Cyclic and Acyclic Amine Oxide Alkyl Derivatives as Potential Adjuvants in Antimicrobial Chemotherapy against Methicillin-Resistant Staphylococcus aureus with an MDR Profile
Abstract
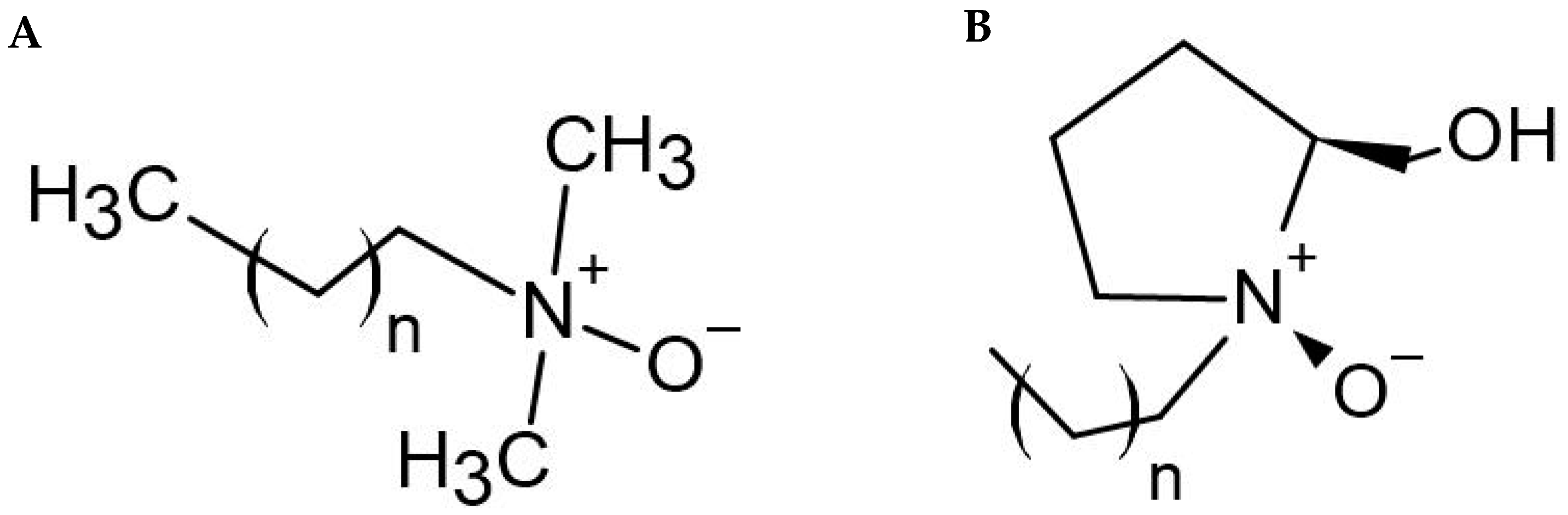
:1. Introduction
2. Results
2.1. In Vitro Susceptibility Test
2.2. Checkerboard Microdilution Assay
2.3. Red Blood Cell Haemolysis and PBMC Cytotoxicity
3. Discussion
4. Materials and Methods
4.1. Organisms
4.2. Antibiotics and Compounds
4.3. In Vitro Susceptibility Test
4.4. Checkerboard Microdilution Assay
4.5. Drug–Drug Interaction Model Analysis
4.6. Human Peripheral Blood Red Cells and Mononuclear Cells (PBMCs) Isolation
4.7. Human Red Blood Cell Haemolytic Assay
4.8. PBMC Cytotoxicity Assay
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). WHO Report on Surveillance of Antibiotic Consumption; WHO: Geneva, Switzerland, 2019.
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: A review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- León-Buitimea, A.; Garza-Cárdenas, C.R.; Garza-Cervantes, J.A.; Lerma-Escalera, J.A.; Morones-Ramírez, J.R. The Demand for New Antibiotics: Antimicrobial Peptides, Nanoparticles, and Combinatorial Therapies as Future Strategies in Antibacterial Agent Design. Front. Microbiol. 2020, 11, 1669. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [Green Version]
- Basak, S.; Singh, P.; Rajurkar, M. Multidrug Resistant and Extensively Drug Resistant Bacteria: A Study. J. Pathog. 2016, 2016, 4065603. [Google Scholar] [CrossRef] [Green Version]
- The World Bank. Drug-Resistant Infections: A Threat to Our Economic Future (Vol. 2): Final Report. Available online: https://documents.worldbank.org/en/publication/documents-reports/documentdetail/323311493396993758/final-report (accessed on 21 April 2021).
- Vallavan, V.; Krishnasamy, G.; Zin, N.M.; Abdul Latif, M. A Review on Antistaphylococcal Secondary Metabolites from Basidiomycetes. Molecules 2020, 25, 5848. [Google Scholar] [CrossRef] [PubMed]
- Aminov, R. History of antimicrobial drug discovery: Major classes and health impact. Biochem. Pharmacol. 2017, 133, 4–19. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K. Platforms for antibiotic discovery. Nat. Rev. Drug Discov. 2013, 12, 371–387. [Google Scholar] [CrossRef] [PubMed]
- Laxminarayan, R.; Matsoso, P.; Pant, S.; Brower, C.; Røttingen, J.A.; Klugman, K.; Davies, S. Access to effective antimicrobials: A worldwide challenge. Lancet 2016, 387, 168–175. [Google Scholar] [CrossRef]
- Abat, C.; Gautret, P.; Raoult, D. Benefits of antibiotics burden in low-income countries. Proc. Natl. Acad. Sci. USA 2018, 115, E8109–E8110. [Google Scholar] [CrossRef] [Green Version]
- Wright, G.D.; Sutherland, A.D. New strategies for combating multidrug-resistant bacteria. Trends Mol. Med. 2007, 13, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Cottarel, G.; Wierzbowski, J. Combination drugs, an emerging option for antibacterial therapy. Trends Biotechnol. 2007, 25, 547–555. [Google Scholar] [CrossRef]
- Phougat, N.; Khatri, S.; Singh, A.; Dangi, M.; Kumar, M.; Dabur, R.; Chhillar, A. Combination Therapy: The Propitious Rationale for Drug Development. Comb. Chem. High. Throughput Screen. 2014, 17, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Bellio, P.; Luzi, C.; Mancini, A.; Cracchiolo, S.; Passacantando, M.; Di Pietro, L.; Perilli, M.; Amicosante, G.; Santucci, S.; Celenza, G. Cerium oxide nanoparticles as potential antibiotic adjuvant. Effects of CeO2 nanoparticles on bacterial outer membrane permeability. Biochim. Biophys. Acta Biomembr. 2018, 1860, 2428–2435. [Google Scholar] [CrossRef] [PubMed]
- Bellio, P.; Brisdelli, F.; Perilli, M.; Sabatini, A.; Bottoni, C.; Segatore, B.; Setacci, D.; Amicosante, G.; Celenza, G. Curcumin inhibits the SOS response induced by levofloxacin in Escherichia coli. Phytomedicine 2014, 21, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Celenza, G.; Segatore, B.; Setacci, D.; Perilli, M.; Brisdelli, F.; Bellio, P.; Piovano, M.; Garbarino, J.A.; Amicosante, G.; Nicoletti, M. Antibacterial activity of selected metabolites from Chilean lichen species against methicillin-resistant staphylococci. Nat. Prod. Res. 2013, 27, 1528–1531. [Google Scholar] [CrossRef] [PubMed]
- Azira, H.; Tazerouti, A.; Canselier, J.P. Study of Foaming Properties and Effect of the Isomeric Distribution of Some Anionic Surfactants. J. Surfactants Deterg. 2008, 11, 279–286. [Google Scholar] [CrossRef] [Green Version]
- Sehgal, P.; Doe, H.; Bakshi, M.S. Solubilisation of phospholipid vesicular structures into mixed micelles of zwitterionic surfactants. J. Surfactants Deterg. 2003, 6, 31–37. [Google Scholar] [CrossRef]
- Lichtenberg, D.; Robson, R.J.; Dennis, E.A. Solubilization of phospholipids by detergents structural and kinetic aspects. BBA Rev. Biomembr. 1983, 737, 285–304. [Google Scholar] [CrossRef]
- Ceccacci, F.; Giansanti, L.; Mancini, G.; Mauceri, A.; Scipioni, A.; Sperduto, C. Transcription of chirality from molecules to complex systems: The role of hydrophobic interactions. Supramol. Chem. 2013, 25, 741–747. [Google Scholar] [CrossRef]
- Costas-Costas, U.; Bravo-Diaz, C.; Gonzalez-Romero, E. Kinetics and mechanism of the reaction between ascorbic acid derivatives and an arenediazonium salt: Cationic micellar effects. Langmuir 2005, 21, 10983–10991. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gao, Y. Effects of length and unsaturation of the alkyl chain on the hydrophobic binding of curcumin with Tween micelles. Food Chem. 2018, 246, 242–248. [Google Scholar] [CrossRef]
- Ceccacci, F.; Giansanti, L.; Mortera, S.L.; Mancini, G.; Sorrenti, A.; Villani, C. Enantiodiscrimination of bilirubin-IXα enantiomers in biomembrane models: Has chirality a role in bilirubin toxicity? Bioorganic Chem. 2008, 36, 252–254. [Google Scholar] [CrossRef] [PubMed]
- Fuangswasdi, A.; Charoensaeng, A.; Sabatini, D.A.; Scamehorn, J.F.; Acosta, E.J.; Osathaphan, K.; Khaodhiar, S. Mixtures of anionic and cationic surfactants with single and twin head groups: Solubilisation and adsolubilization of styrene and ethylcyclohexane. J. Surfactants Deterg. 2006, 9, 29–37. [Google Scholar] [CrossRef]
- Demissie, H.; Duraisamy, R. Effects of electrolytes on the surface and micellar characteristics of Sodium dodecyl sulphate surfactant solution. J. Sci. Innov. Res. 2016, 5, 208–214. [Google Scholar]
- Api, A.M.; Belmonte, F.; Belsito, D.; Biserta, S.; Botelho, D.; Bruze, M.; Burton, G.A.; Buschmann, J.; Cancellieri, M.A.; Dagli, M.L.; et al. RIFM fragrance ingredient safety assessment, dodecyldimethylamine oxide, CAS Registry Number 1643-20-5. Food Chem. Toxicol. 2020, 141, 111424. [Google Scholar] [CrossRef] [PubMed]
- Vlachy, N.; Drechsler, M.; Verbavatz, J.M.; Touraud, D.; Kunz, W. Role of the surfactant headgroup on the counterion specificity in the micelle-to-vesicle transition through salt addition. J. Colloid Interface Sci. 2008, 319, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Karukstis, K.K.; McDonough, J.R. Characterization of the aggregates of N-Alkyl-N-methylpyrrolidinium bromide surfactants in aqueous solution. Langmuir 2005, 21, 5716–5721. [Google Scholar] [CrossRef]
- Zhao, M.; Zheng, L. Micelle formation by N-alkyl-N-methylpyrrolidinium bromide in aqueous solution. Phys. Chem. Chem. Phys. 2011, 13, 1332–1337. [Google Scholar] [CrossRef]
- Tian, Y.; Wei, R.; Cai, B.; Dong, J.; Deng, B.; Xiao, Y. Cationic gemini pyrrolidinium surfactants based sweeping-micellar electrokinetic chromatography for simultaneous detection of nine organic pollutants in environmental water. J. Chromatogr. A 2016, 1475, 95–101. [Google Scholar] [CrossRef]
- Bombelli, C.; Bordi, F.; Ferro, S.; Giansanti, L.; Jori, G.; Mancini, G.; Mazzuca, C.; Monti, D.; Ricchelli, F.; Sennato, S.; et al. New cationic liposomes as vehicles of m-tetrahydroxyphenylchlorin in photodynamic therapy of infectious diseases. Mol. Pharm. 2008, 5, 672–679. [Google Scholar] [CrossRef]
- Goracci, L.; Germani, R.; Rathman, J.F.; Savelli, G. Anomalous behavior of amine oxide surfactants at the air/water interface. Langmuir 2007, 23, 10525–10532. [Google Scholar] [CrossRef] [PubMed]
- Brinchi, L.; Germani, R.; Di Profio, P.; Marte, L.; Savelli, G.; Oda, R.; Berti, D. Viscoelastic solutions formed by worm-like micelles of amine oxide surfactant. J. Colloid Interface Sci. 2010, 346, 100–106. [Google Scholar] [CrossRef] [PubMed]
- García, M.T.; Campos, E.; Ribosa, I. Biodegradability and ecotoxicity of amine oxide based surfactants. Chemosphere 2007, 69, 1574–1578. [Google Scholar] [CrossRef] [PubMed]
- Goldsipe, A.; Blankschtein, D. Molecular-thermodynamic theory of micellisation of multicomponent surfactant mixtures: 2. pH-sensitive surfactants. Langmuir 2007, 23, 5953–5962. [Google Scholar] [CrossRef]
- Łukomska, M.; Rybarczyk-Pirek, A.J.; Jabłoński, M.; Palusiak, M. The nature of NO-bonding in N-oxide group. Phys. Chem. Chem. Phys. 2015, 17, 16375–16387. [Google Scholar] [CrossRef] [PubMed]
- Piasecki, A.; Wójcik, B.; Łuczyński, J.; Piłakowska-Pietras, D.; Witek, S.; Krasowska, A. Bifunctional N-Oxides of Alkyldiamidoamines. J. Surfactants Deterg. 2009, 12, 201–207. [Google Scholar] [CrossRef]
- Bordi, F.; Cerichelli, G.; De Berardinis, N.; Diociaiuti, M.; Giansanti, L.; Mancini, G.; Sennato, S. Synthesis and physicochemical characterisation of new twin-tailed N-oxide based gemini surfactants. Langmuir 2010, 26, 6177–6183. [Google Scholar] [CrossRef] [PubMed]
- Katritzky, A.R.; Duell, B.L.; Seiders, R.P.; Katritzky, A.R. Synthesis and Catalytic Activity of Surfactant Analogues of 4-(Dimethylamino) pyridine. Langmuir 1987, 3, 976–982. [Google Scholar] [CrossRef]
- Karlovská, J.; Uhríková, D.; Kučerka, N.; Teixeira, J.; Devínsky, F.; Lacko, I.; Balgavý, P. Influence of N-dodecyl-N,N-dimethylamine N-oxide on the activity of sarcoplasmic reticulum Ca2+-transporting ATPase reconstituted into diacylphosphatidylcholine vesicles: Effects of bilayer physical parameters. Biophys. Chem. 2006, 119, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Niedziółka, K.; Szymula, M.; Lewińska, A.; Wilk, K.A.; Narkiewicz-Michałek, J. Studies of vitamin C antioxidative activity in the N-oxide surfactant solutions. Colloids Surf. A Physicochem. Eng. Asp. 2012, 413, 33–37. [Google Scholar] [CrossRef]
- Battista, S.; Campitelli, P.; Carlone, A.; Giansanti, L. Influence of structurally related micelle forming surfactants on the antioxidant activity of natural substances. Chem. Phys. Lipids 2019, 225, 104818. [Google Scholar] [CrossRef] [PubMed]
- Battista, S.; Bellio, P.; Celenza, G.; Galantini, L.; Franceschini, I.; Mancini, G.; Giansanti, L. Correlation of Physicochemical and Antimicrobial Properties of Liposomes Loaded with (+)-Usnic Acid. ChemPlusChem 2020, 85, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Battista, S.; Campitelli, P.; Galantini, L.; Köber, M.; Vargas-Nadal, G.; Ventosa, N.; Giansanti, L. Use of N-oxide and cationic surfactants to enhance antioxidant properties of (+)-usnic acid loaded liposomes. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124154. [Google Scholar] [CrossRef]
- Richmon, J. (Ed.) Cationic Surfactants: Organic Chemistry, 1st ed.; CRC Press: Boca Raton, FL, USA, 1990; Volume 34, Available online: https://www.routledge.com/Cationic-Surfactants-Organic-Chemistry/Richmond/p/book/9780824783815 (accessed on 21 April 2021).
- Prabhu, V.S. Synthesis of Tertiary Amine Oxides. U.S. Patent US5866718A, 20 March 1997. [Google Scholar]
- Gunstone, F.D.; Padley, F.B. (Eds.) Lipid Technologies and Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 1997; 848p, Available online: https://www.routledge.com/Lipid-Technologies-and-Applications/Gunstone-Padley/p/book/9780824798383 (accessed on 21 April 2021).
- Birnie, C.R.; Malamud, D.; Schnaare, R.L. Antimicrobial evaluation of N-Alkyl betaines and N-Alkyl-N,N-dimethylamine oxides with variations in chain length. Antimicrob. Agents Chemother. 2000, 44, 2514–2517. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Environment, Eco-Label, Product Groups. 2007. Available online: https://ec.europa.eu/environment/archives/ecolabel/product/pg_did_list_en.htm (accessed on 21 April 2021).
- U.S. Environmental Protection Agency. Safer Chemical Ingredients List. Available online: https://www.epa.gov/saferchoice/safer-ingredients (accessed on 21 April 2021).
- Organisation for Economic Co-Operation and Development. SIAM 22. 18–21 April 2006. SIDS Initial Assessment Profile. Available online: https://www.aciscience.org/docs/Amine_Oxides_SIAP_April_2006.pdf (accessed on 21 April 2021).
- Subik, J.; Takacsova, G.; Psenak, M.; Devinsky, F. Antimicrobial activity of amine oxides: Mode of action and structure-activity correlation. Antimicrob. Agents Chemother. 1977, 12, 139–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devínsky, F.; Kopecka-Leitmanová, A.; Šeršeň, F.; Balgavý, P. Cut-off Effect in Antimicrobial Activity and in Membrane Perturbation Efficiency of the Homologous Series of N,N -Dimethylalkylamine Oxides†. J. Pharm. Pharmacol. 2011, 42, 790–794. [Google Scholar] [CrossRef] [PubMed]
- Berger-Bächi, B. Resistance mechanisms of gram-positive bacteria. Int. J. Med. Microbiol. 2002, 292, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Fuda, C.; Suvorov, M.; Vakulenko, S.B.; Mobashery, S. The Basis for Resistance to β-Lactam Antibiotics by Penicillin-binding Protein 2a of Methicillin-resistant Staphylococcus aureus. J. Biol. Chem. 2004, 279, 40802–40806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, T.; Katayama, Y.; Asada, K.; Mori, N.; Tsutsumimoto, K.; Tiensasitorn, C.; Hiramatsu, K. Structural comparison of three types of staphylococcal cassette chromosome mec integrated in the chromosome in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2001, 45, 1323–1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goffin, C.; Ghuysen, J.-M. Multimodular Penicillin-Binding Proteins: An Enigmatic Family of Orthologs and Paralogs. Microbiol. Mol. Biol. Rev. 1998, 62, 1079–1093. [Google Scholar] [CrossRef] [Green Version]
- Corner, A.M.; Dolan, M.M.; Yankell, S.L.; Malamud, D. C31G, a new agent for oral use with potent antimicrobial and antiadherence properties. Antimicrob. Agents Chemother. 1988, 32, 350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catalone, B.J.; Ferguson, M.L.; Miller, S.R.; Malamud, D.; Kish-Catalone, T.; Thakkar, N.J.; Krebs, F.C.; Howett, M.K.; Wigdahl, B.; Malamud, D. Prolonged exposure to the candidate microbicide C31G differentially reduces cellular sensitivity to agent re-exposure. Biomed. Pharmacother. Biomed. Pharmacother. 2005, 59, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Calis, S.; Yulug, N.; Sumnu, M.; Ayhan, A.; Hincal, A.A. A non-antibiotic antimicrobial mixture (C31G): Evaluation of the antimicrobial efficiency of C31G on vaginal cultures. Boll. Chim. Farm. 1992, 131, 335–338. [Google Scholar] [PubMed]
- Krebs, F.C.; Miller, S.R.; Malamud, D.; Howett, M.K.; Wigdahl, B. Inactivation of human immunodeficiency virus type 1 by nonoxynol-9, C31G, or an alkyl sulfate, sodium dodecyl sulfate. Antivir. Res. 1999, 43, 157–173. [Google Scholar] [CrossRef]
- Michaels, E.B.; Hahn, E.C.; Kenyon, A.J. Effect of C31G, an antimicrobial surfactant, on healing of incised guinea pig wounds. Am. J. Vet. Res. 1983, 44, 1378–1381. [Google Scholar] [PubMed]
- Thompson, K.A.; Malamud, D.; Storey, B.T. Assessment of the antimicrobial agent C31G as a spermicide: Comparison with nonoxynol-9. Contraception 1996, 53, 313–318. [Google Scholar] [CrossRef]
- González-Bello, C. Antibiotic adjuvants—A strategy to unlock bacterial resistance to antibiotics. Bioorg. Med. Chem. Lett. 2017, 27, 4221–4228. [Google Scholar] [CrossRef] [PubMed]
- Chakradhar, S. What’s old is new: Reconfiguring known antibiotics to fight drug resistance. Nat. Med. 2016, 22, 1197–1199. [Google Scholar] [CrossRef] [PubMed]
- Gill, E.E.; Franco, O.L.; Hancock, R.E.W. Antibiotic adjuvants: Diverse strategies for controlling drug-resistant pathogens. Chem. Biol. Drug Des. 2015, 85, 56–78. [Google Scholar] [CrossRef] [PubMed]
- Bernal, P.; Molina-Santiago, C.; Daddaoua, A.; Llamas, M.A. Antibiotic adjuvants: Identification and clinical use. Microb. Biotechnol. 2013, 6, 445–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalan, L.; Wright, G.D. Antibiotic adjuvants: Multicomponent anti-infective strategies. Expert Rev. Mol. Med. 2011, 13. [Google Scholar] [CrossRef] [PubMed]
- Farha, M.A.; Brown, E.D. Discovery of antibiotic adjuvants. Nat. Biotechnol. 2013, 31, 120–122. [Google Scholar] [CrossRef] [PubMed]
- Melander, R.J.; Melander, C. The Challenge of Overcoming Antibiotic Resistance: An Adjuvant Approach? ACS Infect. Dis. 2017, 3, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Celenza, G.; Segatore, B.; Setacci, D.; Bellio, P.; Brisdelli, F.; Piovano, M.; Garbarino, J.A.; Nicoletti, M.; Perilli, M.; Amicosante, G. In vitro antimicrobial activity of pannarin alone and in combination with antibiotics against methicillin-resistant Staphylococcus aureus clinical isolates. Phytomedicine 2012, 19, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Bellio, P.; Segatore, B.; Mancini, A.; Di Pietro, L.; Bottoni, C.; Sabatini, A.; Brisdelli, F.; Piovano, M.; Nicoletti, M.; Amicosante, G.; et al. Interaction between lichen secondary metabolites and antibiotics against clinical isolates methicillin-resistant Staphylococcus aureus strains. Phytomedicine 2015, 22, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Linciano, P.; Vicario, M.; Kekez, I.; Bellio, P.; Celenza, G.; Martín-Blecua, I.; Blázquez, J.; Cendron, L.; Tondi, D. Phenylboronic acids probing molecular recognition against class A and class C β-lactamases. Antibiotics 2019, 8, 171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
| Compounds (Carbon Chain Length) | MIC50 (µM) (Range) | MIC90 (µM) | CMC * (M) |
|---|---|---|---|
| LDAO (12) | 312.50 (39.06–625.00) | 625.00 | a 1.7 × 10−3 |
| TDAO (14) | 19.53 (19.53–39.06) | 39.06 | a 2.7 × 10−4 |
| C12NOX (12) | 156.25 (9.77–156.25) | 156.25 | b 1.4 × 10−5 |
| C14NOX (14) | 4.88 (1.22–19.53) | 9.77 | b 5.8 × 10−6 |
| C16NOX (16) | 2.44 (0.61–19.53) | 4.88 | b 1.5 × 10−6 |
| Compounds (Carbon Chain Length) | Median MIC (µM) (Range) |
|---|---|
| LDAO (12) | 312.50 |
| TDAO (14) | 39.06 |
| C12NOX (12) | 78.13 (78.13–156.25) |
| C14NOX (14) | 19.53 (9.77–19.53) |
| C16NOX (16) | 19.53 (9.77–19.53) |
| Effective Combination | |||||
|---|---|---|---|---|---|
| Compounds (Carbon Chain Length) | Antibiotic | FICImin | INT a | Antibiotic (µg/mL) | Detergents (µM) |
| LDAO (12) | clindamycin | 0.5 | SYN | 16 | 156.25 |
| erythromycin | 0.625 | IND | |||
| gentamicin | 1 | IND | |||
| oxacillin | 0.252 | SYN | 0.0625 | 78.13 | |
| TDAO (14) | clindamycin | 1 | IND | ||
| erythromycin | 1 | IND | |||
| gentamicin | 0.531 | IND | |||
| oxacillin | 0.313 | SYN | 2 | 9.77 | |
| C12NOX (12) | clindamycin | 1 | IND | ||
| erythromycin | 1 | IND | |||
| gentamicin | 1 | IND | |||
| oxacillin | 0.254 | SYN | 0.125 | 19.53 | |
| C14NOX (14) | clindamycin | 1 | IND | ||
| erythromycin | 1 | IND | |||
| gentamicin | 1 | IND | |||
| oxacillin | 0.5 | SYN | 2 | 4.88 | |
| C16NOX (16) | clindamycin | 1 | IND | ||
| erythromycin | 1 | IND | |||
| gentamicin | 1 | IND | |||
| oxacillin | 0.5 | SYN | 2 | 4.88 | |
| Compounds (Carbon Chain Length) | IC20 ± SEM a(µM) | IC50 ± SEM a (µM) | IC80 ± SEM a (µM) | Haemolysis * (µM) |
|---|---|---|---|---|
| LDAO (12) | 192.9 ± 8.6 | 220.1 ± 6.6 | 250.0 ± 11.1 | 625.0 |
| TDAO (14) | 100.3 ± 2.3 | 113.6 ± 1.6 | 128.7 ± 2.9 | 78.2 |
| C12NOX (12) | 154.3 ± 4.1 | 177.3 ± 4.7 | 203.6 ± 5.4 | 625.0 |
| C14NOX (14) | 12.5 ± 1.9 | 20.8 ± 1.4 | 34.7 ± 3.3 | 78.2 |
| C16NOX (16) | 18.3 ± 2.8 | 27.6 ± 2.3 | 41.7 ± 5.1 | 19.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fagnani, L.; Nazzicone, L.; Brisdelli, F.; Giansanti, L.; Battista, S.; Iorio, R.; Petricca, S.; Amicosante, G.; Perilli, M.; Celenza, G.; et al. Cyclic and Acyclic Amine Oxide Alkyl Derivatives as Potential Adjuvants in Antimicrobial Chemotherapy against Methicillin-Resistant Staphylococcus aureus with an MDR Profile. Antibiotics 2021, 10, 952. https://doi.org/10.3390/antibiotics10080952
Fagnani L, Nazzicone L, Brisdelli F, Giansanti L, Battista S, Iorio R, Petricca S, Amicosante G, Perilli M, Celenza G, et al. Cyclic and Acyclic Amine Oxide Alkyl Derivatives as Potential Adjuvants in Antimicrobial Chemotherapy against Methicillin-Resistant Staphylococcus aureus with an MDR Profile. Antibiotics. 2021; 10(8):952. https://doi.org/10.3390/antibiotics10080952
Chicago/Turabian StyleFagnani, Lorenza, Lisaurora Nazzicone, Fabrizia Brisdelli, Luisa Giansanti, Sara Battista, Roberto Iorio, Sabrina Petricca, Gianfranco Amicosante, Mariagrazia Perilli, Giuseppe Celenza, and et al. 2021. "Cyclic and Acyclic Amine Oxide Alkyl Derivatives as Potential Adjuvants in Antimicrobial Chemotherapy against Methicillin-Resistant Staphylococcus aureus with an MDR Profile" Antibiotics 10, no. 8: 952. https://doi.org/10.3390/antibiotics10080952
APA StyleFagnani, L., Nazzicone, L., Brisdelli, F., Giansanti, L., Battista, S., Iorio, R., Petricca, S., Amicosante, G., Perilli, M., Celenza, G., & Bellio, P. (2021). Cyclic and Acyclic Amine Oxide Alkyl Derivatives as Potential Adjuvants in Antimicrobial Chemotherapy against Methicillin-Resistant Staphylococcus aureus with an MDR Profile. Antibiotics, 10(8), 952. https://doi.org/10.3390/antibiotics10080952







