Identifying Mucormycosis Severity in Indian COVID-19 Patients: A Nano-Based Diagnosis and the Necessity for Critical Therapeutic Intervention
Abstract
:1. Introduction
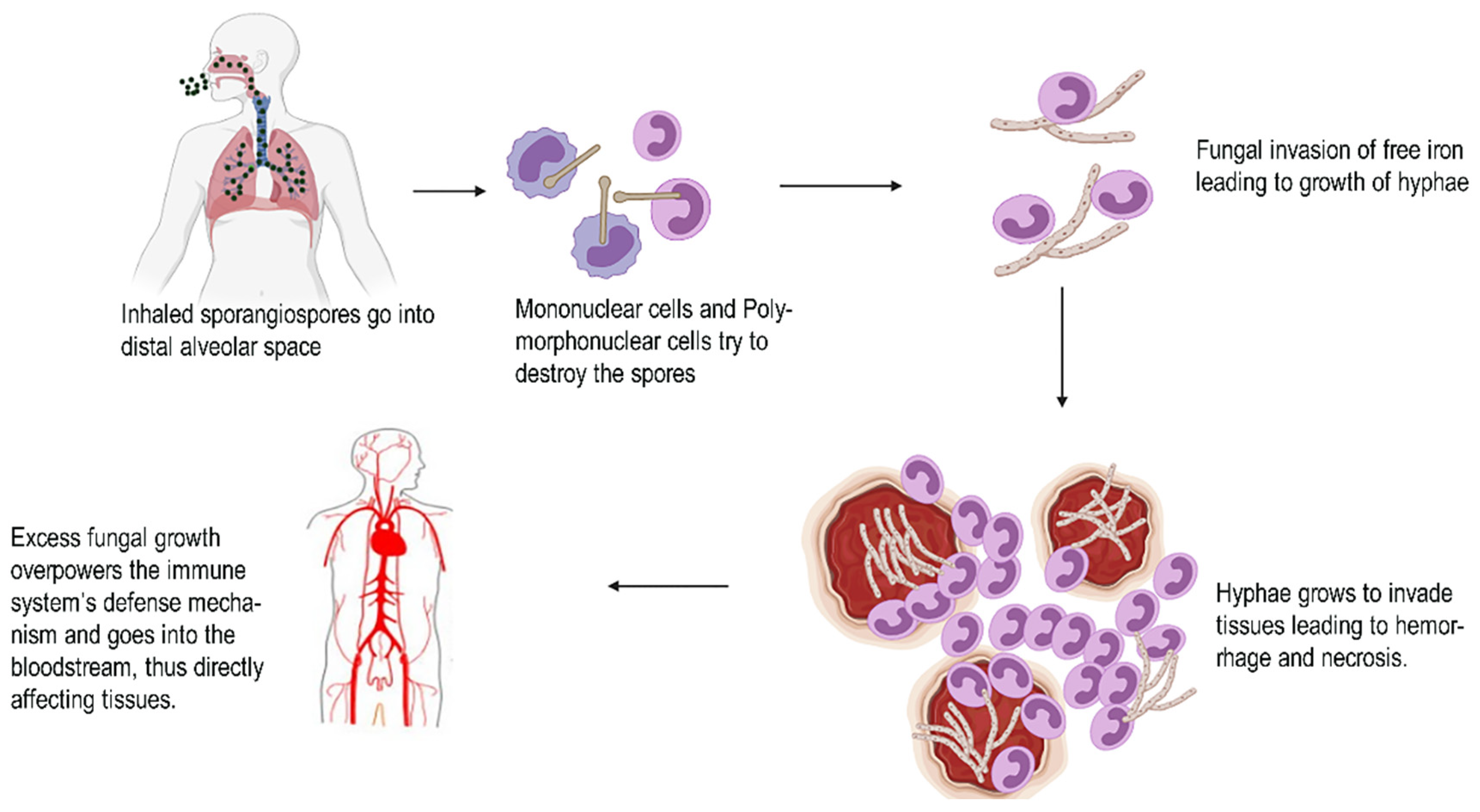
2. Clinical Presentations
- Headaches that are unbearable: When a patient inhales the fungal molds, which target the nasal canals and nerves, the fungal infection can be very dangerous. As a result, a person may have symptoms such as chronic discomfort and headaches.
- Sinusitis—nasal blockage or congestion, nasal discharge (blackish/bloody).
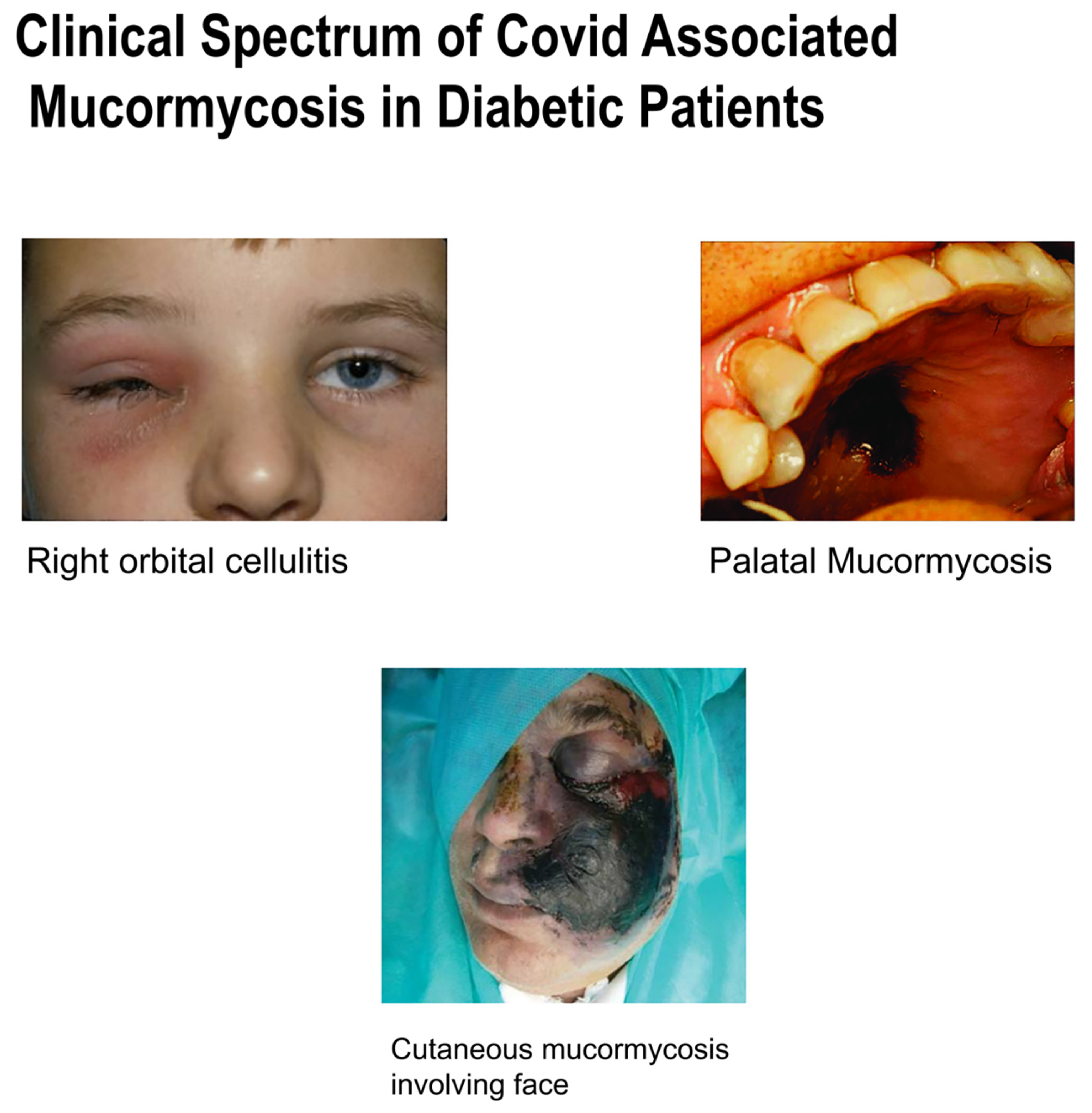
- Local pain on the cheekbone, one-sided facial pain, numbness or swelling; these might be critical indicators of infection right now. Aside from swelling, the fungal infection may impact skin health, causing numerous lesions and necrosis-like signs.
- Blackish discoloration over the bridge of the nose/palate. The infection’s most noticeable symptom is facial deformation. In the most severe cases, the infection can cause black patches to appear around the eyes and nose. In certain circumstances, random fungal infection development might result in a person losing teeth or jaws.
- Tooth decay, jaw involvement.
- Black spots in front of eyes and pain with blackish pupil.
- Experts warn that changes in the eyes or visual distortion might be indicators of the illness progressing. Vision might be affected as the black fungus develops and spreads. Some people may also get bloodshot eyes, have cloudy or impaired vision, or feel swelling in one eye.
- Thrombosis, necrosis, skin lesion [17].
- Doctors warn that important symptoms such as delirium, memory loss, neurological damage, and changed mental state might signal that a patient needs medical treatment.
- Pain in chest, pleural effusion, problem in respiratory symptoms.
- Black fungus is most evident among high-risk patients as per the clinical findings, people on continuous steroid use or immunomodulators and diabetic patients and those who are on prolonged ICU stay post-transplant/malignancy.
3. Pathological Evidences
4. Statistical Data of Mucormycosis Cases and Steps Taken
Mucormycosis Outbreaks
5. COVID-19 and Fungal Co-Infections: A Diagnostic Perspective
- Direct microscopy and culture are used to examine the etiology.
- Histopathology.
- Serology: Similarly, tracheal suction (TA) and bronchoalveolar lavage liquid (BALF) tests for culture and biomarker testing should be done in well-protected environments due to the risk of airborne dissemination and contamination of healthcare specialists. On the other hand, antigen and galactomannan (GM), and counteracting agent, BDG [38], discovery by serum are also necessary for suspected individuals.
- Pathogens can be identified using real-time polymerase chain reaction (PCR) techniques and, if applicable, molecular recognition. PCR methods and atomic acknowledgement on the off chance that appropriate [43] can be perceived.
5.1. Invasive Mucormycosis (IM)
5.2. Nanotechnology Based Mucormycosis Diagnosis
5.2.1. Approaches to Mucormycosis Diagnosis Using Nanotechnology
Fungal Detection Biosensors
Working Principle of Biosensors
- Bio-element;
- Transducer;
- Reference;
- Amplifier;
- Processor;
- Display.
Diagnostics Based on Nucleic Acids
Point-of-Care Tests (POCT)
Detection of Galactomannans
6. Anti-Fungal Therapy as Challenging Aid to COVID-19

| Therapy | Anti-Fungal Strategies | Pros and Cons | Mode of Action |
|---|---|---|---|
| Established therapies | Amphotericin B (AmB) deoxycholate | Toxicity | Depending on the concentration in body fluids and the susceptibility of the fungus, amphotericin B is either fungistatic or fungicidal. The medicine works by binding to sterols (ergosterol) in susceptible fungi’s cell membranes. This results in the formation of a transmembrane channel and a change in membrane permeability, which allows intracellular components to seep out. Amphotericin B and azoles act on ergosterol, the most abundant sterol in the fungus cytoplasmic membrane. Amphotericin B is a polyene that binds permanently to ergosterol, causing membrane rupture and cell death [74]. |
| Liposomal amphotericin B | Less toxic than AmB, most expensive polyene | Also acts by binding the sterols in fungal cell membrane. Binding cell membrane can cause alterations in cell permeability and also causes cell death [75]. | |
| Amphotericin B lipid complex | Less toxic than AmB | Acts by binding to sterols in fungal cell membrane. | |
| Investigational/adjunctive therapies | Itraconazole (Figure 7) | Superior toxicity profile | 14-demythalase, a cytochrome P-450 enzyme required for the conversion of lanosterol to ergosterol, interacts with itraconazole. Because ergosterol is a necessary component of the fungal cell membrane, inhibiting its production causes increased cellular permeability, resulting in cellular contents leakage. Itraconazole can also decrease endogenous respiration, interact with membrane phospholipids, prevent yeasts from transforming into mycelial forms, prevent purine uptake, and affect triglycerides and phospholipid production [76]. |
| Posaconazole | More effective than itraconazole in animal models | Posaconazole is a triazole antifungal drug that works by blocking the cytochrome P-450 dependent enzymes sterols 14 demythalase in fungi by attaching to the heme co factor present on the enzymes [77]. This causes the synthesis of ergosterol, a critical component of the fungal cell membranes to be inhibited, as well as the accumulation of methylated sterol precursors. As a result, the fungal cell development is inhibited and eventually cell death occurs. | |
| Caspofungin | Very low toxicity, virtually no clinical data for mucormycosis | Caspofungin inhibits the synthesis of beta (1, 3) D-glucan, a key component of the cell wall of Aspergillus species and Candida species. In mammalian cells, beta (1-3)-D glucan is not found. The main target is beta (1,3)-glucan synthase [68]. | |
| Iron chelation | Synergistic with ABLC in murine | Induces iron starvation and thus promotes a fungicidal effect. | |
| Hyperbaric oxygen | Benefit in combination with anti-fungals | Suppresses fungal growth in vitro, reduces tissue hypoxia and acidosis caused due to fungal invasion. | |
| Cytokine therapy | Nontoxic, Successful case reports | Cytokines play a key role in signaling molecules that modulate and control immunity, inflammation, and hematopoiesis. Cytokines are a diverse set of proteins, peptides, and glycoproteins released by the immune system of cells [78]. |

Surgical Intervention and Management with Mucormycosis
7. The Interplay between Black Fungus and SARS-COV-2
7.1. Underlying Conditions for Black Fungus in COVID-19
7.1.1. Diabetes and Mucormycosis: A Complex Connection
7.1.2. Problems Affecting the Gastrointestinal Tract during Mucormycosis Infection
7.1.3. Pulmonary Diseases and Mucormycosis: A Complex Connection
7.1.4. Hypertension and Mucormycosis: A Complex Connection
8. Prospects and Recommendations
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wuhan City Health Committee. Wuhan Municipal Health and Health Commission’s Briefing on the Current Pneumonia Epidemic Situation in Our City 2019. Available online: http://wjw.wuhan.gov.cn/front/web/showDetail/2019123108989 (accessed on 14 January 2020).
- Frazier, K.M.; Hooper, J.E.; Mostafa, H.H.; Stewart, C.M. SARS-CoV-2 virus isolated from the mastoid and middle ear: Implications for COVID-19 precautions during ear surgery: Implications for COVID-19 precautions during ear surgery. JAMA Otolaryngol. Head Neck Surg. 2020, 146, 964–966. [Google Scholar] [CrossRef]
- Ferguson, B.J. Definitions of fungal rhinosinusitis. Otolaryngol. Clin. N. Am. 2000, 33, 227–235. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Denning, D.W.; Ferguson, B.J.; Ponikau, J.; Buzina, W.; Kita, H.; Marple, B.; Panda, N.; Vlaminck, S.; Kauffmann-Lacroix, C.; et al. Fungal rhinosinusitis: A categorization and definitional schema addressing current controversies: A categorization and definitional schema addressing current controversies. Laryngoscope 2009, 119, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Mohindra, S.; Gupta, R.; Bakshi, J.; Gupta, S.K. Rhinocerebral mucormycosis: The disease spectrum in 27 patients. Mycoses 2007, 50, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Munir, N.; Jones, N.S. Rhinocerebral mucormycosis with orbital and intracranial extension: A case report and review of optimum management. J. Laryngol. Otol. 2007, 121, 192–195. [Google Scholar] [CrossRef] [PubMed]
- DeShazo, R.D.; Chapin, K.; Swain, R.E. Fungal sinusitis. N. Engl. J. Med. 1997, 337, 254–259. [Google Scholar] [CrossRef]
- Gillespie, M.B.; O’Malley, B.W. An algorithmic approach to the diagnosis and management of invasive fungal rhinosinusitis in the immunocompromised patient. Otolaryngol. Clin. N. Am. 2000, 33, 323–334. [Google Scholar] [CrossRef]
- González Ballester, D.; González-García, R.; Moreno García, C.; Ruiz-Laza, L.; Monje Gil, F. Mucormycosis of the head and neck: Report of five cases with different presentations. J. Craniomaxillofac. Surg. 2012, 40, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in wuhan, china: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Song, G.; Liang, G.; Liu, W. Fungal co-infections associated with global covid-19 pandemic: A clinical and diagnostic perspective from china. Mycopathologia 2020, 185, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with sars-cov-2 pneumonia in wuhan, china: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef] [Green Version]
- Gangneux, J.P.; Bougnoux, M.E.; Dannaoui, E.; Cornet, M.; Zahar, J.R. Invasive fungal diseases during covid-19: We should be prepared. J. Mycol. Med. 2020, 30, 100971. [Google Scholar] [CrossRef] [PubMed]
- ‘Black Fungus’ Disease Linked to Covid Spreads across India. The Guardian. Available online: https://www.theguardian.com/world/2021/may/21/mucormycosis-black-fungus-disease-linked-covid-spreads-india (accessed on 26 May 2021).
- India Sees 259,551 New Covid Cases, ‘Black Fungus’ Adds to Woes. Aljazeera. Available online: https://www.google.com/amp/s/www.aljazeera.com/amp/news/2021/5/21/india-sees-259551-new-covid-cases-black-fungus-adds-to-woes (accessed on 26 May 2021).
- Mucormycosis: Can Black Fungus Infect People Who Don’t Have Covid-19? Here’s What Experts Say. Money Control News. Available online: https://www.moneycontrol.com/news/india/mucormycosis-can-black-fungus-infect-people-without-covid-19-heres-what-experts-say-6929921.html (accessed on 26 May 2021).
- COVID-19 and Black Fungus: What Is Mucormycosis? Health, the Sciences. Available online: https://science.thewire.in/the-sciences/covid-19-and-black-fungus-what-is-mucormycosis/ (accessed on 17 April 2021).
- Mucormycosis in COVID-19 Patients: Everything You Should Know. Hetero Health Care. Available online: https://www.heterohealthcare.com/blog/what-is-the-fungal-infection-mucormycosis-affecting-covid-patients-in-india (accessed on 18 May 2021).
- Mucormycosis/Black Fungus: Why Are COVID-19 Patients at Risk? Available online: https://www.netmeds.com/health-library/post/mucormycosis-black-fungus-why-are-covid-19-patients-at-risk-here-are-icmr-guidelines-for-prevention (accessed on 21 May 2021).
- Mucormycosis. CDC. Available online: https://www.cdc.gov/fungal/diseases/mucormycosis/index.html (accessed on 21 May 2021).
- Mucormycosis; the Black Fungus Hitting Covid Patients. BBC. Available online: https://www.bbc.com/future/article/20210519-mucormycosis-the-black-fungus-hitting-indias-covid-patients (accessed on 25 February 2021).
- Black fungus or Mucormycosis: Costly Mistakes in COVID-19 Treatment Lead to New Challenges. First Point. Available online: https://www.firstpost.com/india/black-fungus-or-mucormycosis-costly-mistakes-in-covid-19-treatment-lead-to-new-challenges-9637481.html (accessed on 20 May 2021).
- Mucormycosis: An Emerging Challenge in COVID-19 Management. Available online: https://health.economictimes.indiatimes.com/news/industry/mucormycosis-an-emerging-challenge-in-covid-19-management/82507750 (accessed on 10 May 2021).
- Katragkou, A.; Walsh, T.J.; Roilides, E. Why is mucormycosis more difficult to cure than more common mycoses? Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2014, 20, 74–81. [Google Scholar] [CrossRef] [Green Version]
- Mehta, S.; Pandey, A. Rhino-orbital mucormycosis associated with COVID-19. Cureus 2020, 12, 10726. [Google Scholar] [CrossRef] [PubMed]
- Prakash, H.; Chakrabarti, A. Global epidemiology of mucormycosis. J. Fungi 2019, 5, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prakash, H.; Ghosh, A.K.; Rudramurthy, S.M.; Singh, P.; Xess, I.; Savio, J.; Pamidimukkala, U.; Jillwin, J.; Varma, S.; Das, A.; et al. A prospective multicenter study on mucormycosis in india: Epidemiology, diagnosis, and treatment. Med. Mycol. 2019, 57, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Kontoyiannis, D.P.; Yang, H.; Song, J.; Kelkar, S.S.; Yang, X.; Azie, N.; Harrington, R.; Fan, A.; Lee, E.; Spalding, J.R. Prevalence, clinical and economic burden of mucormycosis-related hospitalizations in the united states: A retrospective study. BMC Infect. Dis. 2016, 16, 730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guinea, J.; Escribano, P.; Vena, A.; Muñoz, P.; Martínez-Jiménez, M.D.C.; Padilla, B.; Bouza, E. Increasing incidence of mucormycosis in a large spanish hospital from 2007 to 2015: Epidemiology and microbiological characterization of the isolates. PLoS ONE 2017, 12, e0179136. [Google Scholar] [CrossRef] [PubMed]
- Ambrosioni, J.; Bouchuiguir-Wafa, K.; Garbino, J. Emerging invasive zygomycosis in a tertiary care center: Epidemiology and associated risk factors. Int. J. Infect. Dis. 2010, 14, e100-3. [Google Scholar] [CrossRef] [Green Version]
- Muthu, V.; Rudramurthy, S.M.; Chakrabarti, A.; Agarwal, R. Epidemiology and pathophysiology of covid-19-associated mucormycosis: India versus the rest of the world. Mycopathologia 2021, 1–16. [Google Scholar] [CrossRef]
- 760 Black Fungus Patients Still Being Treated in Delhi Hospitals. The Hindustan Times. Available online: https://www.hindustantimes.com/cities/delhi-news/760-black-fungus-patients-still-being-treated-in-delhi-hospitals-101625248850429.html (accessed on 2 July 2021).
- PTI. Over 3000 Affected by Black Fungus, 122 Fatalities in TN: Minister. The Indian Express. Available online: https://indianexpress.com/article/cities/chennai/over-3000-affected-black-fungus-122-fatalities-tn-minister-7392597/lite/ (accessed on 7 July 2021).
- Black Fungus: ICMR Releases Guidelines: Dos and Don’ts. India TV. Available online: https://www.indiatvnews.com/fyi/mucormycosis-epidemic-icmr-releases-guidelines-dos-and-donts-for-black-fungus-infection-symptoms-706050 (accessed on 8 July 2021).
- ICMR Releases Diagnosis and Management Guidelines for COVID-19 Associated Mucormycosis. Firstpost. Available online: https://www.google.com/amp/s/www.firstpost.com/india/icmr-releases-diagnosis-and-management-guidelines-for-covid-19-associated-mucormycosis-9628341.html/amp (accessed on 8 July 2021).
- Mohammadi, R.; Nazeri, M.; Sayedayn, S.M.; Ehteram, H. A successful treatment of rhinocerebralmucormycosis due to Rhizopusoryzae. J. Res. Med. Sci. 2014, 19, 72–74. [Google Scholar] [PubMed]
- Spellberg, B.; Edwards, J.; Ibrahim, A. Novel perspectives on mucormycosis: Pathophysiology, presentation, and management. Clin. Microbiol. Rev. 2005, 18, 556–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roden, M.M.; Zaoutis, T.E.; Buchanan, W.L.; Knudsen, T.A.; Sarkisova, T.A.; Schaufele, R.L.; Walsh, T.J. Epidemiology and outcome of zygomycosis: A review of 929 reported cases. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2005, 41, 634–653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mucormycosis: The ‘Black Fungus’ Maiming Covid Patients in India. BBC. Available online: https://www.bbc.com/news/world-asia-india-57027829 (accessed on 9 May 2021).
- India Today Web Desk. Andhra Pradesh Sees Rise in Black Fungus Cases, Active Case Tally at 677. Available online: https://www.indiatoday.in/india/story/andhra-pradesh-sees-rise-in-black-fungus-cases-1838920-2021-08-10 (accessed on 12 October 2021).
- Guegan, H.; Iriart, X.; Bougnoux, M.E.; Berry, A.; Robert-Gangneux, F.; Gangneux, J.P. Evaluation of MucorGenius® mucorales PCR assay for the diagnosis of pulmonary mucormycosis. J. Infect. 2020, 81, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Sen, M.; Lahane, S.; Lahane, T.P.; Parekh, R.; Honavar, S.G. Mucor in a viral land: A tale of two pathogens. Indian J. Ophthalmol. 2021, 69, 244–252. [Google Scholar] [PubMed]
- Prattes, J.; Valentine, T.; Hoengil, M.; Talakic, E.; Reisinger, A.; Philipp, E. Invasive pulmonary aspergillosis complicating COVID-19 in the ICU—A case report. Med. Mycol. Case Rep. 2021, 11, 2–5. [Google Scholar] [CrossRef]
- Hage, C.A.; Carmona, E.M.; Epelbaum, O.; Evans, S.E.; Gabe, L.M.; Haydour, Q.; Limper, A.H. Microbiological laboratory testing in the diagnosis of fungal infections in pulmonary and critical care practice. An official American thoracic society clinical practice guideline. Am. J. Respir. Crit. Care Med. 2009, 200, 535–550. [Google Scholar] [CrossRef]
- Cornely, O.A.; Alastruey-Izquierdo, A.; Arenz, D.; Chen, S.C.A.; Dannaoui, E.; Hochhegger, B.; Chakrabarti, A. Global guideline for the diagnosis and management of mucormycosis: An initiative of the European confederation of medical mycology in cooperation with the mycoses study group education and research consortium. Lancet Infect. Dis. 2019, 19, e405–e421. [Google Scholar] [CrossRef]
- Skiada, A.; Lass-Floerl, C.; Klimko, N.; Ibrahim, A.; Roilides, E.; Petrikkos, G. Challenges in the diagnosis and treatment of mucormycosis. Med Mycol. 2018, 56, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Bellanger, A.P.; Berceanu, A.; Rocchi, S.; Valot, B.; Fontan, J.; Chauchet, A.; Millon, L. Development of a quantitative PCR detecting Cunninghamella bertholletiae to help in diagnosing this rare and aggressive mucormycosis. Bone Marrow Transplant. 2018, 53, 1180–1183. [Google Scholar] [CrossRef]
- Brunet, K.; Rammaert, B. Mucormycosis treatment: Recommendations, latest advances, and perspectives. J. Mycol. Méd. 2020, 30, 101007. [Google Scholar] [CrossRef] [PubMed]
- Pakdel, F.; Ahmadikia, K.; Salehi, M.; Tabari, A.; Jafari, R.; Mehrparvar, G.; Khodavaisy, S. Mucormycosis in patients with COVID-19: A cross-sectional descriptive multicentre study from Iran. Mycoses 2020, 64, 1238–1252. [Google Scholar] [CrossRef] [PubMed]
- Santana, O.I.; Silva, J.A.G.; Andrade, C.A.S.; Lima, O.M.D. Biosensors for early detection of fungi spoilage and toxigenic and mycotoxins in food. Curr. Opin. Food Sci. 2019, 29, 64–79. [Google Scholar] [CrossRef]
- Hussain, K.; Malavia, D.; Johnson, E.M.; Littlechild, J.; Winlove, C.P.; Vollmer, F.; Gow, N.A.R. Biosensors and diagnostics for fungal detection. J. Fungi. 2020, 6, 349. [Google Scholar] [CrossRef] [PubMed]
- Biosensor Technology: Advantages and Applications. Available online: https://www.azosensors.com/article.aspx?ArticleID=402 (accessed on 11 October 2021).
- Morales-Del Ángel, J.A.; Morales-Chávez, O.D.; Méndez-Sáenz, M.A.; Montemayor-Alatorre, A.; Jasso-Ramírez, N.G.; Treviño-González, J.L. Clinical Implications of facial edema in chronic mucormycosis: A report of 5 cases. Arch. Clin. Med. Case Rep. 2020, 4, 836–844. [Google Scholar]
- Hoffmann, C.; Guillerm, G.; Le Pape, P.; Carausu, L.; Lavergne, R.A.; Nevez, G.; Le Gal, S. Mucorales DNA detection in serum specimens for early diagnosis of mucormycosis. Diagn. Microbiol. Infect. Dis. 2020, 97, 115004. [Google Scholar] [CrossRef]
- Liu, M.; Bruni, G.O.; Taylor, C.M.; Zhang, Z.; Wang, P. Comparative genome-wide analysis of extracellular small RNAs from the mucormycosis pathogen Rhizopus delemar. Sci. Rep. 2018, 8, 5243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Haidar, G.; Singh, N. How we approach combination antifungal therapy for invasive aspergillosis and mucormycosis in transplant recipients. Transplantation 2018, 102, 1815–1823. [Google Scholar] [CrossRef]
- Khan, Z.; Ahmad, S. Diagnostic Algorithm for Invasive Fungal Infections: In Clinical Practice of Medical Mycology in Asia, 1st ed.; Springer: Singapore, 2020; pp. 179–197. [Google Scholar]
- Jafari, M.; Abolmaali, S.S.; Tamaddon, A.M.; Zomorodian, K.; Shahriarirad, B. Nanotechnology approaches for delivery and targeting of Amphotericin B in fungal and parasitic diseases. Nanomedicine 2021, 16, 857–877. [Google Scholar] [CrossRef]
- Zhou, W.; Li, H.; Zhang, Y.; Huang, M.; He, Q.; Li, P.; Zhang, F.; Shi, Y.; Su, X. Diagnostic value of galactomannan antigen test in serum and bronchoalveolar lavage fluid samples from patients with nonneutropenic invasive pulmonary aspergillosis. J. Clin. Microbiol. 2017, 55, 2153–2161. [Google Scholar] [CrossRef] [Green Version]
- Baby, B.; Devan, A.R.; Nair, B.; Nath, L.R. The impetus of COVID-19 in multiple organ affliction apart from respiratory infection: Pathogenesis, diagnostic measures and current treatment strategy. Infect. Disord. Drug Targets 2020, 21, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Gleissner, B.; Schilling, A.; Anagnostopolous, I.; Siehl, I.; Thiel, E. Improved outcome of zygomycosis in patients with hematological diseases? Leuk. Lymphoma 2004, 45, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Timpl, R.; Rohde, H.; Robey, P.G.; Rennard, S.I.; Foidart, J.M.; Martin, G.R. Laminin—A glycoprotein from basement membranes. J. Biol. Chem. 1979, 254, 9933–9937. [Google Scholar] [CrossRef]
- Bouchara, J.P.; Oumeziane, N.A.; Lissitzky, J.C.; Larcher, G.; Tronchin, G.; Chabasse, D. Attachment of spores of the human pathogenic fungus Rhizopus oryzae to extracellular matrix components. Eur. J. Cell Biol. 1996, 70, 76–83. [Google Scholar] [PubMed]
- Liu, M.; Spellberg, B.; Phan, Q.T.; Fu, Y.; Lee, A.S.; Edwards, J.E. The endothelial cell receptor GRP78 is required for mucormycosis pathogenesis in diabetic mice. J. Clin. Investig. 2010, 120, 1914–1924. [Google Scholar] [CrossRef] [Green Version]
- Dadwal, S.S.; Kontoyiannis, D.P. Recent advances in the molecular diagnosis of mucormycosis. Expert Rev. Mol. Diagn. 2018, 18, 845–854. [Google Scholar] [CrossRef]
- Herbrecht, R.; Denning, T.F.; Patterson, J.E.; Bennett, R.E.; Greene, J.W.; Oestmann, W.; Kern, V. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 2002, 347, 408–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, E.A.; Fung, H.B.; Kirschenbaum, H.L. Caspofungin: An echinocandin antifungal agent. Clin. Ther. 2002, 24, 351–377. [Google Scholar] [CrossRef]
- Silva, L.N.; de Mello, T.P.; de Souza Ramos, L. Current challenges and updates on the therapy of fungal infections. Curr. Top. Med. Chem. 2019, 19, 495–499. [Google Scholar] [CrossRef]
- Chowdhary, A.; Sharma, C.; Meis, J.F. Azole resistant aspergillosis: Epidemiology, molecular mechanisms and treatment. J. Infect. Dis. 2017, 216, S436–S444. [Google Scholar] [CrossRef] [Green Version]
- Perlin, D.S. Echinocandin resistance in Candida. Clin. Intect. Dis. 2015, 61, 612–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, S.; Sun, W.; Li, M.; Dong, L.A. Complex COVID 19 case with rheumatoid arthritis treated with toclizumab. Clin. Rheumatol. 2020, 19, 2797–2802. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, J.P.; Mishra, A.P.; Pradhan, P.; Samal, K.C. Misfortune never comes alone—The new “black fungus” accompanying COVID-19 wave. Biot. Res. Today 2021, 3, 318–320. [Google Scholar]
- Sugar, A.M. Use of amphotericin B with azole antifungal drugs: What are we doing? Antimicrob. Agents Chemother. 1995, 39, 1907–1912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adler-Moore, J.; Richard, T.P. AmBisome: Liposomal formulation, structure, mechanism of action and pre-clinical experience. J. Antimicrob. Chemother. 2002, 49, 21–30. [Google Scholar] [CrossRef]
- De Beule, K.; Van Gestel, J. Pharmacology of itraconazole. Drugs 2001, 61, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Hof, H. A new, broad-spectrum azole antifungal: Posaconazole--mechanisms of action and resistance, spectrum of activity. Mycoses 2006, 49, 2–6. [Google Scholar] [CrossRef]
- Brattsand, R.; Linden, M. Cytokine modulation by glucocorticoids: Mechanisms and actions in cellular studies. Aliment. Pharm. Ther. 1996, 10, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B.; Walsh, T.J.; Kontoyiannis, D.P. Recent advances in the management of mucormycosis: From bench to bedside. Clin. Infect. Dis. 2009, 1743–1751. [Google Scholar] [CrossRef]
- Lin, E.; Moua, T.; Limper, A.H. Pulmonary mucormycosis: Clinical features and outcomes. Infection 2017, 45, 443–448. [Google Scholar] [CrossRef]
- Lalayanni, C.; Baliakas, P.; Xochelli, A.; Apostolou, C.; Arabatzis, M.; Velegraki, A. Outbreak of cutaneous zygomycosis associated with the use of adhesive tape in haematology patients. J. Hosp. Infect. 2012, 81, 213–215. [Google Scholar] [CrossRef] [PubMed]
- Szalai, G.; Fellegi, V.; Szabó, Z.; Vitéz, L.C. Mucormycosis mimics sinusitis in a diabetic adult. Ann. N. Y. Acad. Sci. 2006, 10, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Losee, J.E.; Selber, J.; Vega, S.; Hall, C.; Scott, G.; Serletti, J.M. Primary cutaneous mucormycosis: Guide to surgical management. Ann. Plast. Surg. 2002, 49, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Chew, H.H.; Abuzeid, A.; Singh, D. Surgical wound mucormycosis necessitating hand amputation: A case report. J. Orthop. Surg. 2008, 16, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Ingram, P.R.; Suthananthan, A.E.; Rajan, R.; Sieunarine, K.; Gardam, D.J. Cutaneous mucormycosis and motor vehicle accidents: Findings from an Australian case series. Med. Mycol. 2014, 52, 819–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.Y.; Singh, N. Mucormycosis: Its contemporary face and management strategies. Lancet Infect. Dis. 2011, 11, 301–311. [Google Scholar] [CrossRef]
- Polo, J.R.; Luño, J.; Menarguez, C.; Gallego, E.; Robles, R. Peritoneal mucormycosis in a patient receiving continuous ambulatory peritoneal dialysis. Am. J. Kidney Dis. 2009, 13, 237–239. [Google Scholar] [CrossRef]
- Mucormycosis (Zygomycosis) Treatment & Management. Available online: https://emedicine.medscape.com/article/222551-treatment (accessed on 15 October 2021).
- Torres-Narbona, M.; Guinea, A.; Muñoz, P.; Bouza, E. Zigomicetos y zigomicosis en la era de las nuevas terapias antifúngicas [Zygomycetes and zygomycosis in the new era of antifungal therapies]. Rev. Esp. Quim. 2007, 20, 375–386. [Google Scholar]
- Goel, S.; Palaskar, S.; Shetty, V.P.; Bhushan, A. Rhinomaxillary mucormycosis with cerebral extension. J. Oral Maxillofac. Pathol. 2009, 13, 14–17. [Google Scholar]
- Ali, T.; Kaitha, S.; Mohmood, S.; Ftesi, A.; Stone, J.; Bronze, M.S. Clinical use of anti TNF therapy and increased risk of infection. Drug. Health Patient Saf. 2013, 5, 79–99. [Google Scholar] [CrossRef] [Green Version]
- Hadi, H.A.; Suwaidi, J.A. Endothelial dysfunction in diabetes mellitus. Vasc. Health Risk. Manag. 2020, 3, 853–876. [Google Scholar]
- Ibrahim, A.S.; Spellberg, B.; Walsh, T.J.; Kontoyiannis, D.P. Pathogenesis of mucormycosis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021, 54, S16–S22. [Google Scholar] [CrossRef]
- Werthman-Ehrenreich, A. Mucormycosis with orbital compartment syndrome in a patient with COVID-19. Am. J. Emerg. Med. 2021, 42, 264.e5–264.e8. [Google Scholar] [CrossRef]
- Ravani, S.A.; Agrawal, G.A.; Leuva, P.A.; Modi, P.H.; Amin, K.D. Rise of the phoenix: Mucormycosis in COVID-19 times. Indian J. Ophthalmol. 2021, 69, 1563–1568. [Google Scholar] [PubMed]
- Patel, A.; Agarwal, R.; Rudramurthy, S.M.; Shevkani, M.; Xess, I.; Sharma, R. Multicenter epidemiologic study of Coronavirus disease-associated mucormycosis, India. Emerg. Infect. Dis. 2021, 27, 2349–2359. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Kontoyiannis, D.P. Update on mucormycosis pathogenesis. Curr. Opin. Infect. Dis. 2013, 26, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, A.; Ahmed, M.S.; Rahman, M.A.; Islam, S. Mucormycosis or black fungus: An emerging threat in COVID-19. Bangabandhu Sheikh Mujib Med. Univ. J. 2021, 25, 51–56. [Google Scholar] [CrossRef]
- Daria, S.; Asaduzzaman, M.; Shahriar, M.; Islam, M.R. The massive attack of COVID-19 in India is a big concern for Bangladesh: The key focus should be given on the interconnection between the countries. Int. J. Health Plan. Manag. 2021, 36, 1947–1949. [Google Scholar] [CrossRef]
- Ezeokoli, O.T.; Gcilitshana, O.; Pohl, C.H. Risk factors for fungal co-infections in critically ill COVID-19 patients, with a focus on immunosuppressants. J. Fungi. 2021, 7, 545. [Google Scholar] [CrossRef]
- Daria, S.; Islam, M.R. The use of cow dung and urine to cure COVID-19 in India: A public health concern. Int. J. Health Plan. Manag. 2021. 36, 1950–1952.
- Sankar, S.; Gokhale, T.; Choudhury, S.S.; Deb, A.K. COVID 19 and orbital mucormycosis. Indian J. Ophthalmol. 2021, 69, 1002–1004. [Google Scholar]
- Berenner, E.J.; Ungaro, R.C.; Gearry, R.B.; Zhang, X.; Colombel, J.F.; Kappelman, M.D. Corticosteroids, but not TNF antagonists, are associated with adverse COVID 19 outcomes in patient with inflammatory bowel diseases. Gastroenterology 2020, 159, 481–491. [Google Scholar] [CrossRef]
- Artis, W.M.; Fountain, J.A.; Delcher, H.K.; Jones, H.E. A mechanism in susceptibility to mucormycosids in diabetic ketoacidosis: Transferrin and iron availability. Diabetes 1982, 31, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Pagano, L.; Ricci, P.; Tonso, A.; Nosari, A.M.; Cudillo, L.; Montillo, M.; Cenacchi, A. Mucormycosis in patients with haematological malignancies: A retrospective clinical study of 37 cases. Br. J. Haematol. 1999, 2, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.R.; Sharma, B. Opportunistic fungal infections post-COVID: How threatened are we? Homœopath. Links 2021, 34, 91–92. [Google Scholar]
- Mahalaxmi, I.; Jayaramayya, K.; Venkatesan, D.; Subramaniam, M.D.; Renu, K.; Vijayakumar, P.; Vellingiri, B. Mucormycosis: An opportunistic pathogen during COVID-19. Environ. Res. 2021, 725, 111643. [Google Scholar] [CrossRef]
- Forouzesh, M.; Farshid, S.; Ghiasi, N.; Narimani, M.; Valizadeh, R.; Sadighpour, T.; Alimohammadi, S. Mucormycosis (black fungus/zygomycosis) and COVID-19; Does the coexistence of these two increase mortality? Immunopathol. Persa 2021, 7, e0x. [Google Scholar]
- Gandra, S.; Ram, S.; Levitz, S.M. The “black fungus” in India: The emerging syndemic of COVID-19—Associated mucormycosis. Antibiotics 2021, 10, 1079. [Google Scholar]
- Garg, E.; PulinSaluja, A.D.; Khurana, C.; Arora, M.; Rai, R. Mucormycosis: The black fungus–An insidious killer. Ann. Rom. Soc. Cell Biol. 2021, 25, 12978–12992. [Google Scholar]
- Shevade, D.S. Mucormycosis: Black fungus, a deadly post-COVID infection. Microbiology 2021, 2, 1. [Google Scholar]
- Ibrahim, A.S. Host cell invasion in mucormycosis: Role of iron. Curr. Opin. Microbiol. 2011, 14, 406–411. [Google Scholar] [CrossRef] [Green Version]
- Challa, S.; Uppin, S.G.; Hanumanthu, S.; Panigrahi, M.K.; Purohit, A.K.; Sattaluri, S. Fungal rhinosinusitis: A clinicopathological study from South India. Eur. Arch. Otorhinolaryngol. 2010, 267, 1239. [Google Scholar] [CrossRef]
- Sundaram, C.; Mahadevan, A.; Laxmi, V.; Yasha, T.C.; Santosh, V.; Murthy, J.M. Cerebral zygomycosis. Mycoses 2005, 48, 396–407. [Google Scholar] [CrossRef]
- Neblett, F.R.; Benedict, K.; Bos, J.; Bennett, S.D.; Lo, Y.C.; Adebanjo, T. Necrotizing cutaneous mucormycosis after a tornado in Joplin, Missouri, in 2011. N. Engl. J. Med. 2012, 367, 2214–2225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andresen, D.; Donaldson, A.; Choo, L.; Knox, A.; Klaassen, M.; Ursic, C. Multifocal cutaneous mucormycosis complicating polymicrobial wound infections in a tsunami survivor from Sri Lanka. Lancet 2005, 365, 876–878. [Google Scholar] [CrossRef]
- Alqarihi, A.; Gebremariam, T.; Gu, Y.; Swidergall, M.; Alkhazraji, S.; Soliman, S.S.M.; Bruno, V.M.; Edwards, J.E.; Filler, S.G.; Uppuluri, P. GRP78 and integrins play different roles in host cell invasion during Mucormycosis. Mbio 2020, 11, e01087-20. [Google Scholar] [CrossRef] [PubMed]
- Gebremariam, T.; Liu, M.; Luo, G.; Bruno, V.; Phan, Q.T.; Waring, A.J.; Edwards, J.E.; Filler, S.G.; Yeaman, M.R.; Ibrahim, A.S. CotH3 mediates fungal invasion of host cells during mucormycosis. J. Clin. Investig. 2014, 124, 237–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marx, R.E.; Stern, D. Inflammatory, Reactive and Infectious Diseases. In Oral and Maxillofacial Pathology, 2nd ed.; Marx, R.E., Stern, D., Eds.; Quintessence Publishing: Batavia, NY, USA, 2009; Volume 1, p. 1002. [Google Scholar]
- CAsqueiro, J.; Casqueiro, J.; Alves, C. Infections in Patients with Diabetes Mellitus: A Review of Pathogenesis. Indian J. Endocrinol. Metab. 2012, 16 (Suppl. 1), S27–S36. [Google Scholar] [PubMed]
- Ibrahim, A.S.; Edwards, J.E.J.; Filler, S.G. Zygomycosis. In Clinical Mycology; Dismukes, W.E., Pappas, P.G., Sobel, J.D., Eds.; Oxford University Press: New York, NY, USA, 2003; pp. 241–251. [Google Scholar]
- Eucker, J.; Sezer, O.; Graf, B.; Possinger, K. Mucormycoses. Mycoses 2001, 44, 253–260. [Google Scholar] [CrossRef]
- Tribble, D.R.; Rodriguez, C.J. Combat-related invasive fungal wound infections. Curr. Fungal Infect. Rep. 2014, 8, 277–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, A.G.; Adulkar, N.G.; D’Cunha, L.; Rao, P.R.; Bradoo, R.A.; Bapaye, M.M.; Kothari, A.; Dave, T.V.; Shinde, C.A. Rhino-orbital Mucor mycosis following COVID-19 in previously non-diabetic, immunocompetent patients. Orbit 2021, 40, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Kontoyiannis, D.P.; Lewis, R.E. Agents of mucormycosis and entomophthoramycosis. Mandell Douglas Bennett’s Princ. Pract. Infect. Dis. 2015, 2, 2909–2919. [Google Scholar]
- Jung, J.; Kim, M.Y.; Lee, H.J.; Park, Y.S.; Lee, S.O.; Choi, S.H.; Kim, S.H. Comparison of computed tomographic findings in pulmonary mucormycosis and invasive pulmonary aspergillosis. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2015, 21, 684.e11–684.e18. [Google Scholar] [CrossRef] [Green Version]
- Legrand, M.; Gits-Muselli, M.; Boutin, L.; Garcia-Hermoso, D.; Maurel, V.; Soussi, S.; Alanio, A. Detection of circulating Mucorales DNA in critically ill burn patients: Preliminary report of a screening strategy for early diagnosis and treatment. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2016, 63, 1312–1317. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Bhowmik, B.; do Vale Moreira, N.C. COVID-19 and diabetes: Knowledge in progress. Diabetes Res. Clin. Pract. 2020, 162, 108142. [Google Scholar] [CrossRef]
- Ceriello, A.; De Nigris, V.; Prattichizzo, F. Why is hyperglycemia worsening COVID-19 and its prognosis? Diabetes Obes. Metab. 2020, 22, 1951–1952. [Google Scholar] [CrossRef] [PubMed]
- Farah, Y.; Hala, N.; Aisha, N.; Dapk, K.E.; Phadke, R. COVID-19 associated mucor mycosis: A systematic review from diagnostic challenges to management. Disease 2021, 9, 65–69. [Google Scholar]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; Macary, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Kumar, S.D.; Shubham, S.; Kumawat, M.; Verma, V.; Singh, B. Mucormycosis in COVID-19 pandemic: Risk factors and linkages. Clin. Res. Microb. Sci. 2021, 2, 100057. [Google Scholar]
- Fasano, A.; Baudry, B.; Pumplin, D.W.; Wasserman, S.S.; Tall, B.D.; Ketley, J.M.; Kaper, J.B. Vibrio cholerae produces a second enterotoxin, which affects intestinal tight junctions. Proc. Natl. Acad. Sci. USA 1991, 88, 5242–5246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fasano, A.; Shea-Donohue, T. Mechanisms of disease: The role of intestinal barrier function in the pathogenesis of gastrointestinal autoimmune diseases. Nat. Clin. Pract. Gastroenterol. Hepatol. 2005, 2, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, L.; Luo, X.M. Retinoic acid, leaky gut, and autoimmune diseases. Nutrients 2018, 10, 1016. [Google Scholar] [CrossRef] [Green Version]
- Nikitakis, N.G.; Papaioannou, W.; Sakkas, L.I.; Kousvelari, E. The autoimmunity-oral microbiome connection. Oral Dis. 2017, 23, 828–839. [Google Scholar] [CrossRef] [PubMed]
- Garg, D.; Muthu, V.; Sehgal, I.S.; Ramachandran, R.; Kaur, H.; Bhalla, A. coronavirus disease (Covid-19) associated mucormycosis (CAM): Case report and systematic review of literature. Mycopathologia 2021, 186, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Hood, M.I.; Skaar, E.P. Nutritional immunity: Transition metals at the pathogen-host interface. Nat. Rev. Microbiol. 2012, 10, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Roilides, E.; Kontoyiannis, D.P.; Walsh, T.J. Host defenses against zygomycetes. Clin. Infect. Dis. 2012, 54, S61–S66. [Google Scholar] [CrossRef] [Green Version]
- Chamilos, G.; Lewis, R.E.; Lamaris, G.; Walsh, T.J.; Kontoyiannis, D.P. Zygomycetes hyphae trigger an early, robust proinflammatory response in human polymorphonuclear neutrophils through toll-like receptor 2 induction but display relative resistance to oxidativedamage. Antimicrob. Agents Chemother. 2008, 52, 722–724. [Google Scholar] [CrossRef] [Green Version]
- Binder, U.; Maurer, E.; Lass-Flörl, C. Mucormycosis—From the pathogens to the disease. Clin. Microbiol. Infect. 2014, 20, 60–66. [Google Scholar] [CrossRef] [Green Version]
- Death of Women with Black Fungus Symptom Causes a Scare. The Hindu. Available online: https://www.thehindu.com/news/national/andhra-pradesh/death-of-woman-with-black-fungus-symptoms-causes-a-scare/article34600320.ece (accessed on 17 July 2021).
- Uchida, T.; Okamoto, M.; Fujikawa, K.; Yoshikawa, D.; Mizokami, A.; Mihara, T.; Kawakami, A. Gastric mucormycosis complicated by a gastropleural fistula: A case report and review of the literature. Medicine 2019, 98, e18142. [Google Scholar] [CrossRef]
- Dioverti, M.V.; Cawcutt, K.A.; Abidi, M.; Sohail, M.R.; Walker, R.C.; Osmon, D.R. Gastrointestinal mucormycosis in immunocompromised hosts. Mycoses 2015, 58, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Kumar, V.; Gupta, D. Pulmonary mucormycosis: Two of a kind. Eur. J. Intern. Med. 2006, 17, 63–65. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, M.K.; Manju, R.; Kumar, S.V.; Toi, P.C. Pulmonary mucormycosis presenting as nonresolving pneumonia in a patient with diabetes mellitus. Respir. Care 2014, 59, e201–e205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raza, A.F.; Paudel, D.R.; Prabhu, P. Black fungus and COVID-19: Role of otorhinolaryngologists and audiologists. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 3133–3134. [Google Scholar] [CrossRef]





| Test | Uses | Advantages | Disadvantages |
|---|---|---|---|
| Fungal detection biosensors |
|
| |
| Diagnostics based on nucleic acids |
|
|
|
| Point of Care tests |
|
|
|
| Detection of Galactomannans |
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asdaq, S.M.B.; Rajan, A.; Damodaran, A.; Kamath, S.R.; Nair, K.S.; Zachariah, S.M.; Sahu, R.K.; Fattepur, S.; Sreeharsha, N.; Nair, A.; et al. Identifying Mucormycosis Severity in Indian COVID-19 Patients: A Nano-Based Diagnosis and the Necessity for Critical Therapeutic Intervention. Antibiotics 2021, 10, 1308. https://doi.org/10.3390/antibiotics10111308
Asdaq SMB, Rajan A, Damodaran A, Kamath SR, Nair KS, Zachariah SM, Sahu RK, Fattepur S, Sreeharsha N, Nair A, et al. Identifying Mucormycosis Severity in Indian COVID-19 Patients: A Nano-Based Diagnosis and the Necessity for Critical Therapeutic Intervention. Antibiotics. 2021; 10(11):1308. https://doi.org/10.3390/antibiotics10111308
Chicago/Turabian StyleAsdaq, Syed Mohammed Basheeruddin, Arya Rajan, Aswin Damodaran, Shivali R. Kamath, Krishnanjana S. Nair, Subin Mary Zachariah, Ram Kumar Sahu, Santosh Fattepur, Nagaraja Sreeharsha, Anroop Nair, and et al. 2021. "Identifying Mucormycosis Severity in Indian COVID-19 Patients: A Nano-Based Diagnosis and the Necessity for Critical Therapeutic Intervention" Antibiotics 10, no. 11: 1308. https://doi.org/10.3390/antibiotics10111308
APA StyleAsdaq, S. M. B., Rajan, A., Damodaran, A., Kamath, S. R., Nair, K. S., Zachariah, S. M., Sahu, R. K., Fattepur, S., Sreeharsha, N., Nair, A., Jacob, S., Albahrani, H. A., Alkhaldi, E. H., Mohzari, Y., Alrashed, A. A., & Imran, M. (2021). Identifying Mucormycosis Severity in Indian COVID-19 Patients: A Nano-Based Diagnosis and the Necessity for Critical Therapeutic Intervention. Antibiotics, 10(11), 1308. https://doi.org/10.3390/antibiotics10111308








