Antimicrobial Activity of Aztreonam in Combination with Old and New β-Lactamase Inhibitors against MBL and ESBL Co-Producing Gram-Negative Clinical Isolates: Possible Options for the Treatment of Complicated Infections
Abstract
:1. Introduction
2. Results
2.1. Characterization of bla Genes
2.2. Susceptibility Testing
2.2.1. Checkerboard Assays
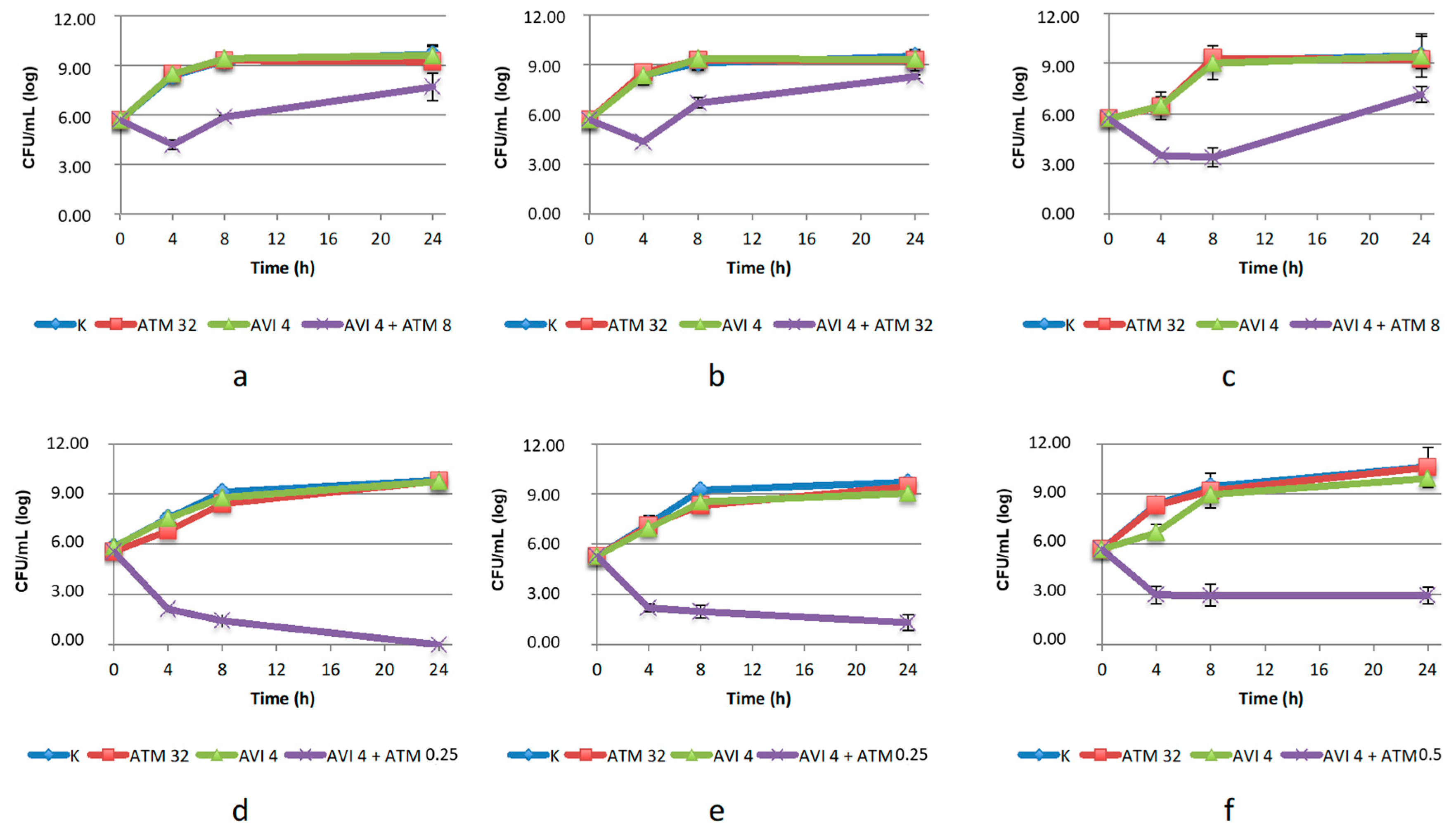
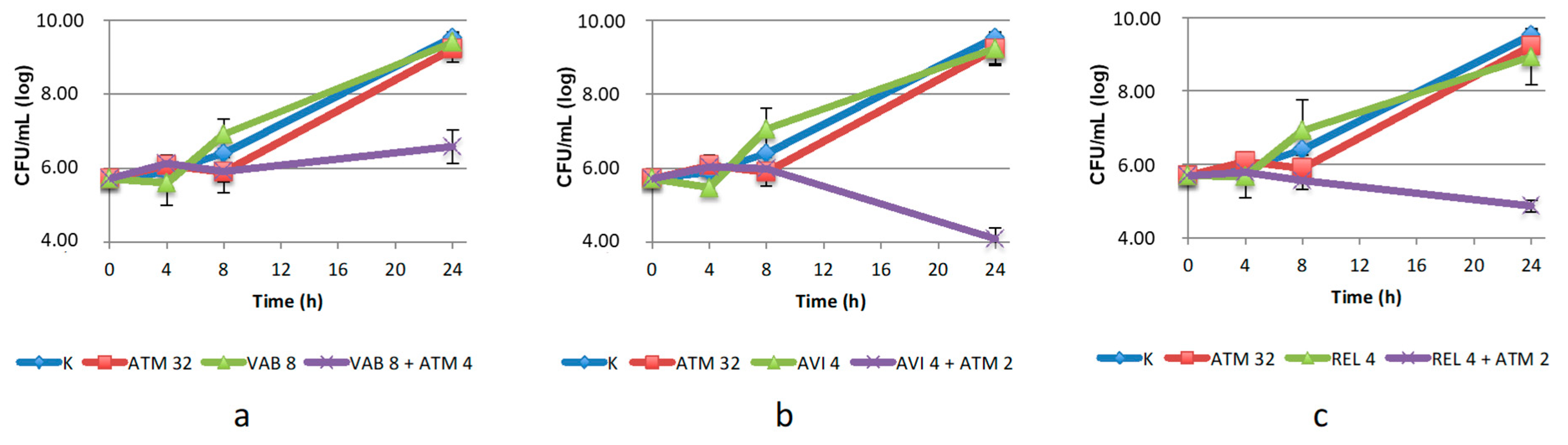
2.2.2. Time–Kill Assay on Enterobacterales
2.2.3. Time–Kill Assay on S. maltophilia
2.2.4. Time-Kill Assays with Aztreonam/Zidebactam 1:1 Ratio
3. Discussion
4. Materials and Methods
4.1. Strains, Culture Media and Chemicals
4.2. Identification, Antimicrobial Susceptibility Testing
4.3. Antimicrobial Resistance Genes Investigations
4.4. Whole-Genome Sequencing
4.5. MIC Evaluation and Checkerboard Assays
4.6. Time–Kill Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bahr, G.; González, L.J.; Vila, A.J. Metallo-β-Lactamases in the Age of Multidrug Resistance: From Structure and Mechanism to Evolution, Dissemination, and Inhibitor Design. Chem. Rev. 2021, 121, 7957–8094. [Google Scholar] [CrossRef]
- Boyd, S.E.; Livermore, D.M.; Hooper, D.C.; Hope, W.W. Metallo-β-Lactamases: Structure, Function, Epidemiology, Treatment Options, and the Development Pipeline. Antimicrob. Agents Chemother. 2020, 64, e00397-20. [Google Scholar] [CrossRef] [PubMed]
- Tooke, C.L.; Hinchliffe, P.; Bragginton, E.C.; Colenso, C.K.; Hirvonen, V.H.A.; Takebayashi, Y.; Spencer, J. β-Lactamases and β-Lactamase Inhibitors in the 21st Century. J. Mol. Biol. 2019, 431, 3472–3500. [Google Scholar] [CrossRef]
- Falcone, M.; Tiseo, G.; Antonelli, A.; Giordano, C.; Di Pilato, V.; Bertolucci, P.; Parisio, E.M.; Leonildi, A.; Aiezza, N.; Baccani, I.; et al. Clinical Features and Outcomes of Bloodstream Infections Caused by New Delhi Metallo-β-Lactamase-Producing Enterobacterales During a Regional Outbreak. Open Forum Infect. Dis. 2020, 7, ofaa011. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Servat, S.; Yero, D.; Huedo, P.; Marquez, R.; Molina, G.; Daura, X.; Gibert, I. Heterogeneous Colistin-Resistance Phenotypes Coexisting in Stenotrophomonas Maltophilia Isolates Influence Colistin Susceptibility Testing. Front. Microbiol. 2018, 9, 2871. [Google Scholar] [CrossRef]
- Mirza, H.C.; Tuncer, Ö.; Ölmez, S.; Şener, B.; Tuğcu, G.D.; Özçelik, U.; Gürsoy, N.C.; Otlu, B.; Büyükçam, A.; Kara, A.; et al. Clinical Strains of Chryseobacterium and Elizabethkingia spp. Isolated from Pediatric Patients in a University Hospital: Performance of MALDI-TOF MS-Based Identification, Antimicrobial Susceptibilities, and Baseline Patient Characteristics. Microb. Drug Resist. 2018, 24, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Mauri, C.; Maraolo, A.E.; Di Bella, S.; Luzzaro, F.; Principe, L. The Revival of Aztreonam in Combination with Avibactam against Metallo-β-Lactamase-Producing Gram-Negatives: A Systematic Review of In Vitro Studies and Clinical Cases. Antibiotics 2021, 10, 1012. [Google Scholar] [CrossRef]
- Carcione, D.; Siracusa, C.; Sulejmani, A.; Leoni, V.; Intra, J. Old and New Beta-Lactamase Inhibitors: Molecular Structure, Mechanism of Action, and Clinical Use. Antibiotics 2021, 10, 995. [Google Scholar] [CrossRef] [PubMed]
- Chew, K.L.; Tay, M.K.L.; Cheng, B.; Lin, R.T.P.; Octavia, S.; Teo, J.W.P. Aztreonam-Avibactam Combination Restores Susceptibility of Aztreonam in Dual-Carbapenemase-Producing Enterobacteriaceae. Antimicrob. Agents Chemother. 2018, 62, e00414-18. [Google Scholar] [CrossRef] [PubMed]
- Sader, H.S.; Carvalhaes, C.G.; Arends, S.J.R.; Castanheira, M.; Mendes, R.E. Aztreonam/Avibactam Activity against Clinical Isolates of Enterobacterales Collected in Europe, Asia and Latin America in 2019. J. Antimicrob. Chemother. 2021, 76, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Mansour, H.; Ouweini, A.E.L.; Chahine, E.B.; Karaoui, L.R. Imipenem/Cilastatin/Relebactam: A New Carbapenem β-Lactamase Inhibitor Combination. Am. J. Health-Syst. Pharm. 2021, 78, 674–683. [Google Scholar] [CrossRef]
- Yahav, D.; Giske, C.G.; Grāmatniece, A.; Abodakpi, H.; Tam, V.H.; Leibovici, L. New β-Lactam-β-Lactamase Inhibitor Combinations. Clin. Microbiol. Rev. 2020, 34, e00115-20. [Google Scholar] [CrossRef] [PubMed]
- Sader, H.S.; Rhomberg, P.R.; Flamm, R.K.; Jones, R.N.; Castanheira, M. WCK 5222 (Cefepime/Zidebactam) Antimicrobial Activity Tested against Gram-Negative Organisms Producing Clinically Relevant β-Lactamases. J. Antimicrob. Chemother. 2017, 72, 1696–1703. [Google Scholar] [CrossRef] [PubMed]
- Thomson, K.S.; AbdelGhani, S.; Snyder, J.W.; Thomson, G.K. Activity of Cefepime-Zidebactam against Multidrug-Resistant (MDR) Gram-Negative Pathogens. Antibiotics 2019, 8, 32. [Google Scholar] [CrossRef]
- Mushtaq, S.; Garello, P.; Vickers, A.; Woodford, N.; Livermore, D.M. Activity of Cefepime/Zidebactam (WCK 5222) against “problem” Antibiotic-Resistant Gram-Negative Bacteria Sent to a National Reference Laboratory. J. Antimicrob. Chemother. 2021, 76, 1511–1522. [Google Scholar] [CrossRef]
- Simoni, S.; Caucci, S.; Brenciani, A.; Morroni, G.; Giovanetti, E.; Menzo, S.; Facinelli, B.; Mingoia, M. Increase and Diversity of Carbapenemase-Producing Escherichia coli Isolates, Italy. Future Microbiol. 2019, 14, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Nucleo, E.; Caltagirone, M.; Marchetti, V.M.; D’Angelo, R.; Fogato, E.; Confalonieri, M.; Reboli, C.; March, A.; Sleghel, F.; Soelva, G.; et al. Colonization of Long-Term Care Facility Residents in Three Italian Provinces by Multidrug-Resistant Bacteria. Antimicrob. Resist. Infect. Control 2018, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Morroni, G.; Sante, L.D.; Simonetti, O.; Brescini, L.; Kamysz, W.; Kamysz, E.; Mingoia, M.; Brenciani, A.; Giovanetti, E.; Bagnarelli, P.; et al. Synergistic Effect of Antimicrobial Peptide LL-37 and Colistin Combination against Multidrug-Resistant Escherichia coli Isolates. Future Microbiol. 2021, 16, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Principe, L.; Mauri, C.; Conte, V.; Pini, B.; Giani, T.; Rossolini, G.M.; Luzzaro, F. First Report of NDM-1-Producing Klebsiella pneumoniae Imported from Africa to Italy: Evidence of the Need for Continuous Surveillance. J. Glob. Antimicrob. Resist. 2017, 8, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Principe, L.; Vecchio, G.; Sheehan, G.; Kavanagh, K.; Morroni, G.; Viaggi, V.; di Masi, A.; Giacobbe, D.R.; Luzzaro, F.; Luzzati, R.; et al. Zinc Chelators as Carbapenem Adjuvants for Metallo-β-Lactamase-Producing Bacteria: In Vitro and In Vivo Evaluation. Microb. Drug Resist. 2020, 26, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Mojica, M.F.; Rossi, M.-A.; Vila, A.J.; Bonomo, R.A. The Urgent Need for Metallo-β-Lactamase Inhibitors: An Unattended Global Threat. Lancet Infect. Dis. 2021. [Google Scholar] [CrossRef]
- Emeraud, C.; Escaut, L.; Boucly, A.; Fortineau, N.; Bonnin, R.A.; Naas, T.; Dortet, L. Aztreonam plus Clavulanate, Tazobactam, or Avibactam for Treatment of Infections Caused by Metallo-β-Lactamase-Producing Gram-Negative Bacteria. Antimicrob. Agents Chemother. 2019, 63, e00010-19. [Google Scholar] [CrossRef] [PubMed]
- Ract, P.; Compain, F.; Robin, F.; Decre, D.; Gallah, S.; Podglajen, I. Synergistic in Vitro Activity between Aztreonam and Amoxicillin-Clavulanate against Enterobacteriaceae-Producing Class B and/or Class D Carbapenemases with or without Extended-Spectrum β-Lactamases. J. Med. Microbiol. 2019, 68, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Papp-Wallace, K.M.; Barnes, M.D.; Alsop, J.; Taracila, M.A.; Bethel, C.R.; Becka, S.A.; van Duin, D.; Kreiswirth, B.N.; Kaye, K.S.; Bonomo, R.A. Relebactam Is a Potent Inhibitor of the KPC-2 β-Lactamase and Restores Imipenem Susceptibility in KPC-Producing Enterobacteriaceae. Antimicrob. Agents Chemother. 2018, 62, e00174-18. [Google Scholar] [CrossRef] [PubMed]
- Maraki, S.; Mavromanolaki, V.E.; Moraitis, P.; Stafylaki, D.; Kasimati, A.; Magkafouraki, E.; Scoulica, E. Ceftazidime-Avibactam, Meropenen-Vaborbactam, and Imipenem-Relebactam in Combination with Aztreonam against Multidrug-Resistant, Metallo-β-Lactamase-Producing Klebsiella pneumoniae. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2021, 40, 1755–1759. [Google Scholar] [CrossRef] [PubMed]
- Biagi, M.; Lamm, D.; Meyer, K.; Vialichka, A.; Jurkovic, M.; Patel, S.; Mendes, R.E.; Bulman, Z.P.; Wenzler, E. Activity of Aztreonam in Combination with Avibactam, Clavulanate, Relebactam, and Vaborbactam against Multidrug-Resistant Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 2020, 64, e00297-20. [Google Scholar] [CrossRef] [PubMed]
- White, R.L.; Burgess, D.S.; Manduru, M.; Bosso, J.A. Comparison of Three Different in Vitro Methods of Detecting Synergy: Time–Kill, Checkerboard, and E Test. Antimicrob. Agents Chemother. 1996, 40, 1914–1918. [Google Scholar] [CrossRef]
- Lin, J.-N.; Lai, C.-H.; Yang, C.-H.; Huang, Y.-H. Elizabethkingia Infections in Humans: From Genomics to Clinics. Microorganisms 2019, 7, 295. [Google Scholar] [CrossRef] [PubMed]
- Jean, S.-S.; Chang, Y.-C.; Lin, W.-C.; Lee, W.-S.; Hsueh, P.-R.; Hsu, C.-W. Epidemiology, Treatment, and Prevention of Nosocomial Bacterial Pneumonia. J. Clin. Med. 2020, 9, 275. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 10.0. 2020. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_10.0_Breakpoint_Tables.pdf (accessed on 1 November 2020).
- Bogaerts, P.; Cuzon, G.; Evrard, S.; Hoebeke, M.; Naas, T.; Glupczynski, Y. Evaluation of a DNA Microarray for Rapid Detection of the Most Prevalent Extended-Spectrum β-Lactamases, Plasmid-Mediated Cephalosporinases and Carbapenemases in Enterobacteriaceae, Pseudomonas and Acinetobacter. Int. J. Antimicrob. Agents 2016, 48, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Nurk, S.; Bankevich, A.; Antipov, D.; Gurevich, A.A.; Korobeynikov, A.; Lapidus, A.; Prjibelski, A.D.; Pyshkin, A.; Sirotkin, A.; Sirotkin, Y.; et al. Assembling Single-Cell Genomes and Mini-Metagenomes from Chimeric MDA Products. J. Comput. Biol. J. Comput. Mol. Cell Biol. 2013, 20, 714–737. [Google Scholar] [CrossRef]
- Zankari, E.; Hasman, H.; Cosentino, S.; Vestergaard, M.; Rasmussen, S.; Lund, O.; Aarestrup, F.M.; Larsen, M.V. Identification of Acquired Antimicrobial Resistance Genes. J. Antimicrob. Chemother. 2012, 67, 2640–2644. [Google Scholar] [CrossRef] [PubMed]
- Inouye, M.; Dashnow, H.; Raven, L.-A.; Schultz, M.B.; Pope, B.J.; Tomita, T.; Zobel, J.; Holt, K.E. SRST2: Rapid Genomic Surveillance for Public Health and Hospital Microbiology Labs. Genome Med. 2014, 6, 90. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Padmanabhan, B.R.; Diene, S.M.; Lopez-Rojas, R.; Kempf, M.; Landraud, L.; Rolain, J.-M. ARG-ANNOT, a New Bioinformatic Tool to Discover Antibiotic Resistance Genes in Bacterial Genomes. Antimicrob. Agents Chemother. 2014, 58, 212–220. [Google Scholar] [CrossRef] [PubMed]
- EUCAST. EUCAST Reading Guide for Broth Microdilution V.3 2021. Available online: https://eucast.org/ast_of_bacteria/mic_determination/ (accessed on 1 June 2021).
- Doern, C.D. When Does 2 plus 2 Equal 5? A Review of Antimicrobial Synergy Testing. J. Clin. Microbiol. 2014, 52, 4124–4128. [Google Scholar] [CrossRef] [PubMed]


| Strain | Metallo-β-Lactamase | Serine-β- Lactamase | MIC ATM | MIC ATM after Addition of | MIC ZID | MIC ATM/ZID 1:1 Ratio | REF | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CLA a | TAZ b | SUL b | VAB c | AVI b | REL b | ZID d | |||||||
| E. coli CP-Ec3 | blaVIM-1 | blaKPC-2 | >32 | >32 | >32 | >32 | >32 | 8 | >32 | 32 | 1 | 1 | [16] |
| E. coli CP-Ec4 | blaVIM-1 | blaTEM-1 * blaCTX-M-15 * blaSHV-12 | >32 | 16 | >32 | >32 | >32 | 32 | >32 | >32 | 1 | 1 | [16] |
| E. coli 482483 | blaNDM-5 | blaTEM-1 * blaCTX-M-15 | >32 | 16 | >32 | >32 | >32 | 8 | >32 | 32 | 1 | 1 | [17] |
| C. amalonaticus N18 | blaVIM-1 | * blaSHV-12 | >32 | 8 | >32 | >32 | >32 | 0.25 | 4 | 0.5 | 4 | 0.5 | [18] |
| K. pneumoniae KL 12 SG | blaNDM-1 | blaTEM-1 * blaCTX-M-15 | >32 | >32 | >32 | >32 | 4 | 0.25 | 4 | 0.25 | 8 | 0.5 | This study |
| K. pneumoniae LC954/14 | blaNDM-1 |
* blaCTX-M-15 * blaSHV-182 | >32 | >32 | >32 | >32 | 8 | 0.25 | 4 | >32 | 1 | 1 | [19] |
| C. indologenes LC650/17 | blaIND-3 | * blaCIA | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | [20] |
| E. meningoseptica LC596/11 | blaB-9 blaGOB-13 | * blaCME-1 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | [20] |
| S. maltophilia | blaL-1 | * blaL-2 | >32 | >32 | 4 | 8 | 2 | 1 | 0.5 | 0.5 | >32 | 0.5 | [20] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morroni, G.; Bressan, R.; Fioriti, S.; D’Achille, G.; Mingoia, M.; Cirioni, O.; Di Bella, S.; Piazza, A.; Comandatore, F.; Mauri, C.; et al. Antimicrobial Activity of Aztreonam in Combination with Old and New β-Lactamase Inhibitors against MBL and ESBL Co-Producing Gram-Negative Clinical Isolates: Possible Options for the Treatment of Complicated Infections. Antibiotics 2021, 10, 1341. https://doi.org/10.3390/antibiotics10111341
Morroni G, Bressan R, Fioriti S, D’Achille G, Mingoia M, Cirioni O, Di Bella S, Piazza A, Comandatore F, Mauri C, et al. Antimicrobial Activity of Aztreonam in Combination with Old and New β-Lactamase Inhibitors against MBL and ESBL Co-Producing Gram-Negative Clinical Isolates: Possible Options for the Treatment of Complicated Infections. Antibiotics. 2021; 10(11):1341. https://doi.org/10.3390/antibiotics10111341
Chicago/Turabian StyleMorroni, Gianluca, Raffaela Bressan, Simona Fioriti, Gloria D’Achille, Marina Mingoia, Oscar Cirioni, Stefano Di Bella, Aurora Piazza, Francesco Comandatore, Carola Mauri, and et al. 2021. "Antimicrobial Activity of Aztreonam in Combination with Old and New β-Lactamase Inhibitors against MBL and ESBL Co-Producing Gram-Negative Clinical Isolates: Possible Options for the Treatment of Complicated Infections" Antibiotics 10, no. 11: 1341. https://doi.org/10.3390/antibiotics10111341







