Systematic Review of Antimicrobial Combination Options for Pandrug-Resistant Acinetobacter baumannii
Abstract
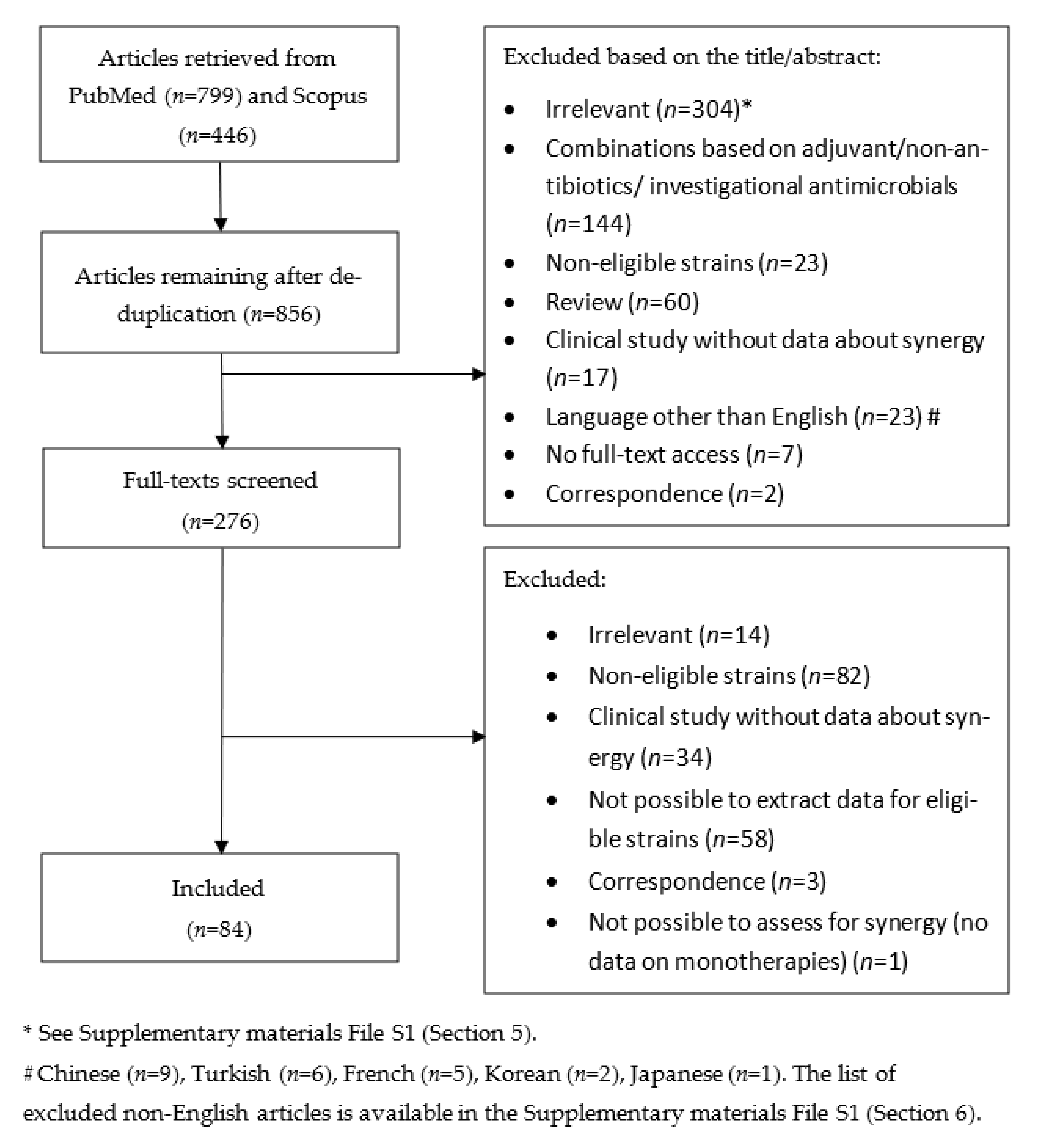
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction
2.4. Definition of Eligible Strains
2.5. Evaluation of In Vivo Feasibility of the Identified Combinations
2.6. Data Synthesis and Analysis
3. Results
3.1. Summary and Characteristics of Reviewed Studies
3.2. Overview of Methods for Assessment of Antimicrobial Combinations
3.3. Overview of Antimicrobial Combinations That have been Evaluated
3.4. Overview of Polymyxin-Based Combinations
3.5. Overview of Non-Polymyxin Based Combinations
3.6. Evaluation of Clinical Relevance of Reported Synergy
3.7. Clinical Studies
4. Discussion
4.1. Summary of Main Findings
4.2. Polymyxin-Based Combinations
4.3. Non-Polymyxin Combinations
4.4. Limitations of the Review and of the Available Evidence
4.5. Strengths of the Review
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Year of Publication | Number of Studies (%) |
|---|---|
| 2017–2021 | 29 (35%) |
| 2021 | 5 (6%) |
| 2020 | 6 (7%) |
| 2019 | 10 (12%) |
| 2018 | 3 (4%) |
| 2017 | 5 (6%) |
| 2012–2016 | 32 (38%) |
| 2016 | 10 (12%) |
| 2015 | 6 (7%) |
| 2014 | 7 (8%) |
| 2013 | 6 (7%) |
| 2012 | 3 (4%) |
| 2007–2011 | 15 (18%) |
| 2011 | 3 (4%) |
| 2010 | 6 (7%) |
| 2009 | 3 (4%) |
| 2008 | 2 (2%) |
| 2007 | 1 (1%) |
| 2002–2006 | 7 (8%) |
| 2005 | 2 (2%) |
| 2004 | 4 (5%) |
| 2003 | 1 (1%) |
| 1995–2001 | 1 (1%) |
| 1996 | 1 (1%) |
| WHO Regions | Number of Studies Per Region (%) |
|---|---|
| Americas | 24 (29%) |
| Brazil | 6 (7%) |
| USA | 12 (14%) |
| Argentina | 3 (4%) |
| Colombia | 1 (1%) |
| Southeast Asia Region | 7 (8%) |
| India | 1 (1%) |
| Thailand | 6 (7%) |
| European Region | 28 (33%) |
| France | 3 (4%) |
| Germany | 1 (1%) |
| Greece | 3 (4%) |
| Italy | 3 (4%) |
| Spain | 7 (8%) |
| Turkey | 7 (8%) |
| Switzerland | 1 (1%) |
| United Kingdom | 1 (1%) |
| Eastern Mediterranean Region | 5 (6%) |
| Iran | 1 (1%) |
| Saudi Arabia | 4 (5%) |
| United Arab Emirates | 2 (2%) |
| Oman | 2 (2%) |
| Kuwait | 2 (2%) |
| Qatar | 2 (2%) |
| Bahrain | 3 (4%) |
| Western Pacific Region | 20 (24%) |
| China | 9 (11%) |
| South Korea | 6 (7%) |
| Taiwan | 3 (3%) |
| Antimicrobial Combinations | CHBD | CHBD: Concentration at which Synergy Was Present | TKA | TKA: Concentration at which Synergy Was Present | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies n | Isolates n | Synergy n (% *) | ≤Breakpoints n (% *) | >Breakpoints n (% *) | Unclear n (% *) | Studies n | Isolates n | Synergy n (% *) | ≤Breakpoints n (% *) | >Breakpoints n (% *) | Unclear n (% *) | |
| SUL-based | ||||||||||||
| SUL/CAZ | 1 | 10 | 7 (70%) | 1 (10%) | 6 (60%) | 0 (0%) | ||||||
| SUL/CIP | 1 | 10 | 8 (80%) | 2 (20%) | 6 (60%) | 0 (0%) | ||||||
| SUL/MEM | 6 | 173 | 72 (42%) | 2 (1%) | 2 (1%) | 68 (39%) | 3 | 54 | 32 (59%) | 0 (0%) | 7 (22%) | 25 (78%) |
| SUL/DOR | 1 | 17 | 4 (24%) | 4 (100%) | 0 (0%) | 0 (0%) | ||||||
| SUL/AVI | 1 | 1 | 1 (100%) | 1 (100%) | 0 (0%) | 0 (0%) | ||||||
| SUL/GEN | 1 | 10 | 8 (80%) | 2 (25%) | 6 (75%) | 0 (0%) | ||||||
| SUL/CST | 1 | 6 | 2 (33%) | 2 (100%) | 0 (0%) | 0 (0%) | ||||||
| SUL/PMB | 1 | 3 | 2 (67%) | 2 (100%) | 0 (0%) | 0 (0%) | ||||||
| SUL/FOF | 2 | 56 | 41 (73%) | 3 (7%) | 37 (90%) | 1 (2%) | 2 | 10 | 7 (70%) | 0 (0%) | 7 (100%) | 0 (0%) |
| SAM-based | ||||||||||||
| SAM/FEP | 1 | 2 | 2 (100%) | 1 (50%) | 1 (50%) | 0 (0%) | ||||||
| SAM/LVX | 1 | 7 | 7 (100%) | 5 (71%) | 2 (29%) | 0 (0%) | ||||||
| SAM/MEM | 2 | 10 | 2 (20%) | 2 (100%) | 0 (0%) | 0 (0%) | 1 | 2 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| SAM/RIF | 1 | 7 | 7 (100%) | 4 (57%) | 1 (14%) | 2 (29%) | ||||||
| IMP-based | ||||||||||||
| IMP/CFS | 1 | 16 | 11 (69%) | 9 (82%) | 2 (18%) | 0 (0%) | ||||||
| IMP/CST | 2 | 10 | 9 (90%) | 2 (20%) | 7 (70%) | 0 (0%) | 1 | 2 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| IMP/RIF | 2 | 28 | 16 (57%) | 11 (39%) | 3 (11%) | 2 (7%) | 5 | 13 | 6 (46%) | 0 (0%) | 6 (46%) | 0 (0%) |
| IMP/FOF | 3 | 45 | 26 (58%) | 9 (20%) | 10 (22%) | 7 (16%) | 1 | 9 | 8 (89%) | 0 (0%) | 8 (89%) | 0 (0%) |
| MEM-based | ||||||||||||
| MEM/SUL | 6 | 173 | 72 (42%) | 2 (1%) | 2 (1%) | 68 (39%) | 3 | 54 | 32 (59%) | 0 (0%) | 7 (13%) | 25 (46%) |
| MEM/SAM | 2 | 10 | 2 (20%) | 2 (20%) | 0 (0%) | 0 (0%) | 1 | 2 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| MEM/AMK | 4 | 47 | 16 34%) | 1 (2%) | 2 (4%) | 13 (28%) | ||||||
| MEM/CST | 6 | 29 | 21 (72%) | 5 (17%) | 11 (3%) | 5 (17%) | 3 | 4 | 4 (100%) | 0 (0%) | 4 (100%) | 0 (0%) |
| MEM/PMB | 1 | 3 | 3 (100%) | 3 (100%) | 0 (0%) | 0 (0%) | 1 | 2 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| MEM/FOF | 4 | 79 | 15 (19%) | 1 (1%) | 14 (18%) | 0 (0%) | ||||||
| MEM/VAN | 1 | 5 | 3 (60%) | 1 (20%) | 0 (0%) | 2 (40%) | ||||||
| DOR-based | ||||||||||||
| DOR/SUL | 1 | 17 | 4 (24%) | 4 (24%) | 0 (0%) | 0 (0%) | ||||||
| DOR/CST | 3 | 6 | 2 (33%) | 1 (17%) | 1 (17%) | 0 (0%) | 5 | 33 | 23 (70%) | 19 (58%) | 4 (12%) | 0 (0%) |
| DOR/TGC | 1 | 3 | 3 (100%) | 1 (33%) | 0 (0%) | 2 (67%) | 1 | 45 | 5 (11%) | 5 (11%) | 0 (0%) | 0 (0%) |
| DOR/RIF | 1 | 1 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 | 5 | 2 (40%) | 1 (20%) | 1 (20%) | 0 (0%) |
| CZA- or AVI-based | ||||||||||||
| AVI/SUL | 1 | 1 | 1 (100%) | 1 (100%) | 0 (0%) | 0 (0%) | ||||||
| CST-based | ||||||||||||
| CST/SUL | 1 | 6 | 2 (33%) | 2 (33%) | 0 (0%) | 0 (0%) | ||||||
| CST/LVX | 2 | 2 | 1 (50%) | 1 (50%) | 0 (0%) | 0 (0%) | 2 | 2 | 1 (50%) | 0 (0%) | 1 (50%) | 0 (0%) |
| CST/IMP | 2 | 10 | 9 (90%) | 2 (20%) | 7 (70%) | 0 (0%) | 1 | 2 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| CST/MEM | 6 | 29 | 21 (72%) | 5 (17%) | 11 (38%) | 5 (17%) | 3 | 4 | 4 (100%) | 0 (0%) | 4 (100%) | 0 (0%) |
| CST/DOR | 3 | 6 | 2 (33%) | 1 (17%) | 1 (17%) | 0 (0%) | 5 | 33 | 23 (70%) | 19 (58%) | 4 (12%) | 0 (0%) |
| CST/TGC | 2 | 10 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 | 12 | 7 (100%) | 7 (100%) | 0 (0%) | 0 (0%) |
| CST/RIF | 5 | 40 | 31 (78%) | 10 (25%) | 10 (25%) | 11 (28%) | 3 | 7 | 7 (100%) | 0 (0%) | 6 (86%) | 1 (14%) |
| CST/SXT | 2 | 8 | 2 (25%) | 1 (13%) | 0 (0%) | 1 (13%) | 1 | 1 | 1 (100%) | 0 (0%) | 1 (100%) | 0 (0%) |
| CST/CHL | 1 | 2 | 2 (100%) | 1 (50%) | 1 (50%) | 0 (0%) | 1 | 2 | 2 (100%) | 0 (0%) | 2 (100%) | 0 (0%) |
| CST/FA | 2 | 6 | 6 (100%) | 1 (17%) | 2 (33%) | 3 (50%) | 2 | 4 | 3 (75%) | 0 (0%) | 3 (75%) | 0 (0%) |
| CST/VAN | 7 | 33 | 29 (88%) | 2 (6%) | 2 (6%) | 25 (67%) | 6 | 20 | 16 (80%) | 13 (65%) | 3 (15%) | 0 (0%) |
| PMB-based | ||||||||||||
| PMB/SUL | 1 | 3 | 2 (67%) | 2 (67%) | 0 (0%) | 0 (0%) | ||||||
| PMB/MEM | 1 | 3 | 3 (100%) | 3 (100%) | 0 (0%) | 0 (0%) | 1 | 2 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| PMB/RIF | 1 | 3 | 3 (100%) | 1 (33%) | 1 (33%) | 1 (33%) | 1 | 3 | 1 (33%) | 1 (33%) | 0 (0%) | 0 (0%) |
| PMB/VAN | 1 | 3 | 3 (100%) | 3 (100%) | 0 (0%) | 0 (0%) | 1 | 3 | 2 (67%) | 0 (0%) | 2 (67%) | 0 (0%) |
| TGC-based | ||||||||||||
| TGC/DOR | 1 | 3 | 3 (100%) | 1 (33%) | 0 (0%) | 2 (67%) | 1 | 45 | 5 (11%) | 5 (11%) | 0 (0%) | 0 (0%) |
| TGC/AMK | 1 | 14 | 2 (14%) | 1 (7%) | 1 (7%) | 0 (0%) | 1 | 1 | 1 (100%) | 1 (100%) | 0 (0%) | 0 (0%) |
| TGC/CST | 2 | 10 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 | 12 | 7 (58%) | 7 (58%) | 0 (0%) | 0 (0%) |
| TGC/RIF | 2 | 16 | 1 (6%) | 0 (0%) | 1 (6%) | 0 (0%) | 2 | 4 | 1 (25%) | 1 (25%) | 0 (0%) | 0 (0%) |
| TGC/FOF | 1 | 4 | 3 (75%) | 1 (25%) | 2 (50%) | 0 (0%) | 1 | 1 | 1 (100%) | 0 (0%) | 1 (100%) | 0 (0%) |
| RIF-based | ||||||||||||
| RIF/SAM | 1 | 7 | 7 (100%) | 4 (57%) | 1 (14%) | 2 (29%) | ||||||
| RIF/CFS | 1 | 7 | 2 (29%) | 1 (14% | 0 (0%) | 1 (14%) | ||||||
| RIF/IMP | 2 | 28 | 16 (57%) | 11 (39%) | 3 (11%) | 2 (7%) | 5 | 13 | 6 (46%) | 0 (0%) | 6 (46%) | 0 (0%) |
| RIF/DOR | 1 | 1 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 | 5 | 2 (40%) | 1 (20%) | 1 (20%) | 0 (0%) |
| RIF/CST | 5 | 40 | 31 (78%) | 10 (25%) | 10 (25%) | 11 (28%) | 3 | 7 | 7 (100%) | 0 (0%) | 6 (85%) | 1 (14%) |
| RIF/PMB | 1 | 3 | 3 (100%) | 1 (33%) | 1 (33%) | 1 (33%) | 1 | 3 | 1 (33%) | 1 (33%) | 0 (0%) | 0 (0%) |
| RIF/TGC | 2 | 16 | 1 (100%) | 0 (0%) | 1 (100%) | 0 (0%) | 2 | 4 | 1 (25%) | 1 (25%) | 0 (0%) | 0 (0%) |
| FOF-based | ||||||||||||
| FOF/SUL | 2 | 56 | 41 (73%) | 3 (5%) | 37 (66%) | 1 (2%) | 2 | 10 | 7 (70%) | 0 (0%) | 7 (70%) | 0 (0%) |
| FOF/IMP | 3 | 45 | 26 (58%) | 9 (20%) | 10 (22%) | 7 (16%0 | 1 | 9 | 8 (89%) | 0 (0%) | 8 (89%) | 0 (0%) |
| FOF/MEM | 4 | 79 | 15 (19%) | 1 (1%) | 14 (18%) | 0 (0%) | ||||||
| FOF/AMK | 2 | 29 | 26 (90%) | 11 (38%) | 15 (52%) | 0 (0%) | ||||||
| FOF/GEN | 2 | 13 | 12 (92%) | 3 (32%) | 9 (69%) | 0 (0%) | ||||||
| FOF/TGC | 1 | 4 | 3 (75%) | 1 (25%) | 2 (50%) | 0 (0%) | 1 | 1 | 1 (100%) | 0 (0%) | 1 (100%) | 0 (0%) |
| Triple combinations | ||||||||||||
| PMB/FOF/MEM | 1 | 3 | 3 (100%) | 3 (100%) | 0 (0%) | 0 (0%) | ||||||
| PMB/SUL/MEM | 1 | 3 | 3 (100%) | 3 (100%) | 0 (0%) | 0 (0%) | ||||||
| CST/DOR/SUL | 1 | 6 | 6 (100%) | 6 (100%) | 0 (0%) | 0 (0%) | ||||||
| CST/VAN/DOR | 1 | 3 | 3 (100%) | 3 (100%) | 0 (0%) | 0 (0%) | ||||||
| Study-Combinations | Method | Synergy % (n/N) | Comments |
|---|---|---|---|
| Lenhard, J.R., 2017 [61,62] | |||
| PMB/MEM | HFIM | 0 (0/1) | Doses simulating human regimens were used (PMB 3.33 mg/kg then 1.43 mg/kg every 12 h, MEM 2 gr every 8 h as 3 h infusions, SAM 8/4 g every 8 h as 3 h infusions). |
| PMB/SAM | 0 (0/1) | ||
| MEM/SAM | 0 (0/1) | ||
| PMB/MEM/SAM | 100 (1/1) | ||
| Yuan, Z., 2010 [102] and Lim, T.P., 2008 [107] | |||
| AMK/LVX | HFIM | 0 (0/1) | Regrowth despite initial killing at 4 h. |
| AMK/FEP | 0 (0/1) | Regrowth despite initial killing at 4 h. | |
| Córdoba, J., 2015 [73] | |||
| CST/IMP | Other dynamic in vitro PK/PD model | 0 (0/1) | Simulation of human treatment regimens |
| CST/DAP | 100 (1/1) | ||
| IMP/ETP | 0 (0/3) | ||
| RIF/CFS | 0 (0/7) | ||
| Housman, S.T., 2013 [87] | Simulated regimens: SAM 9 g q8 h (3 h inf), DOR 2 gr q8 h (4 h inf), TGC 200 mg q12 h (0.5 h inf). | ||
| TGC/DOR | Other dynamic in vitro PK/PD model | 0 (0/2) | |
| SAM/DOR | 0 (0/3) | Increased killing with SAM/DOR vs. monotherapies against all 3 strains but with regrowth by 24 h. | |
| SAM/TGC | 0 (0/1) | ||
| Lee, H.J., 2013 [88] | |||
| CST/RIF | Other dynamic in vitro PK/PD model | 100 (1/1) | Regimens mimicking human serum concentration after usual doses in critically-ill patients. |
| Study-Combinations | Method | Synergy % (n/N) | Comments |
|---|---|---|---|
| Cebrero-Cangueiro, T., 2021 [38] | |||
| MEM/IMP | Intraperitoneal infection mouse model | 0 (0/2) | Decreased bacterial loads with combination vs. monotherapy, but similar mortality and bacterial clearance comparing meropenem monotherapy to combination therapy. |
| Poulakou, G., 2019 [55] | |||
| CST/DAP | Intraperitoneal infection mouse model | 100 (1/1) | The combination significantly improved survival and reduced bacterial loads in tissues compared to monotherapies. |
| Wei, W., 2017 [64] | |||
| CST/LVX | G. mellonella model | 0 (0/1) | Same survival comparing combination therapy to monotherapy |
| Yang, H., 2016 [70] | |||
| CST/VAN | G. mellonella model | 100 (1/1) | Survival rate in G. mellonella model higher with combination, but high survival even with monotherapies. |
| O’Hara, J.A., 2013 [35] | |||
| CST/DOR | G. mellonella model | 0 (0/3) | No synergy |
| CST/VAN | 0 (0/3) | ||
| DOR/VAN | 100 (3/3) | The clinical relevance of the G. mellonella model is unclear because of mechanisms of action likely not relevant to humans; high survival even with DOR and VAN monotherapies, and high survival with DOR/VAN despite lack of in vitro synergy | |
| CST/VAN/DOR | 100 (3/3) | ||
| Queenan, A.M., 2013 [90] | |||
| DOR/CIP | intraperitoneal infection mouse model | 0 (0/1) | No synergy |
| DOR/LVX | 100 (1/1) | Improved survival in the mouse model (the isolate had relatively low MICs: DOR 16 mg/L and LVX 8 mg/L). | |
| Pachón-Ibáñez, M.E., 2011 [94] | |||
| RIF/IMP | Pneumonia mouse model | 0 (0/2) | In the animal model survival with RIF/IMP (80 and 33%) and RIF/SUL (60 and 53%) did not differ significantly compared to RIF monotherapy (73 and 40%). Lung clearance and blood culture sterilization was higher against one of the two strains with RIF/SUL. |
| RIF/SUL | 50 (1/2) | ||
| Pachón-Ibáñez, M.E., 2010 [98] | |||
| SUL/IMP | Pneumonia (mouse) and meningitis (rabbit) models | 100 (1/1) | Higher survival and bacterial clearance in animal model compared to monotherapies. |
| RIF/IMP | 0 (0/1) | Survival not improved comparing RIF monotherapy (71%) to combination therapy (60%), despite improved bacterial clearance. | |
| RIF/SUL | 0 (0/1) | Survival not improved comparing RIF monotherapy (71%) to combination therapy (47%), despite improved bacterial clearance. | |
| Yuan, Z., 2010 [102] | |||
| AMK/LVX | Pneumonia mouse model | 0 (0/1) | Similar survival with AMK monotherapy. |
| AMK/FEP | 1 (1/1) | Improved survival and reduction of tissue bacterial burden in the mouse model. | |
| FEP/LVX | 0 (0/1) | Similar survival with FEP monotherapy. | |
| Song, Y.C., 2009 [105] | |||
| IMP/RIF | Pneumonia mouse model | 100 (3/3) | Synergistic (≥2Δlog reduction in lung baterial loads compared to RIF monotherapy) against all 3 strains, but 100% survival with both monotherapy and combination. |
| RIF/AMK | 0 (0/1) | Not better than monotherapy | |
| IMP/AMK | 0 (0/1) | Not better than monotherapy | |
| Montero, A., 2004 [113] | |||
| IMP/RIF | Pneumonia mouse model | 50 (1/2) | Strain D: no differences compared to monotherapy in the mouse model. Strain E: significantly reduced lung bacterial counts, no significant reduction of bacteremia, similar survival (100% with the combination, 100% with RIF monotherapy). |
References
- Karakonstantis, S.; Kritsotakis, E.I.; Gikas, A. Pandrug-resistant Gram-negative bacteria: A systematic review of current epidemiology, prognosis and treatment options. J. Antimicrob. Chemother. 2019, 75, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Karakonstantis, S.; Gikas, A.; Astrinaki, E.; Kritsotakis, E.I. Excess mortality due to pandrug-resistant Acinetobacter baumannii infections in hospitalized patients. J. Hosp. Infect. 2020, 106, 447–453. [Google Scholar] [CrossRef] [PubMed]
- McCreary, E.K.; Heil, E.L.; Tamma, P.D. New Perspectives on Antimicrobial Agents: Cefiderocol. Antimicrob. Agents Chemother. 2021, 65, e0217120. [Google Scholar] [CrossRef] [PubMed]
- Choby, J.E.; Ozturk, T.; Satola, S.W.; Jacob, J.T.; Weiss, D.S. Widespread cefiderocol heteroresistance in carbapenem-resistant Gram-negative pathogens. Lancet Infect. Dis. 2021, 21, 597–598. [Google Scholar] [CrossRef]
- Karakonstantis, S.; Saridakis, I. Colistin heteroresistance in Acinetobacter spp.: Systematic review and meta-analysis of the prevalence and discussion of the mechanisms and potential therapeutic implications. Int. J. Antimicrob. Agents 2020, 56, 106065. [Google Scholar] [CrossRef]
- Karakonstantis, S.; Kritsotakis, E.I.; Gikas, A. Treatment options for K. pneumoniae, P. aeruginosa and A. baumannii co-resistant to carbapenems, aminoglycosides, polymyxins and tigecycline: An approach based on the mechanisms of resistance to carbapenems. Infection 2020, 48, 835–851. [Google Scholar] [CrossRef]
- Wang, J.; Niu, H.; Wang, R.; Cai, Y. Safety and efficacy of colistin alone or in combination in adults with Acinetobacter baumannii infection: A systematic review and meta-analysis. Int. J. Antimicrob. Agents 2019, 53, 383–400. [Google Scholar] [CrossRef]
- Salameh, M.; Daher, L.M.A.; Chartouny, M.; Hanna, P.A. Colistin monotherapy v/s colistin combination therapy for treatment of Acinetobacter infections, a systematic review. J. Infect. Dev. Ctries. 2018, 12, 23S. [Google Scholar] [CrossRef] [Green Version]
- Paul, M.; Daikos, G.L.; Durante-Mangoni, E.; Yahav, D.; Carmeli, Y.; Benattar, Y.D.; Skiada, A.; Andini, R.; Eliakim-Raz, N.; Nutman, A.; et al. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: An open-label, randomised controlled trial. Lancet Infect. Dis. 2018, 18, 391–400. [Google Scholar] [CrossRef]
- Dickstein, Y.; Lellouche, J.; Amar, M.B.D.; Schwartz, D.; Nutman, A.; Daitch, V.; Yahav, D.; Leibovici, L.; Skiada, A.; Antoniadou, A.; et al. Treatment Outcomes of Colistin- and Carbapenem-resistant Acinetobacter baumannii Infections: An Exploratory Subgroup Analysis of a Randomized Clinical Trial. Clin. Infect. Dis. 2018, 69, 769–776. [Google Scholar] [CrossRef]
- Poulikakos, P.; Tansarli, G.S.; Falagas, M.E. Combination antibiotic treatment versus monotherapy for multidrug-resistant, extensively drug-resistant, and pandrug-resistant Acinetobacter infections: A systematic review. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1675–1685. [Google Scholar] [CrossRef]
- Bae, S.; Kim, M.-C.; Park, S.-J.; Kim, H.S.; Sung, H.; Kim, S.-H.; Lee, S.-O.; Choi, S.-H.; Woo, J.H.; Kim, Y.S.; et al. In Vitro Synergistic Activity of Antimicrobial Agents in Combination against Clinical Isolates of Colistin-Resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2016, 60, 6774–6779. [Google Scholar] [CrossRef] [Green Version]
- Karakonstantis, S. Re: ‘Colistin plus meropenem for carbapenem-resistant Gram-negative infections: In vitro synergism is not associated with better clinical outcomes’ by Nutman et al. Clin. Microbiol. Infect. 2020, 26, 1274. [Google Scholar] [CrossRef]
- Mohammadi, M.; Khayat, H.; Sayehmiri, K.; Soroush, S.; Sayehmiri, F.; Delfani, S.; Bogdanovic, L.; Taherikalani, M. Synergistic Effect of Colistin and Rifampin Against Multidrug Resistant Acinetobacter baumannii: A Systematic Review and Meta-Analysis. Open Microbiol. J. 2017, 11, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Ni, W.; Shao, X.; Di, X.; Cui, J.; Wang, R.; Liu, Y. In vitro synergy of polymyxins with other antibiotics for Acinetobacter baumannii: A systematic review and meta-analysis. Int. J. Antimicrob. Agents 2015, 45, 8–18. [Google Scholar] [CrossRef]
- Scudeller, L.; Righi, E.; Chiamenti, M.; Bragantini, D.; Menchinelli, G.; Cattaneo, P.; Giske, C.G.; Lodise, T.; Sanguinetti, M.; Piddock, L.J.; et al. Systematic review and meta-analysis of in vitro efficacy of antibiotic combination therapy against carbapenem-resistant Gram-negative bacilli. Int. J. Antimicrob. Agents 2021, 57, 106344. [Google Scholar] [CrossRef]
- Jiang, Z.; He, X.; Li, J. Synergy effect of meropenem-based combinations against Acinetobacter baumannii: A systematic review and meta-analysis. Infect. Drug Resist. 2018, 11, 1083–1095. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Yang, X.; Chen, L.; Duan, X.; Jiang, Z. In Vitro Activity of Various Antibiotics in Combination with Tigecycline Against Acinetobacter baumannii: A Systematic Review and Meta-Analysis. Microb. Drug Resist. 2017, 23, 982–993. [Google Scholar] [CrossRef]
- Brennan-Krohn, T.; Kirby, J.E. When One Drug Is Not Enough: Context, Methodology, and Future Prospects in Antibacterial Synergy Testing. Clin. Lab. Med. 2019, 39, 345–358. [Google Scholar] [CrossRef]
- Pillai, S.K.; Moellering, R.C.; Eliopoulos, G.M. Antimicrobial combinations. In Antibiotics in Laboratory Medicine, 5th ed.; Lorian, V., Ed.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2005. [Google Scholar]
- Morrison, A.; Polisena, J.; Husereau, D.; Moulton, K.; Clark, M.; Fiander, M.; Mierzwinski-Urban, M.; Clifford, T.; Hutton, B.; Rabb, D. The effect of English-language restriction on systematic review-based meta-analyses: A systematic review of empirical studies. Int. J. Technol. Assess. Health Care 2012, 28, 138–144. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A.E. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Available online: www.training.cochrane.org/handbook (accessed on 3 November 2021).
- Balk, E.M.; Chung, M.; Chen, M.L.; Chang, L.K.W.; Trikalinos, T.A. Data extraction from machine-translated versus original language randomized trial reports: A comparative study. Syst. Rev. 2013, 2, 97. [Google Scholar] [CrossRef] [Green Version]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Bonapace, C.R.; Bosso, J.A.; Friedrich, L.V.; White, R.L. Comparison of methods of interpretation of checkerboard synergy testing. Diagn. Microbiol. Infect. Dis. 2002, 44, 363–366. [Google Scholar] [CrossRef]
- Brill, M.; Kristoffersson, A.; Zhao, C.; Nielsen, E.; Friberg, L. Semi-mechanistic pharmacokinetic–pharmacodynamic modelling of antibiotic drug combinations. Clin. Microbiol. Infect. 2018, 24, 697–706. [Google Scholar] [CrossRef] [Green Version]
- Doern, C.D. When Does 2 Plus 2 Equal 5? A Review of Antimicrobial Synergy Testing. J. Clin. Microbiol. 2014, 52, 4124–4128. [Google Scholar] [CrossRef] [Green Version]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 31st ed.; CLSI Supplement M100; Clinical Laboratory Standards Institute: Wayne, PA, USA, 2021. [Google Scholar]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 11.0. Available online: http://www.eucast.org/ (accessed on 3 November 2021).
- Sader, H.S.; Carvalhaes, C.; Streit, J.M.; Castanheira, M.; Flamm, R.K. Antimicrobial activity of cefoperazone-sulbactam tested against Gram-Negative organisms from Europe, Asia-Pacific, and Latin America. Int. J. Infect. Dis. 2020, 91, 32–37. [Google Scholar] [CrossRef] [Green Version]
- FDA. Plazomicin Infection. FDA-Identified Interpretive Criteria. Available online: https://www.fda.gov/drugs/development-resources/plazomicin-injection (accessed on 23 June 2021).
- Lepe, J.A.; García-Cabrera, E.; Gil-Navarro, M.V.; Aznar, J. Rifampin breakpoint for Acinetobacter baumannii based on pharmacokinetic-pharmacodynamic models with Monte Carlo simulation. Rev. Esp. Quimioter. Publ. Soc. Esp. Quimioter. 2012, 25, 134–138. [Google Scholar]
- Food and Drug Administration (FDA). Tigecycline–Injection Products 2019. Available online: https://www.fda.gov/drugs/development-resources/tigecycline-injection-products (accessed on 26 June 2019).
- Gordon, N.; Png, K.; Wareham, D.W. Potent Synergy and Sustained Bactericidal Activity of a Vancomycin-Colistin Combination versus Multidrug-Resistant Strains of Acinetobacter baumannii. Antimicrob. Agents Chemother. 2010, 54, 5316–5322. [Google Scholar] [CrossRef] [Green Version]
- O’Hara, J.A.; Ambe, L.A.; Casella, L.G.; Townsend, B.M.; Pelletier, M.R.; Ernst, R.K.; Shanks, R.M.Q.; Doi, Y. Activities of Vancomycin-Containing Regimens against Colistin-Resistant Acinetobacter baumannii Clinical Strains. Antimicrob. Agents Chemother. 2013, 57, 2103–2108. [Google Scholar] [CrossRef] [Green Version]
- Bowler, S.L.; Spychala, C.N.; McElheny, C.L.; Mettus, R.T.; Doi, Y. In Vitro Activity of Fusidic Acid-Containing Combinations against Carbapenem-Resistant Acinetobacter baumannii Clinical Strains. Antimicrob. Agents Chemother. 2016, 60, 5101. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.M.S.; Heffernan, A.J.; Roberts, J.A.; Sime, F.B. Semimechanistic Pharmacokinetic/Pharmacodynamic Modeling of Fosfomycin and Sulbactam Combination against Carbapenem-Resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2021, 65, e02472-20. [Google Scholar] [CrossRef]
- Cebrero-Cangueiro, T.; Nordmann, P.; Carretero-Ledesma, M.; Pachón, J.; Pachón-Ibáñez, M.E. Efficacy of dual carbapenem treatment in a murine sepsis model of infection due to carbapenemase-producing Acinetobacter baumannii. J. Antimicrob. Chemother. 2021, 76, 680–683. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Yan, J.; Reyna, Z.; Slarve, M.; Lu, P.; Spellberg, B.; Luna, B. Synergistic Rifabutin and Colistin Reduce Emergence of Resistance When Treating Acinetobacter baumannii. Antimicrob. Agents Chemother. 2021, 65, e02204-20. [Google Scholar] [CrossRef] [PubMed]
- Terbtothakun, P.; Voravuthikunchai, S.; Chusri, S. Evaluation of the Synergistic Antibacterial Effects of Fosfomycin in Combination with Selected Antibiotics against Carbapenem–Resistant Acinetobacter baumannii. Pharmaceuticals 2021, 14, 185. [Google Scholar] [CrossRef]
- Armengol, E.; Asunción, T.; Viñas, M.; Sierra, J.M. When Combined with Colistin, an Otherwise Ineffective Rifampicin–Linezolid Combination Becomes Active in Escherichia coli, Pseudomonas aeruginosa, and Acinetobacter baumannii. Microorganisms 2020, 8, 86. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Fu, Y.; Zhang, J.; Zhao, Y.; Fan, X.; Yu, L.; Wang, Y.; Zhang, X.; Li, C. The efficacy of colistin monotherapy versus combination therapy with other antimicrobials against carbapenem-resistant Acinetobacter baumannii ST2 isolates. J. Chemother. 2020, 32, 359–367. [Google Scholar] [CrossRef]
- Limsrivanichakorn, S.; Ngamskulrungroj, P.; Leelaporn, A. Activity of Antimicrobial Combinations Against Extensively Drug-Resistant Acinetobacter baumannii as Determined by Checkerboard Method and E-test. Siriraj Med. J. 2020, 72, 214–218. [Google Scholar] [CrossRef]
- Lim, S.M.S.; Naicker, S.; Ayfan, A.; Zowawi, H.; Roberts, J.; Sime, F. Non-polymyxin-based combinations as potential alternatives in treatment against carbapenem-resistant Acinetobacter baumannii infections. Int. J. Antimicrob. Agents 2020, 56, 106115. [Google Scholar] [CrossRef]
- Lim, S.M.S.; Heffernan, A.J.; Roberts, J.A.; Sime, F.B. Pharmacodynamic Analysis of Meropenem and Fosfomycin Combination Against Carbapenem-Resistant Acinetobacter baumannii in Patients with Normal Renal Clearance: Can It Be a Treatment Option? Microb. Drug Resist. 2021, 27, 546–552. [Google Scholar] [CrossRef]
- Nordmann, P.; Perler, J.; Kieffer, N.; Poirel, L. In-vitro evaluation of a dual carbapenem combination against carbapenemase-producing Acinetobacter baumannii. J. Infect. 2020, 80, 121–142. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, C.H.; Brune, A.; Nastro, M.; Vay, C.; Famiglietti, A. In vitro synergistic activity of the sulbactam/avibactam combination against extensively drug-resistant Acinetobacter baumannii. J. Med. Microbiol. 2020, 69, 928–931. [Google Scholar] [CrossRef]
- Gaudereto, J.J.; Neto, L.V.P.; Leite, G.C.; Martins, R.R.; Prado, G.V.B.D.; Rossi, F.; Guimarães, T.; Levin, A.S.; Costa, S.F. Synergistic Effect of Ceftazidime-Avibactam with Meropenem against Panresistant, Carbapenemase-Harboring Acinetobacter baumannii and Serratia marcescens Investigated Using Time-Kill and Disk Approximation Assays. Antimicrob. Agents Chemother. 2019, 63, e02367-18. [Google Scholar] [CrossRef] [Green Version]
- Ghaith, D.; Hassan, R.; Dawoud, M.E.E.-D.; Eweis, M.; Metwally, R.; Zafer, M. Effect of rifampicin–colistin combination against XDR Acinetobacter baumannii harbouring blaOXA 23-like gene and showed reduced susceptibility to colistin at Cairo University Hospital, Cairo, Egypt. Infect. Dis. 2019, 51, 308–311. [Google Scholar] [CrossRef]
- Kara, E.M.; Yılmaz, M.; Çelik, B. In vitro activities of ceftazidime/avibactam alone or in combination with antibiotics against multidrug-resistant Acinetobacter baumannii isolates. J. Glob. Antimicrob. Resist. 2019, 17, 137–141. [Google Scholar] [CrossRef]
- Menegucci, T.C.; Fedrigo, N.H.; Lodi, F.G.; Albiero, J.; Nishiyama, S.A.B.; Mazucheli, J.; Carrara-Marroni, F.E.; Voelkner, N.M.F.; Gong, H.; Sy, S.; et al. Pharmacodynamic Effects of Sulbactam/Meropenem/Polymyxin-B Combination Against Extremely Drug Resistant Acinetobacter baumannii Using Checkerboard Information. Microb. Drug Resist. 2019, 25, 1266–1274. [Google Scholar] [CrossRef]
- Oliva, A.; Garzoli, S.; De Angelis, M.; Marzuillo, C.; Vullo, V.; Mastroianni, C.M.; Ragno, R. In-Vitro Evaluation of Different Antimicrobial Combinations with and without Colistin Against Carbapenem-Resistant Acinetobacter baumannii. Molecules 2019, 24, 886. [Google Scholar] [CrossRef] [Green Version]
- Ozger, H.S.; Cuhadar, T.; Yildiz, S.S.; Gulmez, Z.D.; Dizbay, M.; Tunccan, O.G.; Kalkanci, A.; Simsek, H.; Unaldi, O. In vitro activity of eravacycline in combination with colistin against carbapenem-resistant A. baumannii isolates. J. Antibiot. 2019, 72, 600–604. [Google Scholar] [CrossRef]
- Phee, L.M.; Kloprogge, F.; Morris, R.; Barrett, J.; Wareham, D.W.; Standing, J.F. Pharmacokinetic-pharmacodynamic modelling to investigate in vitro synergy between colistin and fusidic acid against MDR Acinetobacter baumannii. J. Antimicrob. Chemother. 2019, 74, 961–969. [Google Scholar] [CrossRef]
- Poulakou, G.; Renieris, G.; Sabrakos, L.; Zarkotou, O.; Themeli-Digalaki, K.; Perivolioti, E.; Kraniotaki, E.; Giamarellos-Bourboulis, E.J.; Zavras, N. Daptomycin as adjunctive treatment for experimental infection by Acinetobacter baumannii with resistance to colistin. Int. J. Antimicrob. Agents 2018, 53, 190–194. [Google Scholar] [CrossRef]
- Shinohara, D.R.; Menegucci, T.C.; Fedrigo, N.H.; Migliorini, L.B.; Carrara-Marroni, F.E.; Anjos, M.; Cardoso, C.L.; Nishiyama, S.A.B.; Tognim, M.C.B. Synergistic activity of polymyxin B combined with vancomycin against carbapenem-resistant and polymyxin-resistant Acinetobacter baumannii: First in vitro study. J. Med. Microbiol. 2019, 68, 309–315. [Google Scholar] [CrossRef]
- Wang, J.; Ning, Y.; Li, S.; Wang, Y.; Liang, J.; Jin, C.; Yan, H.; Huang, Y. Multidrug-resistant Acinetobacter baumannii strains with NDM-1: Molecular characterization and in vitro efficacy of meropenem-based combinations. Exp. Ther. Med. 2019, 18, 2924–2932. [Google Scholar] [CrossRef]
- Chen, F.; Wang, L.; Wang, M.; Xie, Y.; Xia, X.; Li, X.; Liu, Y.; Cao, W.; Zhang, T.; Li, P.; et al. Genetic characterization and in vitro activity of antimicrobial combinations of multidrug-resistant Acinetobacter baumannii from a general hospital in China. Oncol. Lett. 2018, 15, 2305–2315. [Google Scholar] [CrossRef]
- Singkham-In, U.; Chatsuwan, T. In vitro activities of carbapenems in combination with amikacin, colistin, or fosfomycin against carbapenem-resistant Acinetobacter baumannii clinical isolates. Diagn. Microbiol. Infect. Dis. 2018, 91, 169–174. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, Y.; Cao, W.; Cao, S.; Zhang, J. In vitro evaluation of antimicrobial combinations against imipenem-resistant Acinetobacter baumannii of different MICs. J. Infect. Public Health 2018, 11, 856–860. [Google Scholar] [CrossRef]
- Lenhard, J.R.; Thamlikitkul, V.; Silveira, F.P.; Garonzik, S.M.; Tao, X.; Forrest, A.; Shin, B.S.; Kaye, K.S.; Bulitta, J.B.; Nation, R.L.; et al. Polymyxin-resistant, carbapenem-resistant Acinetobacter baumannii is eradicated by a triple combination of agents that lack individual activity. J. Antimicrob. Chemother. 2017, 72, 1415–1420. [Google Scholar] [CrossRef] [Green Version]
- Lenhard, J.; Smith, N.M.; Bulman, Z.P.; Tao, X.; Thamlikitkul, V.; Shin, B.S.; Nation, R.L.; Li, J.; Bulitta, J.B.; Tsuji, B.T. High-Dose Ampicillin-Sulbactam Combinations Combat Polymyxin-Resistant Acinetobacter baumannii in a Hollow-Fiber Infection Model. Antimicrob. Agents Chemother. 2017, 61, e01268-16. [Google Scholar] [CrossRef] [Green Version]
- Madadi-Goli, N.; Moniri, R.; Bagheri-Josheghani, S.; Dasteh-Goli, N. Sensitivity of levofloxacin in combination with ampicillin-sulbactam and tigecycline against multidrug-resistant Acinetobacter baumannii. Iran. J. Microbiol. 2017, 9, 19–25. [Google Scholar]
- Wei, W.; Yang, H.; Hu, L.; Ye, Y.; Li, J. Activity of levofloxacin in combination with colistin against Acinetobacter baumannii: In vitro and in a Galleria mellonella model. J. Microbiol. Immunol. Infect. 2017, 50, 821–830. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.-J.; Yang, H.-F. Synergy against extensively drug-resistant Acinetobacter baumannii in vitro by two old antibiotics: Colistin and chloramphenicol. Int. J. Antimicrob. Agents 2017, 49, 321–326. [Google Scholar] [CrossRef]
- Hong, D.J.; Kim, J.O.; Lee, H.; Yoon, E.-J.; Jeong, S.H.; Yong, D.; Lee, K. In vitro antimicrobial synergy of colistin with rifampicin and carbapenems against colistin-resistant Acinetobacter baumannii clinical isolates. Diagn. Microbiol. Infect. Dis. 2016, 86, 184–189. [Google Scholar] [CrossRef]
- Laishram, S.; Anandan, S.; Devi, B.Y.; Elakkiya, M.; Priyanka, B.; Bhuvaneshwari, T.; Peter, J.V.; Subramani, K.; Balaji, V. Determination of synergy between sulbactam, meropenem and colistin in carbapenem-resistant Klebsiella pneumoniae and Acinetobacter baumannii isolates and correlation with the molecular mechanism of resistance. J. Chemother. 2016, 28, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Leite, G.C.; Oliveira, M.S.; Perdigão-Neto, L.V.; Rocha, C.K.D.; Guimarães, T.; Rizek, C.; Levin, A.; Costa, S.F. Antimicrobial Combinations against Pan-Resistant Acinetobacter baumannii Isolates with Different Resistance Mechanisms. PLoS ONE 2016, 11, e0151270. [Google Scholar] [CrossRef] [PubMed]
- Menegucci, T.C.; Albiero, J.; Migliorini, L.B.; Alves, J.L.B.; Viana, G.F.; Mazucheli, J.; Carrara-Marroni, F.E.; Cardoso, C.L.; Tognim, M.C.B. Strategies for the treatment of polymyxin B-resistant Acinetobacter baumannii infections. Int. J. Antimicrob. Agents 2016, 47, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Lv, N.; Hu, L.; Liu, Y.; Cheng, J.; Ye, Y.; Li, J. In vivoactivity of vancomycin combined with colistin against multidrug-resistant strains of Acinetobacter baumannii in aGalleriamellonellamodel. Infect. Dis. 2016, 48, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-S.; Lee, Y.; Tseng, K.-C.; Huang, W.-C.; Chuang, M.-F.; Kuo, S.-C.; Lauderdale, T.-L.Y.; Chen, T.-L. In Vivo and In Vitro Efficacy of Minocycline-Based Combination Therapy for Minocycline-Resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2016, 60, 4047–4054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yavaş, S.; Yetkin, M.A.; Kayaaslan, B.; Baştuğ, A.; Aslaner, H.; But, A.; Kanyilmaz, D.; Sari, B.; Akinci, E.; Bodur, H. Investigating the in vitro synergistic activities of several antibiotic combinationsagainst carbapenem-resistant Acinetobacter baumannii isolates. Turk. J. Med. Sci. 2016, 46, 892–896. [Google Scholar] [CrossRef] [PubMed]
- Córdoba, J.; Coronado-Álvarez, N.M.; Parra, D.; Parra-Ruiz, J. In Vitro Activities of Novel Antimicrobial Combinations against Extensively Drug-Resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2015, 59, 7316–7319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Salguero, C.; Rodríguez-Avial, I.; Picazo, J.J.; Culebras, E. Can Plazomicin Alone or in Combination Be a Therapeutic Option against Carbapenem-Resistant Acinetobacter baumannii? Antimicrob. Agents Chemother. 2015, 59, 5959–5966. [Google Scholar] [CrossRef] [Green Version]
- Marie, M.A.M.; Krishnappa, L.G.; Alzahrani, A.J.; Mubaraki, M.A.; Alyousef, A.A. A prospective evaluation of synergistic effect of sulbactam and tazobactam combination with meropenem or colistin against multidrug resistant Acinetobacter baumannii. Bosn. J. Basic Med. Sci. 2015, 15, 24–29. [Google Scholar] [CrossRef] [Green Version]
- Phee, L.M.; Betts, J.; Bharathan, B.; Wareham, D.W. Colistin and Fusidic Acid, a Novel Potent Synergistic Combination for Treatment of Multidrug-Resistant Acinetobacter baumannii Infections. Antimicrob. Agents Chemother. 2015, 59, 4544–4550. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, C.H.; Nastro, M.; Vay, C.; Famiglietti, A. In vitro activity of minocycline alone or in combination in multidrug-resistant Acinetobacter baumannii isolates. J. Med. Microbiol. 2015, 64, 1196–1200. [Google Scholar] [CrossRef] [Green Version]
- Vourli, S.; Frantzeskaki, F.; Meletiadis, J.; Stournara, L.; Armaganidis, A.; Zerva, L.; Dimopoulos, G. Synergistic interactions between colistin and meropenem against extensively drug-resistant and pandrug-resistant Acinetobacter baumannii isolated from ICU patients. Int. J. Antimicrob. Agents 2015, 45, 670–671. [Google Scholar] [CrossRef]
- Galani, I.; Orlandou, K.; Moraitou, H.; Petrikkos, G.; Souli, M. Colistin/daptomycin: An unconventional antimicrobial combination synergistic in vitro against multidrug-resistant Acinetobacter baumannii. Int. J. Antimicrob. Agents 2014, 43, 370–374. [Google Scholar] [CrossRef]
- Majewski, P.; Wieczorek, P.; Ojdana, D.; Sacha, P.; Wieczorek, A.; Tryniszewska, E. In vitro activity of rifampicin alone and in combination with imipenem against multidrug-resistant Acinetobacter baumannii harboring theblaOXA-72resistance gene. Scand. J. Infect. Dis. 2014, 46, 260–264. [Google Scholar] [CrossRef]
- Nastro, M.; Rodríguez, C.H.; Monge, R.; Zintgraff, J.; Neira, L.; Rebollo, M.; Vay, C.; Famiglietti, A. Activity of the colistin–rifampicin combination against colistin-resistant, carbapenemase-producing Gram-negative bacteria. J. Chemother. 2014, 26, 211–216. [Google Scholar] [CrossRef]
- Oleksiuk, L.M.; Nguyen, M.H.; Press, E.G.; Updike, C.L.; O’Hara, J.A.; Doi, Y.; Clancy, C.J.; Shields, R.K. In VitroResponses of Acinetobacter baumannii to Two- and Three-Drug Combinations following Exposure to Colistin and Doripenem. Antimicrob. Agents Chemother. 2014, 58, 1195–1199. [Google Scholar] [CrossRef] [Green Version]
- Percin, D.; Akyol, S.; Kalin, G. In vitro synergism of combinations of colistin with selected antibiotics against colistin-resistant Acinetobacter baumannii. GMS Hyg. Infect. Control 2014, 9, Doc14. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, L.; Li, J.; Zhao, C.; Zhao, J.; Liu, M.; Wang, S.; Lu, C.; Shang, G.; Jia, Y.; et al. Synergistic efficacy of meropenem and rifampicin in a murine model of sepsis caused by multidrug-resistant Acinetobacter baumannii. Eur. J. Pharmacol. 2014, 729, 116–122. [Google Scholar] [CrossRef]
- Wang, Y.; Bao, W.; Guo, N.; Chen, H.; Cheng, W.; Jin, K.; Shen, F.; Xu, J.; Zhang, Q.; Wang, C.; et al. Antimicrobial activity of the imipenem/rifampicin combination against clinical isolates of Acinetobacter baumannii grown in planktonic and biofilm cultures. World J. Microbiol. Biotechnol. 2014, 30, 3015–3025. [Google Scholar] [CrossRef]
- Cetin, E.S.; Tekeli, A.; Ozseven, A.G.; Us, E.; Aridogan, B.C. Determination of In Vitro Activities of Polymyxin B and Rifampin in Combination with Ampicillin/Sulbactam or Cefoperazone/Sulbactam against Multidrug-Resistant Acinetobacter baumannii by the E-test and Checkerboard Methods. Jpn. J. Infect. Dis. 2013, 66, 463–468. [Google Scholar] [CrossRef] [Green Version]
- Housman, S.T.; Hagihara, M.; Nicolau, D.P.; Kuti, J.L. In vitro pharmacodynamics of human-simulated exposures of ampicillin/sulbactam, doripenem and tigecycline alone and in combination against multidrug-resistant Acinetobacter baumannii. J. Antimicrob. Chemother. 2013, 68, 2296–2304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.J.; Bergen, P.J.; Bulitta, J.; Tsuji, B.; Forrest, A.; Nation, R.L.; Li, J. Synergistic Activity of Colistin and Rifampin Combination against Multidrug-Resistant Acinetobacter baumannii in anIn VitroPharmacokinetic/Pharmacodynamic Model. Antimicrob. Agents Chemother. 2013, 57, 3738–3745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Principe, L.; Capone, A.; Mazzarelli, A.; D’Arezzo, S.; Bordi, E.; Di Caro, A.; Petrosillo, N. In Vitro Activity of Doripenem in Combination with Various Antimicrobials Against Multidrug-Resistant Acinetobacter baumannii: Possible Options for the Treatment of Complicated Infection. Microb. Drug Resist. 2013, 19, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Queenan, A.M.; A Davies, T.; He, W.; Lynch, A.S. Assessment of the combination of doripenem plus a fluoroquinolone against non-susceptible Acinetobacter baumannii isolates from nosocomial pneumonia patients. J. Chemother. 2013, 25, 141–147. [Google Scholar] [CrossRef]
- Deveci, A.; Coban, A.Y.; Acicbe, O.; Tanyel, E.; Yaman, G.; Durupinar, B. In vitro effects of sulbactam combinations with different antibiotic groups against clinical Acinetobacter baumannii isolates. J. Chemother. 2012, 24, 247–252. [Google Scholar] [CrossRef]
- Peck, K.R.; Kim, M.J.; Choi, J.Y.; Kim, H.S.; Kang, C.-I.; Cho, Y.K.; Park, D.W.; Lee, H.J.; Lee, M.S.; Ko, K.S. In vitro time-kill studies of antimicrobial agents against blood isolates of imipenem-resistant Acinetobacter baumannii, including colistin- or tigecycline-resistant isolates. J. Med. Microbiol. 2012, 61, 353–360. [Google Scholar] [CrossRef]
- Vidaillac, C.; Benichou, L.; Duval, R. In VitroSynergy of Colistin Combinations against Colistin-Resistant Acinetobacter baumannii, Pseudomonas aeruginosa, and Klebsiella pneumoniae Isolates. Antimicrob. Agents Chemother. 2012, 56, 4856–4861. [Google Scholar] [CrossRef] [Green Version]
- Pachón-Ibáñez, M.E.; Docobo-Pérez, F.; Jiménez-Mejías, M.E.; Ibáñez-Martínez, J.; García-Curiel, A.; Pichardo, C.; Pachón, J. Efficacy of rifampin, in monotherapy and in combinations, in an experimental murine pneumonia model caused by panresistant Acinetobacter baumannii strains. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 895–901. [Google Scholar] [CrossRef] [Green Version]
- Santimaleeworagun, W.; Wongpoowarak, P.; Chayakul, P.; Pattharachayakul, S.; Tansakul, P.; Garey, K.W. In vitro activity of colistin or sulbactam in combination with fosfomycin or imipenem against clinical isolates of carbapenem-resistant Acinetobacter baumannii producing OXA-23 carbapenemases. Southeast Asian J. Trop. Med. Public Health 2011, 42, 890–900. [Google Scholar]
- Tan, T.Y.; Lim, T.P.; Lee, W.H.L.; Sasikala, S.; Hsu, L.Y.; Kwa, A.L.-H. In VitroAntibiotic Synergy in Extensively Drug-Resistant Acinetobacter baumannii: The Effect of Testing by Time-Kill, Checkerboard, and Etest Methods. Antimicrob. Agents Chemother. 2011, 55, 436–438. [Google Scholar] [CrossRef] [Green Version]
- Kiratisin, P.; Apisarnthanarak, A.; Kaewdaeng, S. Synergistic activities between carbapenems and other antimicrobial agents against Acinetobacter baumannii including multidrug-resistant and extensively drug-resistant isolates. Int. J. Antimicrob. Agents 2010, 36, 243–246. [Google Scholar] [CrossRef]
- Pachón-Ibáñez, M.E.; Docobo-Pérez, F.; López-Rojas, R.; Domínguez-Herrera, J.; Jiménez-Mejías, M.E.; García-Curiel, A.; Pichardo, C.; Jiménez, L.; Pachón, J. Efficacy of Rifampin and Its Combinations with Imipenem, Sulbactam, and Colistin in Experimental Models of Infection Caused by Imipenem-Resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2010, 54, 1165–1172. [Google Scholar] [CrossRef] [Green Version]
- Pankuch, G.A.; Seifert, H.; Appelbaum, P.C. Activity of doripenem with and without levofloxacin, amikacin, and colistin against Pseudomonas aeruginosa and Acinetobacter baumannii. Diagn. Microbiol. Infect. Dis. 2010, 67, 191–197. [Google Scholar] [CrossRef]
- Rodriguez, C.H.; De Ambrosio, A.; Bajuk, M.; Spinozzi, M.; Nastro, M.; Bombicino, K.; Radice, M.; Gutkind, G.; Vay, C.; Famiglietti, A. In vitro antimicrobials activity against endemic Acinetobacter baumannii multiresistant clones. J. Infect. Dev. Ctries. 2010, 4, 164–167. [Google Scholar] [CrossRef] [Green Version]
- Urban, C.; Mariano, N.; Rahal, J.J. In Vitro Double and Triple Bactericidal Activities of Doripenem, Polymyxin B, and Rifampin against Multidrug-Resistant Acinetobacter baumannii, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Escherichia coli. Antimicrob. Agents Chemother. 2010, 54, 2732–2734. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Z.; Ledesma, K.R.; Singh, R.; Hou, J.; Prince, R.A.; Tam, V.H. Quantitative Assessment of Combination Antimicrobial Therapy against Multidrug-Resistant Bacteria in a Murine Pneumonia Model. J. Infect. Dis. 2010, 201, 889–897. [Google Scholar] [CrossRef]
- Lim, T.-P.; Tan, T.-Y.; Lee, W.; Sasikala, S.; Tan, T.-T.; Hsu, L.-Y.; Kwa, A.L. In vitro activity of various combinations of antimicrobials against carbapenem-resistant Acinetobacter species in Singapore. J. Antibiot. 2009, 62, 675–679. [Google Scholar] [CrossRef] [Green Version]
- Principe, L.; D’Arezzo, S.; Capone, A.; Petrosillo, N.; Visca, P. In vitro activity of tigecycline in combination with various antimicrobials against multidrug resistant Acinetobacter baumannii. Ann. Clin. Microbiol. Antimicrob. 2009, 8, 18. [Google Scholar] [CrossRef] [Green Version]
- Song, J.Y.; Cheong, H.J.; Lee, J.; Sung, A.K.; Kim, W.J. Efficacy of monotherapy and combined antibiotic therapy for carbapenem-resistant Acinetobacter baumannii pneumonia in an immunosuppressed mouse model. Int. J. Antimicrob. Agents 2009, 33, 33–39. [Google Scholar] [CrossRef]
- Lee, C.-H.; Tang, Y.-F.; Su, L.-H.; Chien, C.-C.; Liu, J.-W. Antimicrobial Effects of Varied Combinations of Meropenem, Sulbactam, and Colistin on a Multidrug-Resistant Acinetobacter baumannii Isolate That Caused Meningitis and Bacteremia. Microb. Drug Resist. 2008, 14, 233–237. [Google Scholar] [CrossRef] [Green Version]
- Lim, T.-P.; Ledesma, K.R.; Chang, K.-T.; Hou, J.-G.; Kwa, A.L.; Nikolaou, M.; Quinn, J.P.; Prince, R.A.; Tam, V.H. Quantitative Assessment of Combination Antimicrobial Therapy against Multidrug-Resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2008, 52, 2898–2904. [Google Scholar] [CrossRef] [Green Version]
- Lee, N.-Y.; Wang, C.-L.; Chuang, Y.-C.; Yu, W.-L.; Lee, H.-C.; Chang, C.-M.; Wang, L.-R.; Ko, W.-C. Combination Carbapenem-Sulbactam Therapy for Critically Ill Patients with Multidrug-Resistant Acinetobacter baumannii Bacteremia: Four Case Reports and an In Vitro Combination Synergy Study. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2007, 27, 1506–1511. [Google Scholar] [CrossRef]
- Sader, H.S.; Rhomberg, P.; Jones, R.N. In Vitro Activity of β-Lactam Antimicrobial Agents in Combination with Aztreonam Tested Against Metallob-β-Lactamase-Producing Pseudomonas aeruginosa and Acinetobacter baumannii. J. Chemother. 2005, 17, 622–627. [Google Scholar] [CrossRef]
- Sader, H.S.; Jones, R.N. Comprehensive in vitro evaluation of cefepime combined with aztreonam or ampicillin/sulbactam against multi-drug resistant Pseudomonas aeruginosa and Acinetobacter spp. Int. J. Antimicrob. Agents 2005, 25, 380–384. [Google Scholar] [CrossRef]
- Choi, J.Y.; Park, Y.S.; Cho, C.H.; Shin, S.Y.; Song, Y.G.; Yong, D.; Lee, K.; Kim, J.M. Synergic in-vitro activity of imipenem and sulbactam against Acinetobacter baumannii. Clin. Microbiol. Infect. 2004, 10, 1098–1101. [Google Scholar] [CrossRef] [Green Version]
- Jung, R.; Husain, M.; Choi, M.K.; Fish, D.N. Synergistic Activities of Moxifloxacin Combined with Piperacillin-Tazobactam or Cefepime against Klebsiella pneumoniae, Enterobacter cloacae, and Acinetobacter baumannii Clinical Isolates. Antimicrob. Agents Chemother. 2004, 48, 1055–1057. [Google Scholar] [CrossRef] [Green Version]
- Montero, A.; Ariza, J.; Corbella, X.; Doménech, A.; Cabellos, C.; Ayats, J.; Tubau, F.; Borraz, C.; Gudiol, F. Antibiotic combinations for serious infections caused by carbapenem-resistant Acinetobacter baumannii in a mouse pneumonia model. J. Antimicrob. Chemother. 2004, 54, 1085–1091. [Google Scholar] [CrossRef]
- Yoon, J.; Urban, C.; Terzian, C.; Mariano, N.; Rahal, J.J. In Vitro Double and Triple Synergistic Activities of Polymyxin B, Imipenem, and Rifampin against Multidrug-Resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2004, 48, 753–757. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Cuenca, F.; Martínez, L.M.; Pascual, A.; Perea, E.J. In vitro Activity of Azithromycin in Combination with Amikacin, Ceftazidime, Ciprofloxacin or Imipenem against Clinical Isolates of Acinetobacter baumannii. Chemotherapy 2003, 49, 24–26. [Google Scholar] [CrossRef]
- Roussel-Delvallez, M.; Wallet, F.; Delpierre, F.; Courcol, R. In Vitro Bactericidal Effect of a β-lactam + Aminoglycoside Combination Against Multiresistant Pseudomonas aeruginosa and Acinetobacter baumannii. J. Chemother. 1996, 8, 365–368. [Google Scholar] [CrossRef]
- Park, G.C.; Choi, J.A.; Jang, S.J.; Jeong, S.H.; Kim, C.-M.; Choi, I.S.; Kang, S.H.; Park, G.; Moon, D.S. In Vitro Interactions of Antibiotic Combinations of Colistin, Tigecycline, and Doripenem Against Extensively Drug-Resistant and Multidrug-Resistant Acinetobacter baumannii. Ann. Lab. Med. 2016, 36, 124–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.M.S.; Heffernan, A.J.; Zowawi, H.M.; Roberts, J.A.; Sime, F.B. Semi-mechanistic PK/PD modelling of meropenem and sulbactam combination against carbapenem-resistant strains of Acinetobacter baumannii. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 1943–1952. [Google Scholar] [CrossRef]
- Jaruratanasirikul, S.; Wongpoowarak, W.; Wattanavijitkul, T.; Sukarnjanaset, W.; Samaeng, M.; Nawakitrangsan, M.; Ingviya, N. Population Pharmacokinetics and Pharmacodynamics Modeling to Optimize Dosage Regimens of Sulbactam in Critically Ill Patients with Severe Sepsis Caused by Acinetobacter baumannii. Antimicrob. Agents Chemother. 2016, 60, 7236–7244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaruratanasirikul, S.; Nitchot, W.; Wongpoowarak, W.; Samaeng, M.; Nawakitrangsan, M. Population pharmacokinetics and Monte Carlo simulations of sulbactam to optimize dosage regimens in patients with ventilator-associated pneumonia caused by Acinetobacter baumannii. Eur. J. Pharm. Sci. 2019, 136, 104940. [Google Scholar] [CrossRef] [PubMed]
- Asuphon, O.; Montakantikul, P.; Houngsaitong, J.; Kiratisin, P.; Sonthisombat, P. Optimizing intravenous fosfomycin dosing in combination with carbapenems for treatment of Pseudomonas aeruginosa infections in critically ill patients based on pharmacokinetic/pharmacodynamic (PK/PD) simulation. Int. J. Infect. Dis. 2016, 50, 23–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pasteran, F.; Cedano, J.; Baez, M.; Albornoz, E.; Rapoport, M.; Osteria, J.; Montaña, S.; Le, C.; Ra, G.; Bonomo, R.; et al. A New Twist: The Combination of Sulbactam/Avibactam Enhances Sulbactam Activity against Carbapenem-Resistant Acinetobacter baumannii (CRAB) Isolates. Antibiotics 2021, 10, 577. [Google Scholar] [CrossRef] [PubMed]
- Savoldi, A.; Carrara, E.; Piddock, L.J.V.; Franceschi, F.; Ellis, S.; Chiamenti, M.; Bragantini, D.; Righi, E.; Tacconelli, E. The role of combination therapy in the treatment of severe infections caused by carbapenem resistant gram-negatives: A systematic review of clinical studies. BMC Infect. Dis. 2021, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kofteridis, D.P.; Andrianaki, A.M.; Maraki, S.; Mathioudaki, A.; Plataki, M.; Alexopoulou, C.; Ioannou, P.; Samonis, G.; Valachis, A. Treatment pattern, prognostic factors, and outcome in patients with infection due to pan-drug-resistant gram-negative bacteria. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 965–970. [Google Scholar] [CrossRef]
- Assimakopoulos, S.F.; Karamouzos, V.; Lefkaditi, A.; Sklavou, C.; Kolonitsiou, F.; Christofidou, M.; Fligou, F.; Gogos, C.; Marangos, M. Triple combination therapy with high-dose ampicillin/sulbactam, high-dose tigecycline and colistin in the treatment of ventilator-associated pneumonia caused by pan-drug resistant Acinetobacter baumannii: A case series study. Infez. Med. 2019, 27, 11–16. [Google Scholar]
- Qureshi, Z.A.; Hittle, L.E.; O’Hara, J.A.; Rivera, J.I.; Syed, A.; Shields, R.K.; Pasculle, A.W.; Ernst, R.; Doi, Y. Colistin-Resistant Acinetobacter baumannii: Beyond Carbapenem Resistance. Clin. Infect. Dis. 2015, 60, 1295–1303. [Google Scholar] [CrossRef] [Green Version]
- Park, H.J.; Cho, J.H.; Kim, H.J.; Han, S.H.; Jeong, S.H.; Byun, M.K. Colistin monotherapy versus colistin/rifampicin combination therapy in pneumonia caused by colistin-resistant Acinetobacter baumannii: A randomised controlled trial. J. Glob. Antimicrob. Resist. 2019, 17, 66–71. [Google Scholar] [CrossRef]
- Hernan, R.C.; Karina, B.; Gabriela, G.; Marcela, N.; Carlos, V.; Angela, F. Selection of colistin-resistant Acinetobacter baumannii isolates in postneurosurgical meningitis in an intensive care unit with high presence of heteroresistance to colistin. Diagn. Microbiol. Infect. Dis. 2009, 65, 188–191. [Google Scholar] [CrossRef]
- Cai, Y.; Chai, D.; Wang, R.; Liang, B.; Bai, N. Colistin resistance of Acinetobacter baumannii: Clinical reports, mechanisms and antimicrobial strategies. J. Antimicrob. Chemother. 2012, 67, 1607–1615. [Google Scholar] [CrossRef]
- Luna, B.; Trebosc, V.; Lee, B.; Bakowski, M.; Ulhaq, A.; Yan, J.; Lu, P.; Cheng, J.; Nielsen, T.; Lim, J.; et al. A nutrient-limited screen unmasks rifabutin hyperactivity for extensively drug-resistant Acinetobacter baumannii. Nat. Microbiol. 2020, 5, 1134–1143. [Google Scholar] [CrossRef]
- Trebosc, V.; Schellhorn, B.; Schill, J.; Lucchini, V.; Bühler, J.; Bourotte, M.; Butcher, J.J.; Gitzinger, M.; Lociuro, S.; Kemmer, C.; et al. In vitro activity of rifabutin against 293 contemporary carbapenem-resistant Acinetobacter baumannii clinical isolates and characterization of rifabutin mode of action and resistance mechanisms. J. Antimicrob. Chemother. 2020, 75, 3552–3562. [Google Scholar] [CrossRef]
- Fan, B.; Guan, J.; Wang, X.; Cong, Y. Activity of Colistin in Combination with Meropenem, Tigecycline, Fosfomycin, Fusidic Acid, Rifampin or Sulbactam against Extensively Drug-Resistant Acinetobacter baumannii in a Murine Thigh-Infection Model. PLoS ONE 2016, 11, e0157757. [Google Scholar] [CrossRef]
- Elsayed, E.; Elarabi, M.A.; Sherif, D.A.; Elmorshedi, M.; El-Mashad, N. Extensive drug resistant Acinetobacter baumannii: A comparative study between non-colistin based combinations. Int. J. Clin. Pharm. 2020, 42, 80–88. [Google Scholar] [CrossRef]
- Xie, J.; Roberts, J.A.; Alobaid, A.S.; Roger, C.; Wang, Y.; Yang, Q.; Sun, J.; Dong, H.; Wang, X.; Xing, J.; et al. Population Pharmacokinetics of Tigecycline in Critically Ill Patients with Severe Infections. Antimicrob. Agents Chemother. 2017, 61, e00345-17. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Wu, Y.; Cao, L.; Yao, D.; Long, M. Is Meropenem as a Monotherapy Truly Incompetent for Meropenem-Nonsusceptible Bacterial Strains? A Pharmacokinetic/Pharmacodynamic Modeling With Monte Carlo Simulation. Front. Microbiol. 2019, 10, 2777. [Google Scholar] [CrossRef] [Green Version]
- Van Wart, S.A.; Andes, D.; Ambrose, P.G.; Bhavnani, S.M. Pharmacokinetic–pharmacodynamic modeling to support doripenem dose regimen optimization for critically ill patients. Diagn. Microbiol. Infect. Dis. 2009, 63, 409–414. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karakonstantis, S.; Ioannou, P.; Samonis, G.; Kofteridis, D.P. Systematic Review of Antimicrobial Combination Options for Pandrug-Resistant Acinetobacter baumannii. Antibiotics 2021, 10, 1344. https://doi.org/10.3390/antibiotics10111344
Karakonstantis S, Ioannou P, Samonis G, Kofteridis DP. Systematic Review of Antimicrobial Combination Options for Pandrug-Resistant Acinetobacter baumannii. Antibiotics. 2021; 10(11):1344. https://doi.org/10.3390/antibiotics10111344
Chicago/Turabian StyleKarakonstantis, Stamatis, Petros Ioannou, George Samonis, and Diamantis P. Kofteridis. 2021. "Systematic Review of Antimicrobial Combination Options for Pandrug-Resistant Acinetobacter baumannii" Antibiotics 10, no. 11: 1344. https://doi.org/10.3390/antibiotics10111344
APA StyleKarakonstantis, S., Ioannou, P., Samonis, G., & Kofteridis, D. P. (2021). Systematic Review of Antimicrobial Combination Options for Pandrug-Resistant Acinetobacter baumannii. Antibiotics, 10(11), 1344. https://doi.org/10.3390/antibiotics10111344







