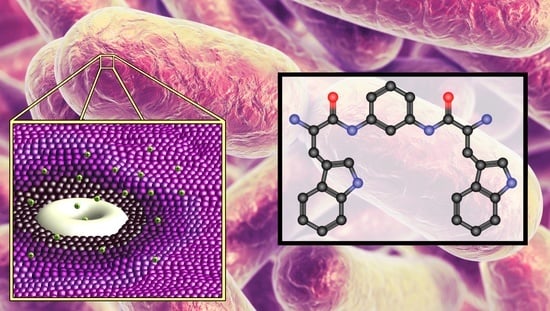
Bis(Tryptophan) Amphiphiles Form Ion Conducting Pores and Enhance Antimicrobial Activity against Resistant Bacteria
Abstract
:1. Introduction
2. Results and Discussion
2.1. Channel and Biological Activity
2.2. Membrane Permeability
2.3. Checkerboard Experiments
2.4. Amino Acid Derivatives
2.5. Synthesis and Characterization
2.6. Bacterial Strains Used
2.7. Antimicrobial Activity
2.8. In-Depth MIC Screen
3. Experimental Section
3.1. Planar Bilayer Conductance
3.2. Fluorescence Assays
3.3. Determination of Minimum Inhibitory Concentrations (MICs)
3.4. Compound Preparation
General Procedures
- Procedure 1. Coupling with HBTU
- Procedure 2. Boc Deprotection with HCl/Dioxane
- Procedure 3. Boc Deprotection with TFA
- Procedure 4. Cbz Deprotection
- Procedure 5. RO-t-Bu Deprotection with TFA
- Procedure 6. RO-CH2Ph (RO-Bn) Deprotection
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CDC. Antibiotic Resistance Threats in the United States 2019; Department of Health and Human Services: Washington, DC, USA, 2019; p. 150.
- Antibiotic/Antimicrobial Resistance (AR/AMR). Available online: https://www.cdc.gov/drugresistance/index.html (accessed on 17 July 2021).
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report Early Implementation 2020; World Health Organization: New York, NY, USA, 2020; p. 134. [Google Scholar]
- World Health Organization. Antimicrobial Resistance Global Report on Surveillance; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- Shlaes, D.M. Antibiotics: The Perfect Storm; Springer: New York, NY, USA, 2010; 106p. [Google Scholar]
- Ling, L.L.; Schneider, T.; Peoples, A.J.; Spoering, A.L.; Engels, I.; Conlon, B.P.; Mueller, A.; Schäberle, T.F.; Hughes, D.E.; Epstein, S.; et al. A new antibiotic kills pathogens without detectable resistance. Nature 2015, 517, 455–459. [Google Scholar] [CrossRef]
- WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. Available online: https://www.who.int/news-room/detail/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 31 May 2021).
- Purdy Drew, K.R.; Sanders, L.K.; Culumber, Z.W.; Zribi, O.; Wong, G.C. Cationic amphiphiles increase activity of aminoglycoside antibiotic tobramycin in the presence of airway polyelectrolytes. J. Am. Chem. Soc. 2009, 131, 486–493. [Google Scholar] [CrossRef]
- Bruno, M.J.; Rusinova, R.; Gleason, N.J.; Koeppe, R.E., II; Andersen, O.S. Interactions of drugs and amphiphiles with membranes: Modulation of lipid bilayer elastic properties by changes in acyl chain unsaturation and protonation. Faraday Discuss. 2013, 161, 461–480. [Google Scholar] [CrossRef] [Green Version]
- Dalton, C.J.; Haxell, M.A.; McArthur, H.A.I.; Wax, R. (Eds.) Peptide Antibiotics: Discovery, Modes of Action, and Applications; Marcel Dekker: New York, NY, USA, 2002; 296p. [Google Scholar]
- Cheah, S.E.; Li, J.; Tsuji, B.T.; Forrest, A.; Bulitta, J.B.; Nation, R.L. Colistin and Polymyxin B Dosage Regimens against Acinetobacter baumannii: Differences in Activity and the Emergence of Resistance. Antimicrob. Agents Chemother. 2016, 60, 3921–3933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karaiskos, I.; Souli, M.; Galani, I.; Giamarellou, H. Colistin: Still a lifesaver for the 21st century? Expert Opin. Drug Metab. Toxicol. 2016, 13, 59–71. [Google Scholar] [CrossRef]
- Yahav, D.; Farbman, L.; Leibovici, L.; Paul, M. Colistin: New lessons on an old antibiotic. Clin. Microbiol. Infect. 2012, 18, 18–29. [Google Scholar] [CrossRef] [Green Version]
- Biswas, S.; Brunel, J.M.; Dubus, J.C.; Reynaud-Gaubert, M.; Rolain, J.M. Colistin: An update on the antibiotic of the 21st century. Expert Rev. Anti. Infect. Ther. 2012, 10, 917–934. [Google Scholar] [CrossRef]
- Kumar, M.; Saha, S.; Subudhi, E. More Furious Than Ever: Escherichia coli-Acquired Co-resistance toward Colistin and Carbapenems. Clin. Infect. Dis. 2016, 63, 1267–1268. [Google Scholar]
- Nicolet, S.; Goldenberger, D.; Schwede, T.; Page, M.; Creus, M. Plasmid-mediated colistin resistance in a patient infected with Klebsiella pneumoniae. Lancet Infect. Dis. 2016, 16, 998–999. [Google Scholar] [CrossRef]
- Kadar, B.; Kocsis, B.; Toth, A.; Kristof, K.; Felso, P.; Kocsis, B.; Boddi, K.; Szabo, D. Colistin resistance associated with outer membrane protein change in Klebsiella pneumoniae and Enterobacter asburiae. Acta Microbiol. Immunol. Hung. 2017, 64, 217–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jasim, R.; Baker, M.A.; Zhu, Y.; Han, M.; Schneider-Futschik, E.K.; Hussein, M.; Hoyer, D.; Li, J.; Velkov, T. A Comparative Study of Outer Membrane Proteome between Paired Colistin-Susceptible and Extremely Colistin-Resistant Klebsiella pneumoniae Strains. ACS Infect. Dis. 2018, 4, 1692–1704. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, M.; Chen, Y.; Bian, X.; Li, Y.; Shi, J.; Zhang, J. Synergistic killing by meropenem and colistin combination of carbapenem-resistant Acinetobacter baumannii isolates from Chinese patients in an in vitro pharmacokinetic/pharmacodynamic model. Int. J. Antimicrob. Agents 2016, 48, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Albur, M.S.; Noel, A.; Bowker, K.; MacGowan, A. The combination of colistin and fosfomycin is synergistic against NDM-1-producing Enterobacteriaceae in in vitro pharmacokinetic/pharmacodynamic model experiments. Int. J. Antimicrob. Agents 2015, 46, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Naghmouchi, K.; Baah, J.; Hober, D.; Jouy, E.; Rubrecht, C.; Sane, F.; Drider, D. Synergistic effect between colistin and bacteriocins in controlling Gram-negative pathogens and their potential to reduce antibiotic toxicity in mammalian epithelial cells. Antimicrob. Agents Chemother. 2013, 57, 2719–2725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falagas, M.E.; Rafailidis, P.I. Nephrotoxicity of colistin: New insight into an old antibiotic. Clin. Infect. Dis. 2009, 48, 1729–1731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dudhani, R.V.; Nation, R.L.; Li, J. Evaluating the stability of colistin and colistin methanesulphonate in human plasma under different conditions of storage. J Antimicrob. Chemother. 2010, 65, 1412–1415. [Google Scholar] [CrossRef] [Green Version]
- Gokel, G.W.; Dishong, D.M.; Diamond, C.J. Lariat Ethers. Synthesis and Cation Binding of Macrocyclic Polyethers Possessing Axially Disposed Secondary Donor Groups. J.C.S. Chem. Commun. 1980, 22, 1053–1054. [Google Scholar]
- Gokel, G.W.; Echegoyen, L. Advances in Bio-Organic Frontiers; Dugas, H., Ed.; Springer: Berlin, Germany, 1990; Volume 1, p. 116. [Google Scholar]
- Gokel, G.W.; Schall, O.F. Lariat Ethers. In Comprehensive Supramolecular Chemistry; Gokel, G.W., Ed.; Pergamon: Oxford, UK, 1996; Volume 1, p. 97. [Google Scholar]
- Gokel, G.W. Hydraphiles: Design, Synthesis, and Analysis of a Family of Synthetic, Cation-Conducting Channels. Chem. Commun. 2000, 1, 1–9. [Google Scholar] [CrossRef]
- Gandour, R.D.; Fronczek, F.R.; Gatto, V.J.; Minganti, C.; Schultz, R.A.; White, B.D.; Arnold, K.A.; Mazzocchi, D.; Miller, S.R.; Gokel, G.W. Solid-State Structural Chemistry of Lariat Ether and BiBLE Cation Complexes: Metal Ion Identity and Coordination Number Determine Cavity Size. J. Am. Chem. Soc. 1986, 108, 4078–4088. [Google Scholar] [CrossRef]
- Hernandez, J.C.; Trafton, J.E.; Gokel, G.W. A direct comparison of extraction and homogeneous binding constants as predictors of efficacy in alkali metal cation transport. Tetrahedron Lett. 1991, 32, 6269–6272. [Google Scholar] [CrossRef]
- Negin, S.; Patel, M.B.; Gokel, M.R.; Meisel, J.W.; Gokel, G.W. Antibiotic Potency against E. coli Is Enhanced by Channel-Forming Alkyl Lariat Ethers. ChemBioChem 2016, 17, 2153–2161. [Google Scholar] [CrossRef] [PubMed]
- Gokel, G.W.; Negin, S. Synthetic Membrane Active Amphiphiles. Adv. Drug Deliv. Rev. 2012, 64, 784–796. [Google Scholar] [CrossRef] [PubMed]
- Atkins, J.L.; Patel, M.B.; Cusumano, Z.; Gokel, G.W. Enhancement of antimicrobial activity by synthetic ion channel synergy. Chem. Commun. 2010, 46, 8166–8167. [Google Scholar] [CrossRef]
- Gokel, G.W.; Gokel, M.R.; Negin, S.; Patel, M.B. Molecules That Inhibit Efflux Pumps in Multi-Drug Resistant Bacteria and Uses Thereof. U.S. Patent 10,463,044 B2, 5 November 2019. [Google Scholar]
- Meisel, J.W.; Patel, M.B.; Garrad, E.; Gokel, G.W. Reversal of Tetracycline Resistance in Escherichia coli by Non-cytotoxic bis(Tryptophan)s. J. Am. Chem. Soc. 2016, 138, 10571–10577. [Google Scholar] [CrossRef]
- de Planque, M.R.; Bonev, B.B.; Demmers, J.A.; Greathouse, D.V.; Koeppe, R.E., II; Separovic, F.; Watts, A.; Killian, J.A. Interfacial anchor properties of tryptophan residues in transmembrane peptides can dominate over hydrophobic matching effects in peptide-lipid interactions. Biochemistry 2003, 42, 5341–5348. [Google Scholar] [CrossRef]
- de Planque, M.R.R.; Kruijtzer, J.A.W.; Liskamp, R.M.J.; Marsh, D.; Greathouse, D.V.; Koeppe, R.E., II; De Kruijff, B.; Killian, J.A. Different membrane anchoring positions of tryptophan and lysine in synthetic transmembrane α-helical peptides. J. Biol. Chem. 1999, 274, 20839–20846. [Google Scholar] [CrossRef] [Green Version]
- Wallace, B.A. Gramicidin channels and pores. Annu. Rev. Biophys. 1990, 19, 127–157. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Lee, K.-C.; Cross, T.A. Tryptophans in membrane proteins: Indole ring orientations and functional implications in the gramicidin channel. Biochemistry 1993, 32, 7035–7047. [Google Scholar] [CrossRef]
- Doyle, D.A.; Cabral, J.M.; Pfuetzner, R.A.; Kuo, A.; Gulbis, J.M.; Cohen, S.L.; Chait, B.T.; MacKinnon, R. The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science 1998, 280, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Abel, E.; Fedders, M.F.; Gokel, G.W. Vesicle formation from N-alkylindoles: Implications for tryptophan-water interactions. J. Am. Chem. Soc. 1995, 117, 1265–1270. [Google Scholar] [CrossRef]
- Weiss, T.M.; van der Wel, P.C.; Killian, J.A.; Koeppe, R.E., II; Huang, H.W. Hydrophobic mismatch between helices and lipid bilayers. Biophys. J. 2003, 84, 379–385. [Google Scholar] [CrossRef] [Green Version]
- Wimley, W.C. Describing the mechanism of antimicrobial peptide action with the interfacial activity model. ACS Chem. Biol. 2010, 5, 905–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakmann, B.; Neher, E. Single-Channel Recording; Kluwer Academic Publishers: New York, NY, USA, 1995; 700p. [Google Scholar]
- Yamamoto, T.; Umegawa, Y.; Yamagami, M.; Suzuki, T.; Tsuchikawa, H.; Hanashima, S.; Matsumori, N.; Murata, M. The Perpendicular Orientation of Amphotericin B Methyl Ester in Hydrated Lipid Bilayers Supports the Barrel-Stave Model. Biochemistry 2019, 58, 2282–2291. [Google Scholar] [CrossRef]
- Cantor, R.S. Size distribution of barrel-stave aggregates of membrane peptides: Influence of the bilayer lateral pressure profile. Biophys. J. 2002, 82, 2520–2525. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Harroun, T.A.; Weiss, T.M.; Ding, L.; Huang, H.W. Barrel-stave model or toroidal model? A case study on melittin pores. Biophys. J. 2001, 81, 1475–1485. [Google Scholar] [CrossRef] [Green Version]
- Providence, L.L.; Andersen, O.S.; Greathouse, D.V.; Koeppe, R.E., II; Bittman, R. Gramicidin channel function does not depend on phospholipid chirality. Biochemistry 1995, 34, 16404–16411. [Google Scholar] [CrossRef] [PubMed]
- Ohki, S.; Arnold, K. Surface dielectric constant, surface hydrophobicity, and membrane fusion. J. Membr. Biol. 1990, 114, 195–203. [Google Scholar] [CrossRef]
- Tallarida, R.J. Drug Synergism and Dose-Effect Data Analysis; Chapman & Hall: Boca Raton, FL, USA, 2000; 267p. [Google Scholar]
- Chou, T.-C. Drug combination studies and their synergy quantification using the Chou-Talalay Method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [Green Version]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimic. Chemother. 2001, 48 (Suppl. S1), 5–16. [Google Scholar] [CrossRef] [Green Version]
- MIC Clinical and Laboratory Standards Institute: M07-A9, Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 9th ed.; Approved Standard; CLSI: Wayne, PA, USA, 2012; ISBN 1-56238-784-7. Available online: www.clsi.org (accessed on 1 November 2021).
- Gokel, G.W.; Barbour, L.J.; Ferdani, R.; Hu, J. Lariat ether receptor systems show experimental evidence for alkali metal cation-pi interactions. Acc. Chem. Res. 2002, 35, 878–886. [Google Scholar] [CrossRef]
- Gokel, G.W. The aromatic sidechains of amino acids as neutral donor groups for alkali metal cations. Chem. Commun. 2003, 23, 2847–2852. [Google Scholar] [CrossRef] [PubMed]









| Cmpd | Spacer (Aaa) | S. aureus 1199B | TetR E. coli |
|---|---|---|---|
| 1 | meta-C6H4(L-Trp)2 | 128 | 48 |
| 2 | meta-C6H4(D-Trp)2 | 16 | 28 |
| 3 | ortho-C6H4(L-Trp)2 | 64 | 56 |
| 4 | para-C6H4(L-Trp)2 | 64 | 120 |
| 5 | (CH2)4(L-Trp)2 | 128 | >128 |
| 6 | (CH2)12(L-Trp)2 | 4 | 10 |
| 7 | meta-C6H4(L-Phe)2 | >128 | >128 |
| 8 | meta-C6H4(L-Tyr)2 | >128 | >128 |
| 9 | meta-C6H4(L-Leu)2 | >128 | >128 |
| 10 | meta-C6H4(L-Ala)2 | >128 | >128 |
| 11 | meta-C6H4(L-Pro)2 | >128 | >128 |
| 12 | meta-C6H4(L-Thr)2 | >128 | >128 |
| 13 | meta-C6H4(L-Lys)2 | >128 | >128 |
| (control) | Tetracycline | <1 | ~900 |
| (control) | Norfloxacin | 64 | N/D b |
| (control) | Ethidium bromide | 16 | 128 |
| (control) | CCCP a | 8 | 64 |
| (control) | Reserpine | >128 | >128 |
| Organism | Phenotype | Controls | W-nC12-W | |
|---|---|---|---|---|
| Vancomycin | Meropenem | |||
| S. aureus | CLSI control a | 0.3 | - | 9 |
| MRSA | USA 100 b | 0.7 | - | 9 |
| MRSA | USA 300 c | 0.3 | - | 9 |
| E. faecalis | CLSI control a | 1.4 | - | 18 |
| E. faecium | VanA d | 22 | - | 9 |
| E. faecalis | VanB e | >44 | - | 18 |
| S. pneumoniae | CLSI control a | 0.2 | - | 9 |
| S. pneumoniae | R: Pen f | 0.2 | - | 9 |
| S. pneumoniae | R: Levo g | 0.2 | - | 9 |
| K. pneumoniae | KPC-3 h | - | >167 | >18 |
| K. pneumoniae | KPC-3 h | - | 83 | >18 |
| K. pneumoniae | VIM i | - | 10 | >18 |
| P. aeruginosa | CLSI control a | - | 1.3 | >18 |
| P. aeruginosa | UNC-D j | - | 21 | >18 |
| P. aeruginosa | VIM k | - | >167 | >18 |
| E. coli | MN VA Med center l | - | ≤0.3 | >18 |
| E. coli | MN VA Med center l | - | ≤0.3 | >18 |
| E. coli | MI VA Med m | - | ≤0.3 | >18 |
| Example a | X-Axis | MIC X (µM) | Y-Axis | MIC Y (µM) | Enhancement b | FIC Index c |
|---|---|---|---|---|---|---|
| A | Norfloxacin | 0.5 | W-nC12-W (6) | 2 | 128 | 0.51 |
| B | Norfloxacin | 0.5 | w-mC6H4-w (2) | 8 | 128 | 0.51 |
| C | Norfloxacin | 4 | W-mC6H4-W (1) | 32 | 16 | 0.31 |
| D | Ethidium Br | 2 | W-nC12-W (6) | 1 | 8 | 0.37 |
| E | Ethidium Br | 4 | w-mC6H4-w (2) | 4 | 4 | 0.5 |
| F | Ethidium Br | 1 | W-mC6H4-W (1) | 32 | 16 | 0.31 |
| G | Norfloxacin | 0.5 | CCCP d | 4 | 128 | 0.51 |
| H | Ethidium Br | 0.25 | CCCP d | 4 | 128 | 0.510 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, M.; Negin, S.; Meisel, J.; Yin, S.; Gokel, M.; Gill, H.; Gokel, G. Bis(Tryptophan) Amphiphiles Form Ion Conducting Pores and Enhance Antimicrobial Activity against Resistant Bacteria. Antibiotics 2021, 10, 1391. https://doi.org/10.3390/antibiotics10111391
Patel M, Negin S, Meisel J, Yin S, Gokel M, Gill H, Gokel G. Bis(Tryptophan) Amphiphiles Form Ion Conducting Pores and Enhance Antimicrobial Activity against Resistant Bacteria. Antibiotics. 2021; 10(11):1391. https://doi.org/10.3390/antibiotics10111391
Chicago/Turabian StylePatel, Mohit, Saeedeh Negin, Joseph Meisel, Shanheng Yin, Michael Gokel, Hannah Gill, and George Gokel. 2021. "Bis(Tryptophan) Amphiphiles Form Ion Conducting Pores and Enhance Antimicrobial Activity against Resistant Bacteria" Antibiotics 10, no. 11: 1391. https://doi.org/10.3390/antibiotics10111391
APA StylePatel, M., Negin, S., Meisel, J., Yin, S., Gokel, M., Gill, H., & Gokel, G. (2021). Bis(Tryptophan) Amphiphiles Form Ion Conducting Pores and Enhance Antimicrobial Activity against Resistant Bacteria. Antibiotics, 10(11), 1391. https://doi.org/10.3390/antibiotics10111391