), Ciprofloxacin (Intermediate) | None | chuA, iss, kpsE, kpsM I_K5 |
| SKMMR2019-7-10PV (AN0044) | Dead Seal | 372 | Intermediate | Florfenicol (Intermediate) | None | None |
| 19Pv16JulWI-07 Isolate #1 (AN0047) | Dead Seal | 372 | Intermediate | Florfenicol (Intermediate) | None | cea, focC, sfaE, focG, focI, fyuA, gad, hra, ibeA, iroN, irp2, iss kpsE, kpsM II_K24, mchB, mchF, ompT, papA_F13, terC |
| 19Pv29JulWI-09 Isolate #2 (AN0041) | Dead Seal | 372 | Intermediate | Florfenicol (Intermediate), Amoxicillin (Intermediate) | None | None |
| GG 14-6 Cef | Fresh Water | 405 | Resistant | Aztreonam, Cefepime, Cefotaxime, Ceftazidime, Ciprofloxacin, Doxycycline, Levofloxacin, Minocycline, Ticarcillin/Clavulanic Acid, SXT | sulI, mph(A), blaCTX-M-15, aadA2, qepA4, dfrA12, catA1, tet(B), qepA, gyrA S83L, gyrA D87N | chuA, fyuA, irp2, kpsM II_K5, sitA, traT |
| GRNRA2E | River Otter | 538 | Resistant | Cefotaxime, Sulfisoxazole, Ampicillin (Intermediate), Imipenem (Intermediate), Meropenem (Intermediate), Amikacin (Intermediate) | aac(2′)-Iia | ibeA, neuC, ompT |
| CRC-1702 (AN0006) | Porpoise | 569 | Intermediate | Florfenicol (Intermediate) Chloramphenicol (Intermediate) | None | chuA, fyuA, ibeA, iss kpsE kpsM II_K1, neuC, ompT, sitA, usp |
| GG 14-5 Cef | Fresh Water | 616 | Resistant | Aztreonam, Cefotaxime, Ceftazidime (Intermediate), Cefepime | blaCTX-M-15, qnrS1, mph(A) | gad, terC, traT |
| 343066-013-868 | South Puget Sound | 641 | Intermediate | Aztreonam (Intermediate) | None | gad, lpfA, ompT, traT |
| PCO1 | Fresh Water | 681 | Intermediate | Ceftriaxone (Intermediate) | None | chuA, cia, cibB, iss, ompT, traT |
| EPA Dock G Cip 1#5 | Live Seal | 744 | Resistant | Ciprofloxacin, Doxycycline (Intermediate), Levofloxacin | aph(3″)-Ib, aph(6)-Id, catA1, floR, sulII, tet(A), gyrA S83L, gyrA D87N | gad |
| SKMMR2020-01-025 Fecal #1 | Live Seal | 744 | Resistant | Ciprofloxacin, Levofloxacin | aph(3″)-Ib, aph(6)-Id, mdf(A), catA1, floR, sulII, tet(A), gyrA S83L, gyrA D87N | gad |
| 351565-001-1202 | North Puget Sound | 744 | Resistant | Ciprofloxacin, Doxycycline, Levofloxacin, Minocycline, SXT | sulI, dfrA17, tet(A), sulII, tet(B), blaTEM-1B, aph(3″)-Ib, mph(A), aadA5, catA1, aph(6)-Id, gyrA S83L, gyrA D87N | cvaC, etsC, gad, hlyF, iroN, iss, mchF, traT |
| 339942-003-511 | North Puget Sound | 746 | Resistant | Cefotaxime, Doxycycline (Intermediate), Gentamicin (Intermediate) | aac(3)-Via, aph(3″)-Ib, aadA1, aph(6)-Id, sulI, blaSHV-12,tet(A) | cib, cma, fyuA, gad, hlyF, iroN, irp2, iss, neuC, terC, traT |
| EPA Dock G#1 | Live Seal | 772 | Resistant | Doxycycline, SXT, Minocycline (Intermediate) | aadA5, sulII, tet(B), dfrA17 | cma, gad, irp2, terC |
| 343389-008-981 | North Puget Sound | 942 | Intermediate | Amikacin (Intermediate), Ticarcillin/Clavulanic Acid (Intermediate) | None | lpfA, sitA, terC |
| 354777-001-1214 | Strait of Juan de Fuca | 967 | Intermediate | Aztreonam (Intermediate) | None | cba, chuA, cma, ibeA, kpsM II_K5 |
| BR1F | River Otter | 1079 | Resistant | Ampicillin, Gentamicin, Tetracycline, Minocycline | aac(3)-IV, aac(3)-Iva, aadA1, aph(4)-Ia, aph(6)-Id, blaTEM-1B, lnu(F), tet(B) | gad, lpfA, terC |
| BR1E | River Otter | 1079 | Resistant | Doxycycline, Gentamicin, Tobramycin, Minocycline (Intermediate) | aac(3)-IV, aph(4)-Ia, aph(6)-Id, blaTEM-1B, lnu(F), tet(B) | gad, lpfA, terC |
| GRN1A | River Otter | 1246 | Intermediate | Ampicillin (Intermediate), Sulfisoxazole (Intermediate) | None | gad, lpfA, terC |
| 2019-SJ013 (AN0032) | Dead Seal | 1718 | Intermediate | Florfenicol (Intermediate) | None | gad, terC |
| EJC-2019-03 (AN0009) | Porpoise | 1723 | Intermediate | Florfenicol (Intermediate), Amoxicillin (Intermediate), Chloramphenicol (Intermediate) | None | cma, gad, ipfA, traT |
| CWG3J | River Otter | 2144 | Resistant | Chloramphenicol, Tetracycline, Sulfisoxazole, Minocycline (Intermediate) | aadA1, cmlA1, sulIII, tet(A) | cib, gad, lpfA, ompT |
| GRNRA2F | River Otter | 2164 | Resistant | Cefotaxime, Imipenem, Meropenem (Intermediate), Kanamycin (Intermediate), Sulfisoxazole (Intermediate) | None | gad, iss, lpfA, ompT, terC |
| GRNRA4F | River Otter | 2521 | Resistant | Sulfisoxazole, Cefotaxime (Intermediate), Ampicillin (Intermediate), Imipenem (Intermediate), Meropenem (Intermediate), Kanamycin (Intermediate) | None | gad, iss, lpfA, ompT, terC |
| 345996-003-1186 | North Puget Sound | 2522 | Intermediate | Aztreonam (Intermediate) | None | gad, lpfA |
| CWG5A | River Otter | 2607 | Intermediate | Cefotaxime (Intermediate), Imipenem (Intermediate), Kanamycin (Intermediate) | None | gad, lss, lpfA, ompT, terC |
| WDFW2019-112 (AN0071) | Dead Seal | 3018 | Intermediate | Florfenicol (Intermediate) | None | None |
| 336039-006-31 | South Puget Sound | 7706 | Intermediate | Ciprofloxacin (Intermediate) | None | gad, iss |
| HASE 6 CEF | Live Seal | 9001 | Resistant | Ampicillin, Amoxicillin/Clavulanic Acid, Ceftriaxone, Aztreonam, Cefotaxime, Ceftazidime, Ticarcillin/Clavulanic Acid (Intermediate) | blaCMY-2 | astA, hlyF, hra, traT |
| 339940-002-477 | Central Puget Sound | 10718 | Resistant | Cefotaxime, Ceftazidime, Ticarcillin/Clavulanic Acid (Intermediate) | blaCMY-2 | gad, lpfA, ompT, terC |
| Sample Source (n = 37) | ST10 | ST10 Resistant | ST38 | ST38 Resistant | ST58 | ST58 Resistant | ST69 | ST69 Resistant | ST73 | ST73 Resistant | ST117 | ST117 Resistant | ST131 | ST131 Resistant | ST405 | ST405 Resistant | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Marine Water (Total) | 10 | 1 | 2 | 0 | 2 | 2 | 2 | 0 | 1 | 0 | 3 | 0 | 1 | 0 | 0 | 0 | 21 |
| North Puget Sound | 4 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 7 |
| Central Puget Sound | 4 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 6 |
| South Puget Sound | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| Strait of Juan de Fuca | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 4 |
| Fresh water | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 |
| Marine water by beaches | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 |
| Harbor Seal (Total) | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 4 | 0 | 2 | 2 | 1 | 0 | 0 | 0 | 8 |
| Dead Seal | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 4 | 0 | 2 | 2 | 1 | 1 | 0 | 0 | 8 |
| Live Seal | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Harbor Porpoise | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| River Otter | 2 | 2 | 0 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 5 |
| Sole | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 12 | 3 | 2 | 0 | 4 | 2 | 4 | 1 | 5 | 0 | 6 | 2 | 3 | 2 | 1 | 1 | 37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vingino, A.; Roberts, M.C.; Wainstein, M.; West, J.; Norman, S.A.; Lambourn, D.; Lahti, J.; Ruiz, R.; D’Angeli, M.; Weissman, S.J.; et al. Surveillance for Antibiotic-Resistant E. coli in the Salish Sea Ecosystem. Antibiotics 2021, 10, 1201. https://doi.org/10.3390/antibiotics10101201
Vingino A, Roberts MC, Wainstein M, West J, Norman SA, Lambourn D, Lahti J, Ruiz R, D’Angeli M, Weissman SJ, et al. Surveillance for Antibiotic-Resistant E. coli in the Salish Sea Ecosystem. Antibiotics. 2021; 10(10):1201. https://doi.org/10.3390/antibiotics10101201
Chicago/Turabian StyleVingino, Alexandria, Marilyn C. Roberts, Michelle Wainstein, James West, Stephanie A. Norman, Dyanna Lambourn, Jeffery Lahti, Ryan Ruiz, Marisa D’Angeli, Scott J. Weissman, and et al. 2021. "Surveillance for Antibiotic-Resistant E. coli in the Salish Sea Ecosystem" Antibiotics 10, no. 10: 1201. https://doi.org/10.3390/antibiotics10101201
APA StyleVingino, A., Roberts, M. C., Wainstein, M., West, J., Norman, S. A., Lambourn, D., Lahti, J., Ruiz, R., D’Angeli, M., Weissman, S. J., & Rabinowitz, P. (2021). Surveillance for Antibiotic-Resistant E. coli in the Salish Sea Ecosystem. Antibiotics, 10(10), 1201. https://doi.org/10.3390/antibiotics10101201