Green and Chemical Silver Nanoparticles and Pomegranate Formulations to Heal Infected Wounds in Diabetic Rats
Abstract
:1. Introduction
2. Results
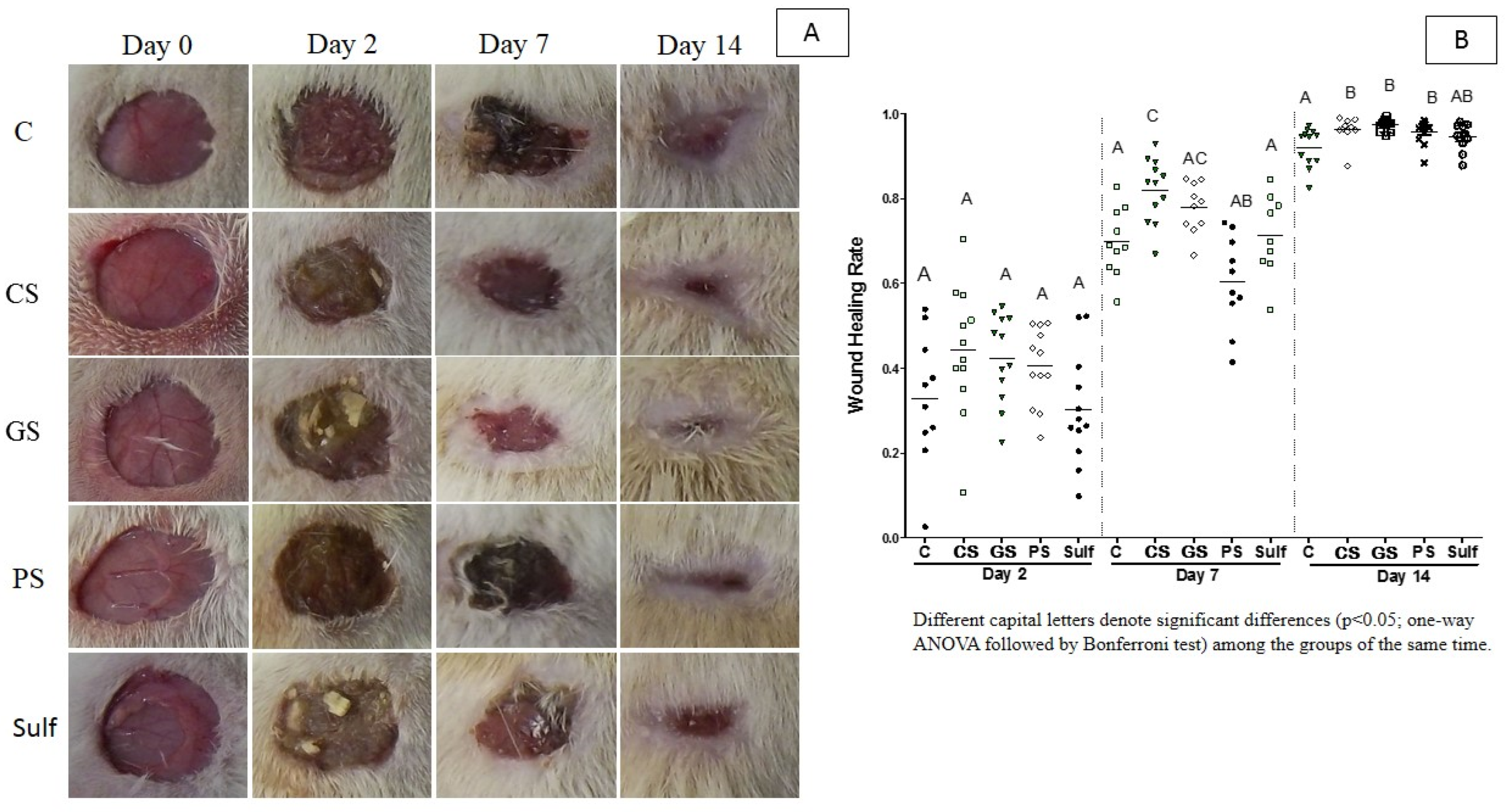
2.1. Wound Healing Activity
2.2. CFU Determination from Ulcers
2.3. Quantitative Image Evaluation for Inflammatory Infiltrate, Angiogenesis, and Fibroplasia
2.4. Dosage of Enzyme Myeloperoxidase (MPO)
2.5. Collagen Evaluation
3. Discussion
4. Materials and Methods
4.1. Spray Formulations
4.2. Ethics Statement
4.3. In Vivo Models for Wound Healing Activity
4.4. Wound Healing Activity
4.5. Material Collection for Future Studies
4.6. CFU Determination from Ulcers
4.7. Quantitative Image Evaluation of Inflammatory Infiltrate, Angiogenesis, and Fibroplasia
4.8. Dosage of Myeloperoxidase Enzyme (MPO)
4.9. Evaluation of Collagen Levels
4.10. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ragab, T.I.M.; Nada, A.A.; Ali, E.A.; Shalaby, A.S.G.; Soliman, A.A.F.; Emam, M.; El Raey, M.A. Soft Hydrogel Based on Modified Chitosan Containing P. Granatum Peel Extract and Its Nano-Forms: Multiparticulate Study on Chronic Wounds Treatment. Int. J. Biol. Macromol. 2019, 135, 407–421. [Google Scholar] [CrossRef]
- Broughton, G.I.I.; Janis, J.E.; Attinger, C.E. The Basic Science of Wound Healing. Plast. Reconstr. Surg. 2006, 117, 12S. [Google Scholar] [CrossRef]
- Poljšak, N.; Kreft, S.; Kočevar Glavač, N. Vegetable Butters and Oils in Skin Wound Healing: Scientific Evidence for New Opportunities in Dermatology. Phytother. Res. 2020, 34, 254–269. [Google Scholar] [CrossRef]
- Kováč, I.; Ďurkáč, J.; Hollý, M.; Jakubčová, K.; Peržeľová, V.; Mučaji, P.; Švajdlenka, E.; Sabol, F.; Legáth, J.; Belák, J.; et al. Plantago Lanceolata L. Water Extract Induces Transition of Fibroblasts into Myofibroblasts and Increases Tensile Strength of Healing Skin Wounds. J. Pharm. Pharmacol. 2015, 67, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Kokane, D.D.; More, R.Y.; Kale, M.B.; Nehete, M.N.; Mehendale, P.C.; Gadgoli, C.H. Evaluation of Wound Healing Activity of Root of Mimosa Pudica. J. Ethnopharmacol. 2009, 124, 311–315. [Google Scholar] [CrossRef]
- Ovington, L.G. Advances in Wound Dressings. Clin. Dermatol. 2007, 25, 33–38. [Google Scholar] [CrossRef]
- Dorsett-Martin, W.A.; Wysocki, A.B. Rat models of skin wound healing. In Sourcebook of Models for Biomedical Research; Conn, P.M., Ed.; Humana Press: Totowa, NJ, USA, 2008; pp. 631–638. [Google Scholar]
- Ashcroft, G.S.; Mills, S.J.; Lei, K.; Gibbons, L.; Jeong, M.-J.; Taniguchi, M.; Burow, M.; Horan, M.A.; Wahl, S.M.; Nakayama, T. Estrogen Modulates Cutaneous Wound Healing by Downregulating Macrophage Migration Inhibitory Factor. J. Clin. Investig. 2003, 111, 1309–1318. [Google Scholar] [CrossRef] [Green Version]
- Nada, A.A.; Hassabo, A.G.; Awad, H.M.; Fayad, W.; Shaffie, N.M.; Sleem, A.A.; Zeid, N.Y.A. Biomaterials Based on Essential Fatty Acids and Carbohydrates for Chronic Wounds. J. Basic Appl. Pharm. Sci. 2015, 5, 13–21. [Google Scholar]
- Huang, Y.-Y.; Lin, C.-W.; Yang, H.-M.; Hung, S.-Y.; Chen, I.-W. Survival and Associated Risk Factors in Patients with Diabetes and Amputations Caused by Infectious Foot Gangrene. J. Foot Ankle Res. 2018, 11, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeyaraman, K.; Berhane, T.; Hamilton, M.; Chandra, A.P.; Falhammar, H. Amputations in Patients with Diabetic Foot Ulcer: A Retrospective Study from a Single Centre in the Northern Territory of Australia. ANZ J. Surg. 2019, 89, 874–879. [Google Scholar] [CrossRef] [PubMed]
- Gottrup, F. A Specialized Wound-Healing Center Concept: Importance of a Multidisciplinary Department Structure and Surgical Treatment Facilities in the Treatment of Chronic Wounds. Am. J. Surg. 2004, 187, 38S–43S. [Google Scholar] [CrossRef]
- Wild, S.H.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global Prevalence of Diabetes: Estimates for the Year 2000 and Projections for 2030: Response to Rathman and Giani. Diabetes Care 2004, 27, 2569–2570. [Google Scholar] [CrossRef] [Green Version]
- Van Acker, H.; Van Dijck, P.; Coenye, T. Molecular Mechanisms of Antimicrobial Tolerance and Resistance in Bacterial and Fungal Biofilms. Trends Microbiol. 2014, 22, 326–333. [Google Scholar] [CrossRef]
- Weinstein, R.A.; Mayhall, C.G. The Epidemiology of Burn Wound Infections: Then and Now. Clin. Infect. Dis. 2003, 37, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Moser, C.; Wang, H.-Z.; Høiby, N.; Song, Z.-J. Strategies for Combating Bacterial Biofilm Infections. Int. J. Oral Sci. 2015, 7, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Wen, Y.-M. The Role of Bacterial Biofilm in Persistent Infections and Control Strategies. Int. J. Oral Sci. 2011, 3, 66–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, K.K.; Surekha, D.B.; Tripathi, M.; Anjum, M.M.; Muthu, M.S.; Tilak, R.; Agrawal, A.K.; Singh, S. Antibiofilm Potential of Silver Sulfadiazine-Loaded Nanoparticle Formulations: A Study on the Effect of DNase-I on Microbial Biofilm and Wound Healing Activity. Mol. Pharm. 2019, 16, 3916–3925. [Google Scholar] [CrossRef]
- James, G.A.; Swogger, E.; Wolcott, R.; Pulcini, E.D.; Secor, P.; Sestrich, J.; Costerton, J.W.; Stewart, P.S. Biofilms in Chronic Wounds. Wound Repair Regen. 2008, 16, 37–44. [Google Scholar] [CrossRef]
- Monteiro, D.R.; Gorup, L.F.; Takamiya, A.S.; Ruvollo-Filho, A.C.; de Camargo, E.R.; Barbosa, D.B. The Growing Importance of Materials That Prevent Microbial Adhesion: Antimicrobial Effect of Medical Devices Containing Silver. Int. J. Antimicrob. Agents 2009, 34, 103–110. [Google Scholar] [CrossRef]
- Berretta, A.A.; Nascimento, A.P.; Bueno, P.C.P.; de Oliveira Lima Leite Vaz, M.M.; Marchetti, J.M. Propolis Standardized Extract (EPP-AF®), an Innovative Chemically and Biologically Reproducible Pharmaceutical Compound for Treating Wounds. Int. J. Biol. Sci. 2012, 8, 512–521. [Google Scholar] [CrossRef]
- Elzayat, E.M.; Auda, S.H.; Alanazi, F.K.; Al-Agamy, M.H. Evaluation of Wound Healing Activity of Henna, Pomegranate and Myrrh Herbal Ointment Blend. Saudi Pharm J. 2018, 26, 733–738. [Google Scholar] [CrossRef]
- Nayak, S.B.; Isik, K.; Marshall, J.R. Wound-Healing Potential of Oil of Hypercium Perforatum in Excision Wounds of Male Sprague Dawley Rats. Adv. Wound Care 2017, 6, 401–406. [Google Scholar] [CrossRef] [Green Version]
- Dwivedi, D.; Dwivedi, M.; Malviya, S.; Singh, V. Evaluation of Wound Healing, Anti-Microbial and Antioxidant Potential of Pongamia Pinnata in Wistar Rats. Afr. J. Tradit. Complement. Altern. Med. 2017, 7, 79–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayouni, E.A.; Miled, K.; Boubaker, S.; Bellasfar, Z.; Abedrabba, M.; Iwaski, H.; Oku, H.; Matsui, T.; Limam, F.; Hamdi, M. Hydroalcoholic Extract Based-Ointment from Punica Granatum L. Peels with Enhanced in Vivo Healing Potential on Dermal Wounds. Phytomedicine 2011, 18, 976–984. [Google Scholar] [CrossRef]
- Kazemian, H.; Ghafourian, S.; Sadeghifard, N.; Houshmandfar, R.; Badakhsh, B.; Taji, A.; Shavalipour, A.; Mohebi, R.; Ebrahim-Saraie, H.S.; Houri, H.; et al. In Vivo Antibacterial and Wound Healing Activities of Roman Chamomile (Chamaemelum Nobile). Infect. Disord. Drug Targets 2018, 18, 41–45. [Google Scholar] [CrossRef]
- Maurya, H.; Semwal, M.; Dubey, S.K. Pharmacological Evaluation of Chrozophora Tinctoria as Wound Healing Potential in Diabetic Rat’s Model. Biomed. Res. Int. 2016, 2016, 7475124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mo, J.; Panichayupakaranant, P.; Kaewnopparat, N.; Nitiruangjaras, A.; Reanmongkol, W. Wound Healing Activities of Standardized Pomegranate Rind Extract and Its Major Antioxidant Ellagic Acid in Rat Dermal Wounds. J. Nat. Med. 2014, 68, 377–386. [Google Scholar] [CrossRef]
- Chidambara Murthy, K.N.; Reddy, V.K.; Veigas, J.M.; Murthy, U.D. Study on Wound Healing Activity of Punica Granatum Peel. J. Med. Food 2004, 7, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.B.; Rodrigues, V.; Maharaj, S.; Bhogadi, V.S. Wound Healing Activity of the Fruit Skin of Punica Granatum. J. Med. Food 2013, 16, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Mofidfar, M.; Kim, E.S.; Larkin, E.L.; Long, L.; Jennings, W.D.; Ahadian, S.; Ghannoum, M.A.; Wnek, G.E. Antimicrobial Activity of Silver Containing Crosslinked Poly(Acrylic Acid) Fibers. Micromachines 2019, 10, 829. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, D.B.; Monteiro, D.R.; Takamyia, A.S.; Camargo, E.R.; Hunt, A.M.A.; Delbem, A.C.B.; Pessan, J.P. Silver and phosphate nanoparticles: Antimicrobial approach and caries prevention application. In Nanobiomaterials in Clinical Dentistry, 2nd ed.; Elsevier: Cambridge, MA, USA, 2019; pp. 225–242. [Google Scholar]
- Syafiuddin, A.; Salim, M.R.; Beng Hong Kueh, A.; Hadibarata, T.; Nur, H. A Review of Silver Nanoparticles: Research Trends, Global Consumption, Synthesis, Properties, and Future Challenges: A Review of Silver Nanoparticles. J. Chin. Chem. Soc. 2017, 64, 732–756. [Google Scholar] [CrossRef]
- Rónavári, A.; Kovács, D.; Igaz, N.; Vágvölgyi, C.; Boros, I.M.; Kónya, Z.; Pfeiffer, I.; Kiricsi, M. Biological Activity of Green-Synthesized Silver Nanoparticles Depends on the Applied Natural Extracts: A Comprehensive Study. Int. J. Nanomed. 2017, 12, 871–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eby, D.M.; Luckarift, H.R.; Johnson, G.R. Hybrid Antimicrobial Enzyme and Silver Nanoparticle Coatings for Medical Instruments. ACS Appl. Mater. Interfaces 2009, 1, 1553–1560. [Google Scholar] [CrossRef]
- Mohite, B.V.; Patil, S.V. In Situ Development of Nanosilver-Impregnated Bacterial Cellulose for Sustainable Released Antimicrobial Wound Dressing. J. Appl. Biomater. Funct. Mater. 2016, 14, e53–e58. [Google Scholar] [CrossRef] [Green Version]
- Hebeish, A.; El-Rafie, M.H.; El-Sheikh, M.A.; Seleem, A.A.; El-Naggar, M.E. Antimicrobial Wound Dressing and Anti-Inflammatory Efficacy of Silver Nanoparticles. Int. J. Biol. Macromol. 2014, 65, 509–515. [Google Scholar] [CrossRef]
- Lázaro-Martínez, J.L.; Álvaro-Afonso, F.J.; Sevillano-Fernández, D.; Molines-Barroso, R.J.; García-Álvarez, Y.; García-Morales, E. Clinical and Antimicrobial Efficacy of a Silver Foam Dressing With Silicone Adhesive in Diabetic Foot Ulcers With Mild Infection. Int. J. Low. Extrem. Wounds 2019, 18, 269–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlachou, E.; Chipp, E.; Shale, E.; Wilson, Y.T.; Papini, R.; Moiemen, N.S. The Safety of Nanocrystalline Silver Dressings on Burns: A Study of Systemic Silver Absorption. Burns 2007, 33, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Asz, J.; Asz, D.; Moushey, R.; Seigel, J.; Mallory, S.B.; Foglia, R.P. Treatment of Toxic Epidermal Necrolysis in a Pediatric Patient with a Nanocrystalline Silver Dressing. J. Pediatr. Surg. 2006, 41, e9–e12. [Google Scholar] [CrossRef]
- Yang, J.-Y.; Huang, C.-Y.; Chuang, S.-S.; Chen, C.-C. A Clinical Experience of Treating Exfoliative Wounds Using Nanocrystalline Silver-Containing Dressings (Acticoat®). Burns 2007, 33, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Sibbald, R.G.; Contreras-Ruiz, J.; Coutts, P.; Fierheller, M.; Rothman, A.; Woo, K. Bacteriology, Inflammation, and Healing: A Study of Nanocrystalline Silver Dressings in Chronic Venous Leg Ulcers. Adv. Ski. Wound Care 2007, 20, 549–558. [Google Scholar] [CrossRef]
- Smith, J.N.; Thomas, D.G.; Jolley, H.; Kodali, V.K.; Littke, M.H.; Munusamy, P.; Baer, D.R.; Gaffrey, M.J.; Thrall, B.D.; Teeguarden, J.G. All That Is Silver Is Not Toxic: Silver Ion and Particle Kinetics Reveals the Role of Silver Ion Aging and Dosimetry on the Toxicity of Silver Nanoparticles. Part. Fibre Toxicol. 2018, 15, 47. [Google Scholar] [CrossRef]
- Roy, N.; Gaur, A.; Jain, A.; Bhattacharya, S.; Rani, V. Green Synthesis of Silver Nanoparticles: An Approach to Overcome Toxicity. Environ. Toxicol. Pharmacol. 2013, 36, 807–812. [Google Scholar] [CrossRef]
- Fernandes, R.A.; Berretta, A.A.; Torres, E.C.; Buszinski, A.F.M.; Fernandes, G.L.; Mendes-Gouvêa, C.C.; de Souza-Neto, F.N.; Gorup, L.F.; de Camargo, E.R.; Barbosa, D.B. Antimicrobial Potential and Cytotoxicity of Silver Nanoparticles Phytosynthesized by Pomegranate Peel Extract. Antibiotics 2018, 7, 51. [Google Scholar] [CrossRef] [Green Version]
- Nema, N.; Arjariya, S.; Bairagi, S.; Jha, M.; Kharya, M.D. In Vivo Topical Wound Healing Activity of Punica Granatum Peel Extract on Rats. Am. J. Phytomedicine Clin. Ther. 2013, 1, 195–200. [Google Scholar]
- Rohl, J.; Zaharia, A.; Rudolph, M. The Role of Inflammation in Cutaneous Repair. Wound Pract. Res. J. Aust. Wound Manag. 2015, 23, 8–12, 14–15. [Google Scholar]
- Olczyk, P.; Mencner, Ł.; Komosinska-Vassev, K. Diverse Roles of Heparan Sulfate and Heparin in Wound Repair. Biomed. Res. Int. 2015, 2015, 549417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, H.-M.; Chen, C.; Jiang, J.-Y.; Zheng, Y.-L.; Cai, W.-F.; Wang, B.; Ling, Z.; Tang, L.; Wang, Y.-H.; Shi, G.-G. The N-Butyl Alcohol Extract from Hibiscus Rosa-Sinensis L. Flowers Enhances Healing Potential on Rat Excisional Wounds. J. Ethnopharmacol. 2017, 198, 291–301. [Google Scholar] [CrossRef]
- Lukiswanto, B.S.; Miranti, A.; Sudjarwo, S.A.; Primarizky, H.; Yuniarti, W.M. Evaluation of Wound Healing Potential of Pomegranate (Punica Granatum) Whole Fruit Extract on Skin Burn Wound in Rats (Rattus Norvegicus). J. Adv. Vet. Anim. Res. 2019, 6, 202–207. [Google Scholar] [CrossRef]
- Ullah, I.; Cosar, G.; Abamor, E.S.; Bagirova, M.; Shinwari, Z.K.; Allahverdiyev, A.M. Comparative Study on the Antileishmanial Activities of Chemically and Biologically Synthesized Silver Nanoparticles (AgNPs). 3 Biotech. 2018, 8, 98. [Google Scholar] [CrossRef]
- Arya, G.; Kumari, R.M.; Sharma, N.; Gupta, N.; Kumar, A.; Chatterjee, S.; Nimesh, S. Catalytic, Antibacterial and Antibiofilm Efficacy of Biosynthesised Silver Nanoparticles Using Prosopis Juliflora Leaf Extract along with Their Wound Healing Potential. J. Photochem. Photobiol. B 2019, 190, 50–58. [Google Scholar] [CrossRef]
- Hadrup, N.; Sharma, A.K.; Loeschner, K. Toxicity of Silver Ions, Metallic Silver, and Silver Nanoparticle Materials after in Vivo Dermal and Mucosal Surface Exposure: A Review. Regul. Toxicol. Pharmacol. 2018, 98, 257–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veerasamy, R.; Xin, T.Z.; Gunasagaran, S.; Xiang, T.F.W.; Yang, E.F.C.; Jeyakumar, N.; Dhanaraj, S.A. Biosynthesis of Silver Nanoparticles Using Mangosteen Leaf Extract and Evaluation of Their Antimicrobial Activities. J. Saudi Chem. Soc. 2011, 15, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Burdușel, A.-C.; Gherasim, O.; Grumezescu, A.M.; Mogoantă, L.; Ficai, A.; Andronescu, E. Biomedical Applications of Silver Nanoparticles: An Up-to-Date Overview. Nanomaterials 2018, 8, 681. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Lee, P.-Y.; Ho, C.-M.; Lui, V.C.H.; Chen, Y.; Che, C.-M.; Tam, P.K.H.; Wong, K.K.Y. Silver Nanoparticles Mediate Differential Responses in Keratinocytes and Fibroblasts during Skin Wound Healing. ChemMedChem 2010, 5, 468–475. [Google Scholar] [CrossRef] [Green Version]
- Yuniarti, W.M.; Primarizky, H.; Lukiswanto, B.S. The Activity of Pomegranate Extract Standardized 40% Ellagic Acid during the Healing Process of Incision Wounds in Albino Rats (Rattus Norvegicus). Vet. World 2018, 11, 321–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanani, Z.A.N.; Yee, F.C.; Nor-Khaizura, M.A.R. Effect of Pomegranate (Punica Granatum L.) Peel Powder on the Antioxidant and Antimicrobial Properties of Fish Gelatin Films as Active Packaging. Food Hydrocoll. 2019, 89, 253–259. [Google Scholar] [CrossRef]
- Ismail, T.; Sestili, P.; Akhtar, S. Pomegranate Peel and Fruit Extracts: A Review of Potential Anti-Inflammatory and Anti-Infective Effects. J. Ethnopharmacol. 2012, 143, 397–405. [Google Scholar] [CrossRef]
- Salama, A.A.; Ismael, N.M.; Bedewy, M. The Anti-Inflammatory and Antiatherogenic In Vivo Effects of Pomegranate Peel Powder: From Waste to Medicinal Food. J. Med. Food 2020, 24, 145–150. [Google Scholar]
- Adibhesami, M.; Ahmadi, M.; Farshid, A.A.; Sarrafzadeh-Rezaei, F.; Dalir-Naghadeh, B. Effects of Silver Nanoparticles on Staphylococcus Aureus Contaminated Open Wounds Healing in Mice: An Experimental Study. Vet. Res. Forum. 2017, 8, 23–28. [Google Scholar]
- Paosen, S.; Jindapol, S.; Soontarach, R.; Voravuthikunchai, S.P. Eucalyptus citriodora leaf extract-mediated biosynthesis of silver nanoparticles: Broad antimicrobial spectrum and mechanisms of action against hospital-acquired pathogens. APMIS 2019, 27, 764–778. [Google Scholar] [CrossRef]
- Kean, R.; Rajendran, R.; Haggarty, J.; Townsend, E.M.; Short, B.; Burgess, K.E.; Lang, S.; Millington, O.; Mackay, W.G.; Williams, C.; et al. Candida Albicans Mycofilms Support Staphylococcus Aureus Colonization and Enhances Miconazole Resistance in Dual-Species Interactions. Front. Microbiol. 2017, 8, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wongsuk, T.; Pumeesat, P.; Luplertlop, N. Fungal Quorum Sensing Molecules: Role in Fungal Morphogenesis and Pathogenicity. J. Basic Microbiol. 2016, 56, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.; Atkin, L.; Swanson, T.; Tachi, M.; Tan, Y.K.; de Ceniga, M.V.; Weir, D.; Wolcott, R.; Ĉernohorská, J.; Ciprandi, G.; et al. Defying Hard-to-Heal Wounds with an Early Antibiofilm Intervention Strategy: Wound Hygiene. J. Wound Care 2020, 29, S1–S26. [Google Scholar] [CrossRef] [Green Version]
- Aslam, M.N.; Lansky, E.P.; Varani, J. Pomegranate as a Cosmeceutical Source: Pomegranate Fractions Promote Proliferation and Procollagen Synthesis and Inhibit Matrix Metalloproteinase-1 Production in Human Skin Cells. J. Ethnopharmacol. 2006, 103, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Saeidinia, A.; Keihanian, F.; Lashkari, A.P.; Lahiji, H.G.; Mobayyen, M.; Heidarzade, A.; Golchai, J. Partial-Thickness Burn Wounds Healing by Topical Treatment: A Randomized Controlled Comparison between Silver Sulfadiazine and Centiderm. Medicine 2017, 96, e6168. [Google Scholar] [CrossRef]
- Demling, R.H.; Leslie DeSanti, M.D. The Rate of Re-Epithelialization across Meshed Skin Grafts Is Increased with Exposure to Silver. Burns 2002, 28, 264–266. [Google Scholar] [CrossRef]
- Mastrogiovanni, F.; Bernini, R.; Basiricò, L.; Bernabucci, U.; Campo, M.; Romani, A.; Santi, L.; Lacetera, N. Antioxidant and Anti-Inflammatory Effects of Pomegranate Peel Extracts on Bovine Mammary Epithelial Cells BME-UV1. Nat. Prod. Res. 2020, 34, 1465–1469. [Google Scholar] [CrossRef] [PubMed]
- El-Missiry, M.A.; Amer, M.A.; Hemieda, F.A.E.; Othman, A.I.; Sakr, D.A.; Abdulhadi, H.L. Cardioameliorative Effect of Punicalagin against Streptozotocin-Induced Apoptosis, Redox Imbalance, Metabolic Changes and Inflammation. Egypt. J. Basic Appl. Sci. 2015, 2, 247–260. [Google Scholar] [CrossRef] [Green Version]
- Vijayakumar, S.; Malaikozhundan, B.; Saravanakumar, K.; Durán-Lara, E.F.; Wang, M.-H.; Vaseeharan, B. Garlic Clove Extract Assisted Silver Nanoparticle--Antibacterial, Antibiofilm, Antihelminthic, Anti-Inflammatory, Anticancer and Ecotoxicity Assessment. J. Photochem. Photobiol. B 2019, 198, 111558. [Google Scholar] [CrossRef] [PubMed]
- Mengshol, J.A.; Vincenti, M.P.; Brinckerhoff, C.E. IL-1 Induces Collagenase-3 (MMP-13) Promoter Activity in Stably Transfected Chondrocytic Cells: Requirement for Runx-2 and Activation by p38 MAPK and JNK Pathways. Nucleic Acids Res. 2001, 29, 4361–4372. [Google Scholar] [CrossRef] [Green Version]
- Larrosa, M.; González-Sarrías, A.; Yáñez-Gascón, M.J.; Selma, M.V.; Azorín-Ortuño, M.; Toti, S.; Tomás-Barberán, F.; Dolara, P.; Espín, J.C. Anti-Inflammatory Properties of a Pomegranate Extract and Its Metabolite Urolithin-A in a Colitis Rat Model and the Effect of Colon Inflammation on Phenolic Metabolism. J. Nutr. Biochem. 2010, 21, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; West, A.P.; Ghosh, S. NF-κ B and the Immune Response. Oncogene 2006, 25, 6758–6780. [Google Scholar] [CrossRef] [Green Version]
- Gorup, L.F.; Longo, E.; Leite, E.R.; Camargo, E.R. Moderating Effect of Ammonia on Particle Growth and Stability of Quasi-Monodisperse Silver Nanoparticles Synthesized by the Turkevich Method. J. Colloid Interface Sci. 2011, 360, 355–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shukla, K.; Dikshit, P.; Tyagi, M.K.; Shukla, R.; Gambhir, J.K. Ameliorative Effect of Withania Coagulans on Dyslipidemia and Oxidative Stress in Nicotinamide–streptozotocin Induced Diabetes Mellitus. Food Chem. Toxicol. 2012, 50, 3595–3599. [Google Scholar] [CrossRef]
- Robson, M.C.; Hill, D.P.; Woodske, M.E.; Steed, D.L. Wound Healing Trajectories as Predictors of Effectiveness of Therapeutic Agents. Arch. Surg. 2000, 135, 773–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berretta, A.A.; Arruda, C.; Miguel, F.G.; Baptista, N.; Nascimento, A.P.; Marquele-Oliveira, F.; Hori, J.I.; da Silva Barud, H.; Damaso, B.; Ramos, C.; et al. Functional Properties of Brazilian Propolis: From Chemical Composition Until the Market. In Superfood and Functional Food—An Overview of Their Processing and Utilization; IntechOpen: London, UK, 2017; pp. 55–98. [Google Scholar]
- Reddy, G.K.; Kesava Reddy, G.; Enwemeka, C.S. A Simplified Method for the Analysis of Hydroxyproline in Biological Tissues. Clin. Biochem. 1996, 29, 225–229. [Google Scholar] [CrossRef]
- Woessner, J.F., Jr. The Determination of Hydroxyproline in Tissue and Protein Samples Containing Small Proportions of This Imino Acid. Arch. Biochem. Biophys. 1961, 93, 440–447. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scappaticci, R.A.F.; Berretta, A.A.; Torres, E.C.; Buszinski, A.F.M.; Fernandes, G.L.; dos Reis, T.F.; de Souza-Neto, F.N.; Gorup, L.F.; de Camargo, E.R.; Barbosa, D.B. Green and Chemical Silver Nanoparticles and Pomegranate Formulations to Heal Infected Wounds in Diabetic Rats. Antibiotics 2021, 10, 1343. https://doi.org/10.3390/antibiotics10111343
Scappaticci RAF, Berretta AA, Torres EC, Buszinski AFM, Fernandes GL, dos Reis TF, de Souza-Neto FN, Gorup LF, de Camargo ER, Barbosa DB. Green and Chemical Silver Nanoparticles and Pomegranate Formulations to Heal Infected Wounds in Diabetic Rats. Antibiotics. 2021; 10(11):1343. https://doi.org/10.3390/antibiotics10111343
Chicago/Turabian StyleScappaticci, Renan Aparecido Fernandes, Andresa Aparecida Berretta, Elina Cassia Torres, Andrei Felipe Moreira Buszinski, Gabriela Lopes Fernandes, Thaila Fernanda dos Reis, Francisco Nunes de Souza-Neto, Luiz Fernando Gorup, Emerson Rodrigues de Camargo, and Debora Barros Barbosa. 2021. "Green and Chemical Silver Nanoparticles and Pomegranate Formulations to Heal Infected Wounds in Diabetic Rats" Antibiotics 10, no. 11: 1343. https://doi.org/10.3390/antibiotics10111343
APA StyleScappaticci, R. A. F., Berretta, A. A., Torres, E. C., Buszinski, A. F. M., Fernandes, G. L., dos Reis, T. F., de Souza-Neto, F. N., Gorup, L. F., de Camargo, E. R., & Barbosa, D. B. (2021). Green and Chemical Silver Nanoparticles and Pomegranate Formulations to Heal Infected Wounds in Diabetic Rats. Antibiotics, 10(11), 1343. https://doi.org/10.3390/antibiotics10111343








