High Genomic Identity between Clinical and Environmental Strains of Herbaspirillum frisingense Suggests Pre-Adaptation to Different Hosts and Intrinsic Resistance to Multiple Drugs
Abstract
1. Introduction
2. Results
2.1. Genome of H. frisingense AU14559
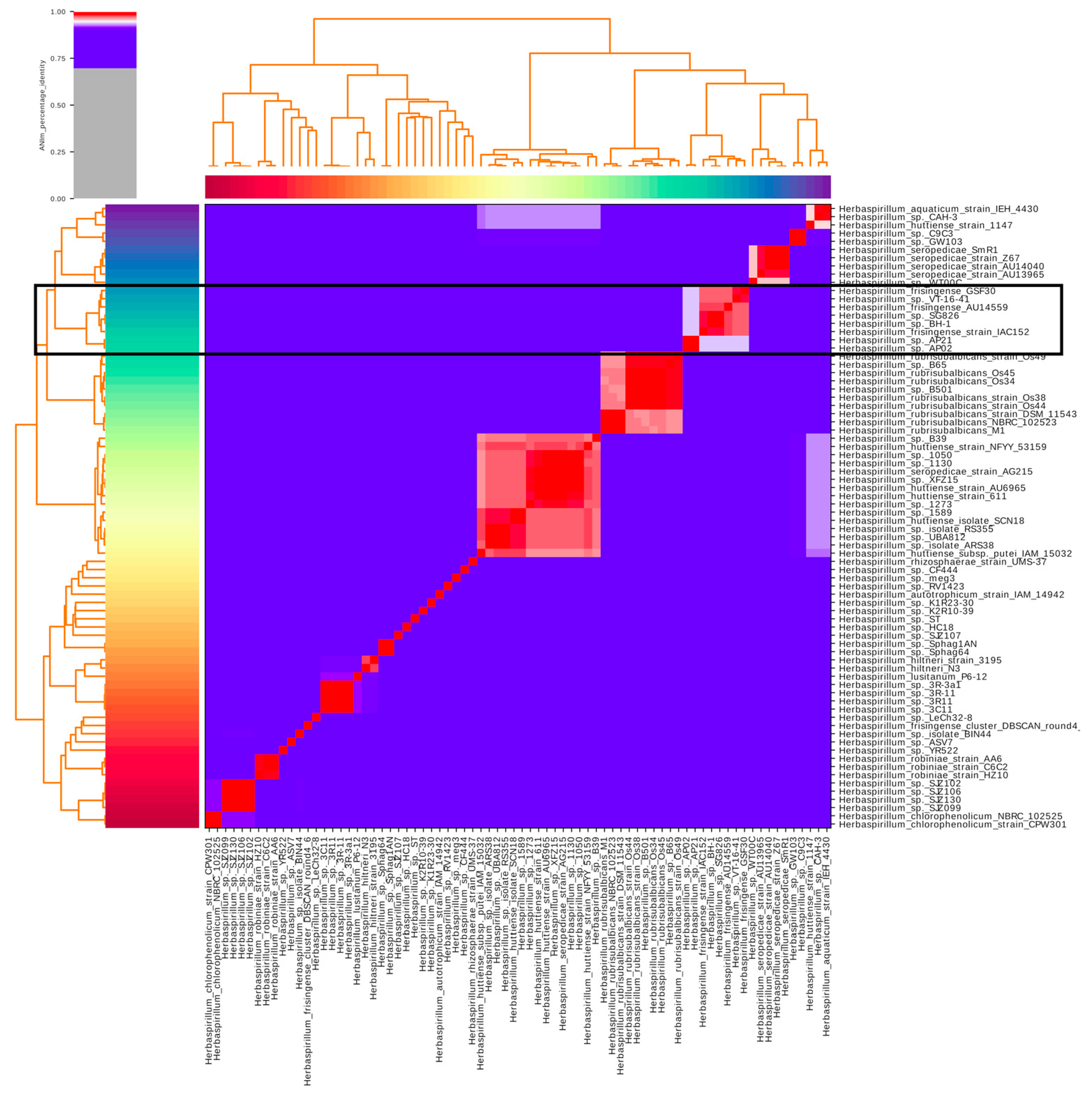
2.2. Identification of Publicly Available H. frisingense Genomes
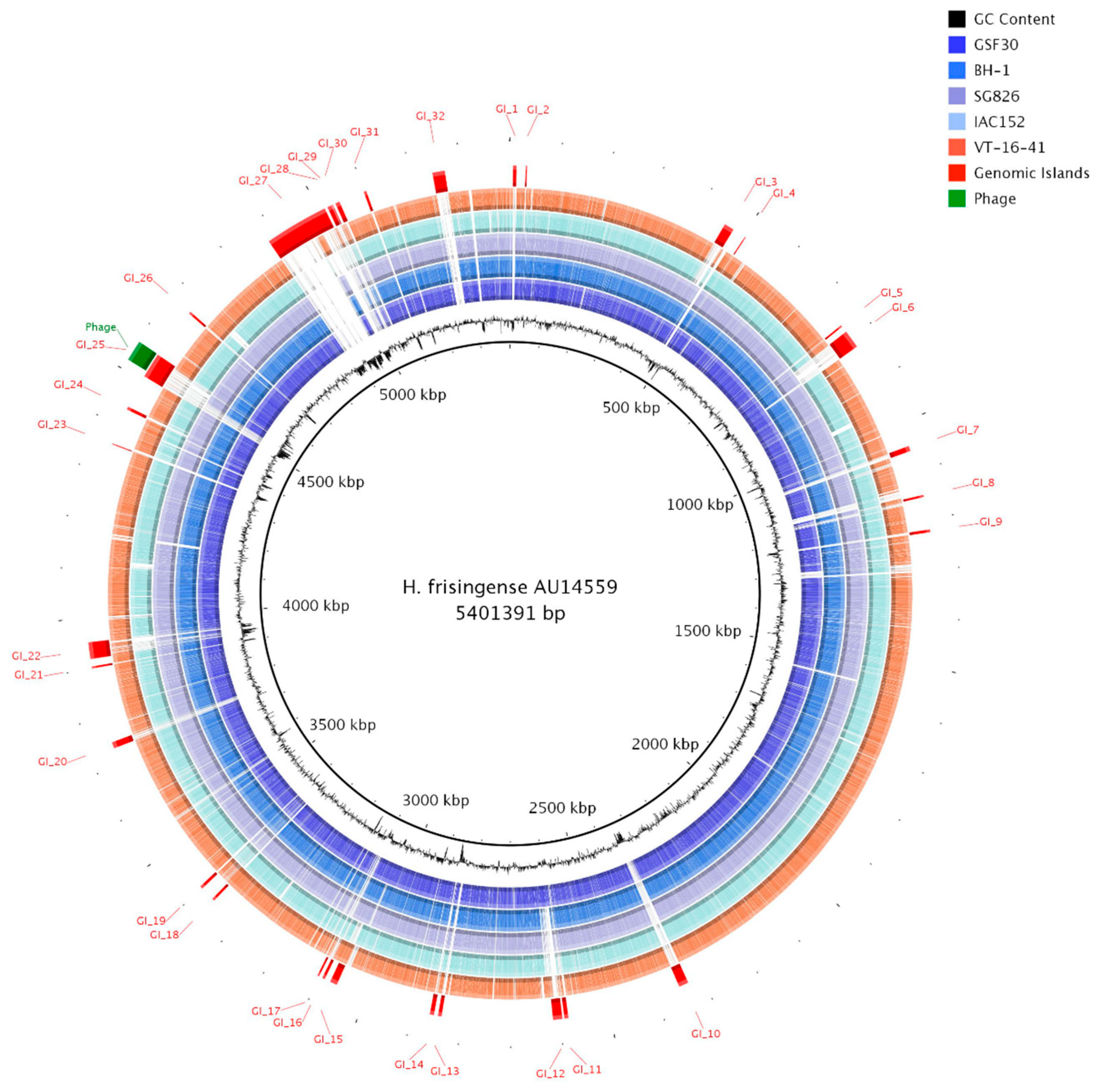
2.3. Identification of Genomic Islands in H. frisingense AU14559
2.4. Identification of Core and Accessory Genomes Using Clustering Analysis
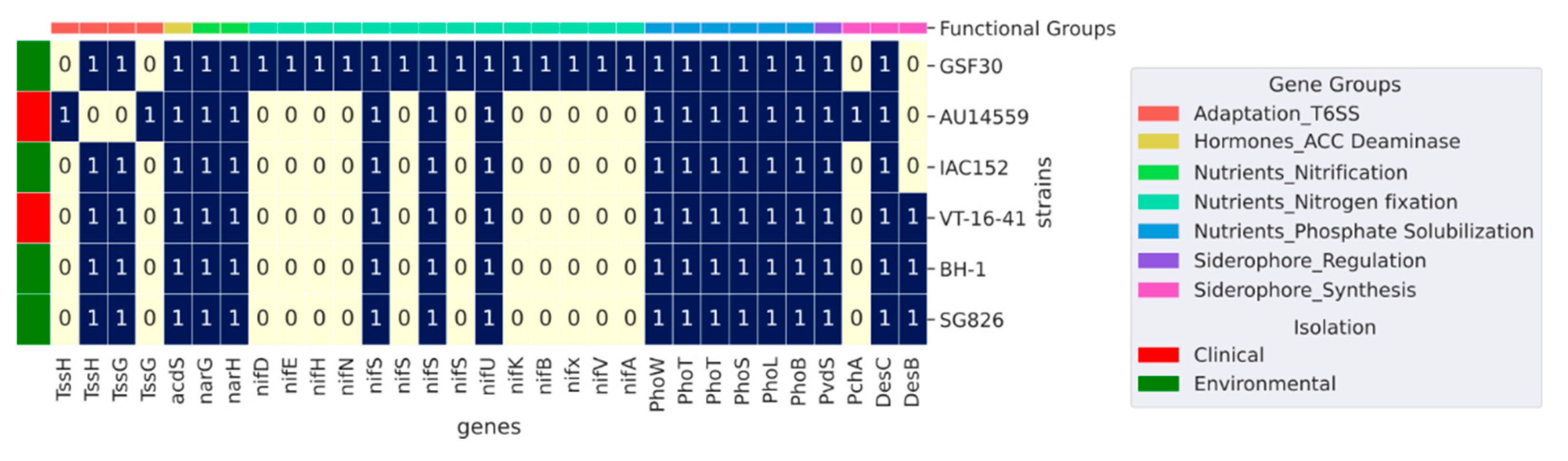
2.5. Plant Growth-Promoting Genes
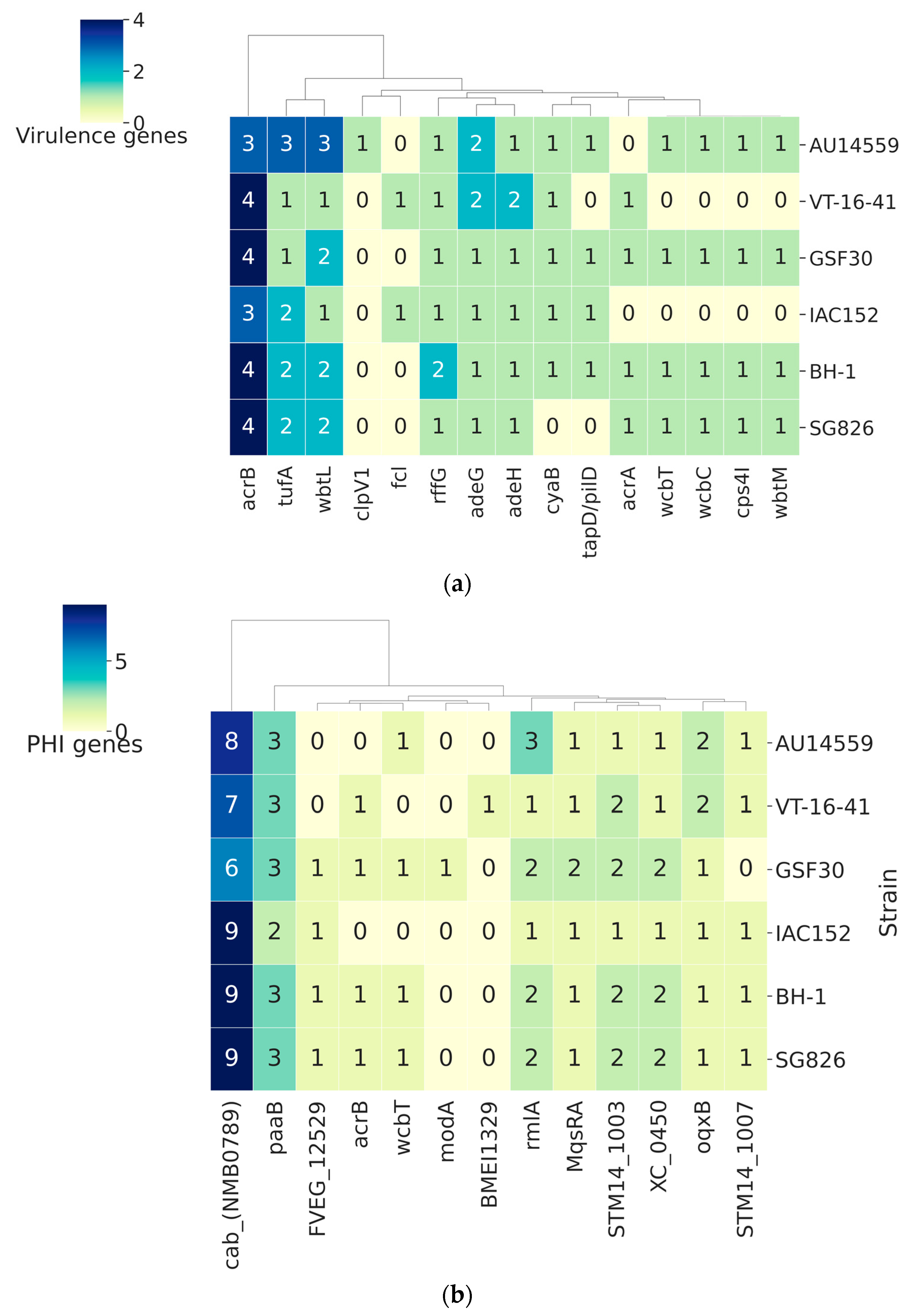
2.6. Host–Pathogen Interaction
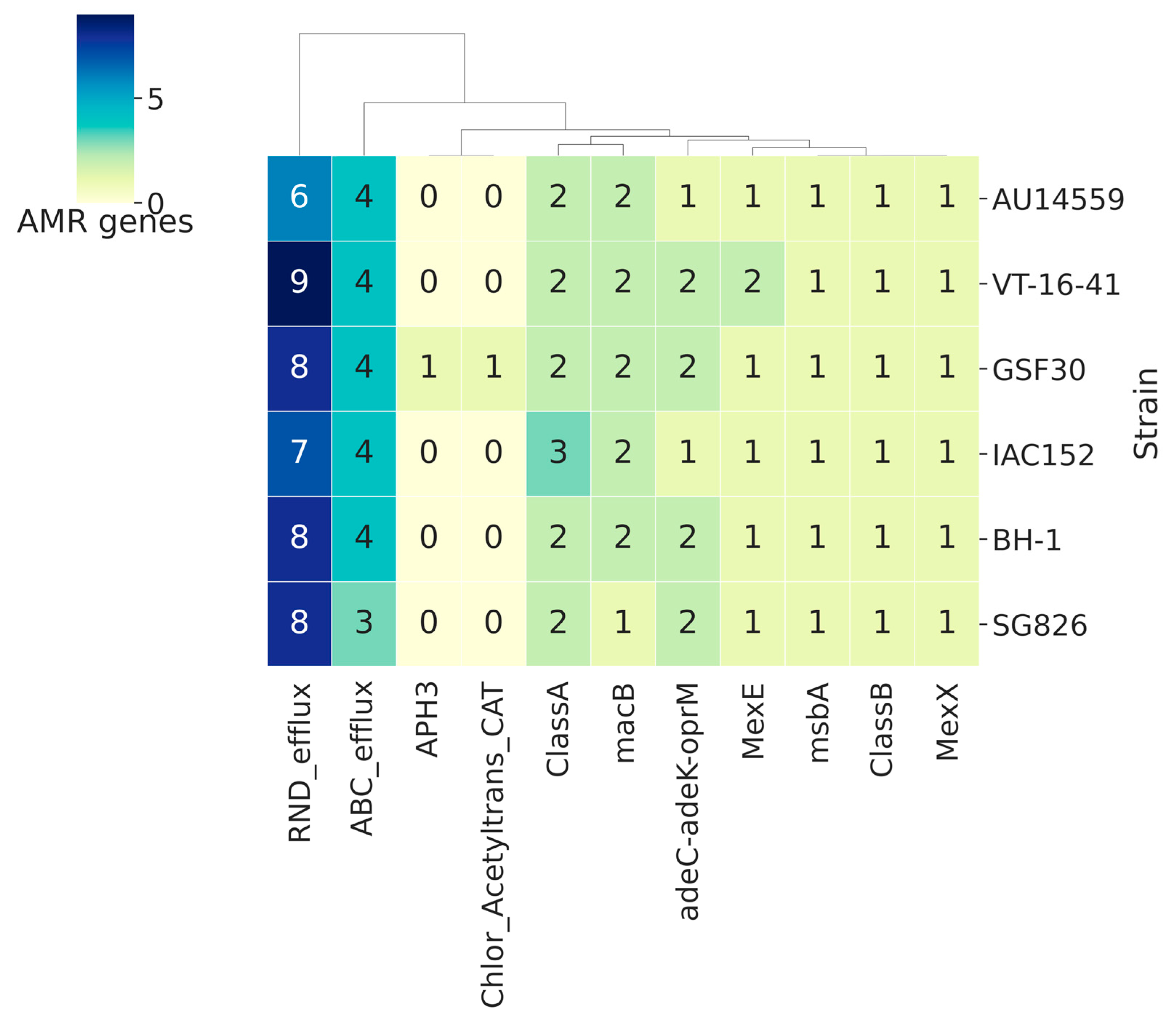
2.7. Antibiotic Resistance Genes in H. frisingense
3. Discussion
4. Materials and Methods
4.1. Purification and Sequencing of Genomic DNA
4.2. Genome Assembly
4.3. Genome Comparisons
4.4. Prospecting for Plant Growth-Promoting Genes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Souza, V.; Piro, V.C.; Faoro, H.; Tadra-Sfeir, M.Z.; Chicora, V.K.; Guizelini, D.; Weiss, V.; Vialle, R.A.; Monteiro, R.A.; Steffens, M.B.R.; et al. Draft Genome Sequence of Herbaspirillum huttiense subsp. putei IAM 15032, a Strain Isolated from Well Water. Genome Announc. 2013, 1, 1. [Google Scholar] [CrossRef] [PubMed]
- Baldani, I.; Baldani, V.L.D. Characterization of Herbaspirillurn Seropedicae Gen. Nov. Sp. Nov. a Root-Associated Nitrogen-Fixing Bacterium. Int. J. Syst. Evol. Microbiol. 1986, 36, 8. [Google Scholar]
- Guizelini, D.; Saizaki, P.M.; Coimbra, N.A.R.; Weiss, V.A.; Faoro, H.; Sfeir, M.Z.T.; Baura, V.A.; Monteiro, R.A.; Chubatsu, L.; Souza, E.M.; et al. Complete Genome Sequence of Herbaspirillum hiltneri N3 (DSM 17495), Isolated from Surface-Sterilized Wheat Roots. Genome Announc. 2015, 3, e01288-15. [Google Scholar] [CrossRef]
- Straub, D.; Rothballer, M.; Hartmann, A.; Ludewig, U. The genome of the endophytic bacterium H. frisingense GSF30T identifies diverse strategies in the Herbaspirillum genus to interact with plants. Front. Microbiol. 2013, 4, 168. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Kwon, M.J.; Kim, M.; Byun, J.-H.; Yong, D.; Lee, K. Septicemia Caused by Herbaspirillum huttiense Secondary to Pneumonia. Ann. Lab. Med. 2019, 39, 340–342. [Google Scholar] [CrossRef] [PubMed]
- Berardino, M.A.-D.; Rodríguez-Czaplicki, E.; Sánchez-Hellín, V. Herbaspirillum huttiense pneumonia in a patient with essential thrombocythaemia. Rev. Esp. Quimioter. 2019, 32, 83–84. [Google Scholar]
- Chemaly, R.F.; Dantes, R.; Shah, D.; Shah, P.K.; Pascoe, N.; Ariza-Heredia, E.; Perego, C.; Nguyen, D.B.; Nguyen, K.; Modarai, F.; et al. Cluster and Sporadic Cases of Herbaspirillum Species Infections in Patients With Cancer. Clin. Infect. Dis. 2015, 60, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Suwantarat, N.; Adams, L.L.; Romagnoli, M.; Carroll, K.C. Fatal case of Herbaspirillum seropedicae bacteremia secondary to pneumonia in an end-stage renal disease patient with multiple myeloma. Diagn. Microbiol. Infect. Dis. 2015, 82, 331–333. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.P.; Stanley, T.; Atuan, M.; McKey, J.; Lipuma, J.J.; Rogers, B.; Jerris, R. Use of matrix assisted laser desorption ionisation–time of flight mass spectrometry in a paediatric clinical laboratory for identification of bacteria commonly isolated from cystic fibrosis patients. J. Clin. Pathol. 2012, 65, 835–838. [Google Scholar] [CrossRef] [PubMed]
- Coenye, T.; Goris, J.; Spilker, T.; Vandamme, P.; LiPuma, J.J. Characterization of Unusual Bacteria Isolated from Respiratory Secretions of Cystic Fibrosis Patients and Description of Inquilinus limosus gen. nov., sp. nov. J. Clin. Microbiol. 2002, 40, 2062–2069. [Google Scholar] [CrossRef] [PubMed]
- Campana, S.; Taccetti, G.; Ravenni, N.; Favari, F.; Cariani, L.; Sciacca, A.; Savoia, D.; Collura, A.; Fiscarelli, E.V.; De Intinis, G.; et al. Transmission of Burkholderia cepacia Complex: Evidence for New Epidemic Clones Infecting Cystic Fibrosis Patients in Italy. J. Clin. Microbiol. 2005, 43, 5136–5142. [Google Scholar] [CrossRef] [PubMed]
- Dhital, R.; Paudel, A.; Bohra, N.; Shin, A.K. Herbaspirillum Infection in Humans: A Case Report and Review of Literature. Case Rep. Infect. Dis. 2020, 2020, 9545243. [Google Scholar] [CrossRef] [PubMed]
- Regunath, H.; Kimball, J.; Smith, L.P.; Salzer, W. Severe Community-Acquired Pneumonia with Bacteremia Caused by Herbaspirillum aquaticum or Herbaspirillum huttiense in an Immune-Competent Adult. J. Clin. Microbiol. 2015, 53, 3086–3088. [Google Scholar] [CrossRef] [PubMed]
- Ziga, E.D.; Druley, T.; Carey-Ann, B.D. Herbaspirillum Species Bacteremia in a Pediatric Oncology Patient. J. Clin. Microbiol. 2010, 48, 4320–4321. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.J.; Oehler, R.L. Lower Extremity Cellulitis and Bacteremia with Herbaspirillum seropedicae Associated With Aquatic Exposure in a Patient with Cirrhosis. Infect. Dis. Clin. Pract. 2005, 13, 277–279. [Google Scholar] [CrossRef]
- Tetz, V.; Tetz, G. Draft Genome Sequence of the Uropathogenic Herbaspirillum frisingense Strain ureolyticus VT-16-41. Genome Announc. 2017, 5, e00279-17. [Google Scholar] [CrossRef] [PubMed]
- Spilker, T.; Uluer, A.Z.; Marty, F.M.; Yeh, W.W.; Levison, J.H.; Vandamme, P.; LiPuma, J.J. Recovery of Herbaspirillum Spe-cies from Persons with Cystic Fibrosis. J. Clin. Microbiol. 2008, 46, 2774–2777. [Google Scholar] [CrossRef] [PubMed]
- Kuramae, E.E.; Derksen, S.; Schlemper, T.R.; Dimitrov, M.R.; Costa, O.Y.A.; Da Silveira, A.P.D. Sorghum Growth Promotion by Paraburkholderia tropica and Herbaspirillum frisingense: Putative Mechanisms Revealed by Genomics and Metagenomics. Microorganisms 2020, 8, 725. [Google Scholar] [CrossRef]
- Waterworth, S.C.; Flórez, L.V.; Rees, E.R.; Hertweck, C.; Kaltenpoth, M.; Kwan, J.C. Horizontal Gene Transfer to a Defensive Symbiont with a Reduced Genome in a Multipartite Beetle Microbiome. mBio 2020, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Juhas, M.; van der Meer, J.R.; Gaillard, M.; Harding, R.M.; Hood, D.W.; Crook, D.W. Genomic islands: Tools of bacterial horizontal gene transfer and evolution. FEMS Microbiol. Rev. 2009, 33, 376–393. [Google Scholar] [CrossRef] [PubMed]
- Filho, A.C.D.S.; Raittz, R.; Guizelini, D.; De Pierri, C.; Augusto, D.W.; Dos Santos-Weiss, I.C.R.; Marchaukoski, J.N. Comparative Analysis of Genomic Island Prediction Tools. Front. Genet. 2018, 9, 619. [Google Scholar] [CrossRef] [PubMed]
- Van Heel, A.J.; De Jong, A.; Song, C.; Viel, J.; Kok, J.; Kuipers, O.P. BAGEL4: A user-friendly web server to thoroughly mine RiPPs and bacteriocins. Nucleic Acids Res. 2018, 46, W278–W281. [Google Scholar] [CrossRef] [PubMed]
- Tonkin-Hill, G.; MacAlasdair, N.; Ruis, C.; Weimann, A.; Horesh, G.; Lees, J.A.; Gladstone, R.A.; Lo, S.; Beaudoin, C.; Floto, R.A.; et al. Producing Polished Prokaryotic Pangenomes with the Panaroo Pipeline. Genome Biol. 2020, 21, 180. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.; Bajeli, S.; Kaushal, D.; Radotra, B.D.; Kumar, A. Biofilm Formation in the Lung Contributes to Virulence and Drug Tolerance of Mycobacterium Tuberculosis. Nat. Commun. 2021, 12, 1606. [Google Scholar] [CrossRef]
- Monteiro, R.A.; Balsanelli, E.; Tuleski, T.; Faoro, H.; Cruz, L.; Wassem, R.; De Baura, V.A.; Tadra-Sfeir, M.Z.; Weiss, V.; DaRocha, W.D.; et al. Genomic comparison of the endophyte Herbaspirillum seropedicaeSmR1 and the phytopathogen Herbaspirillum rubrisubalbicansM1 by suppressive subtractive hybridization and partial genome sequencing. FEMS Microbiol. Ecol. 2012, 80, 441–451. [Google Scholar] [CrossRef]
- Schulze, A.; Mitterer, F.; Pombo, J.P.; Schild, S. Biofilms by Bacterial Human Pathogens: Clinical Relevance—Development, Composition and Regulation—Therapeutical Strategies. Microb. Cell 2021, 8, 28–56. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial Biofilm and Associated Infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Bohin, J.-P. Osmoregulated periplasmic glucans in Proteobacteria. FEMS Microbiol. Lett. 2000, 186, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Guidolin, L.S.; Arce-Gorvel, V.; Ciocchini, A.E.; Comerci, D.J.; Gorvel, J.-P. Cyclic β-glucans at the bacteria-host cells interphase: One sugar ring to rule them all. Cell. Microbiol. 2018, 20, e12850. [Google Scholar] [CrossRef] [PubMed]
- Aramaki, T.; Blanc-Mathieu, R.; Endo, H.; Ohkubo, K.; Kanehisa, M.; Goto, S.; Ogata, H. KofamKOALA: KEGG Ortholog assignment based on profile HMM and adaptive score threshold. Bioinformatics 2020, 36, 2251–2252. [Google Scholar] [CrossRef]
- Yu, K.-W.; Xue, P.; Fu, Y.; Yang, L. T6SS Mediated Stress Responses for Bacterial Environmental Survival and Host Adaptation. Int. J. Mol. Sci. 2021, 22, 478. [Google Scholar] [CrossRef] [PubMed]
- Ballard, A.L.; Ferguson, S.J. Respiratory nitrate reductase from Paracoccus denitrificans. Evidence for two b-type haems in the gamma subunit and properties of a water-soluble active enzyme containing alpha and beta subunits. JBIC J. Biol. Inorg. Chem. 1988, 174, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Morgan, P.W.; Drew, M.C. Ethylene and Plant Responses to Stress. Physiol. Plant. 1997, 100, 620–630. [Google Scholar] [CrossRef]
- Molefe, R.R.; Amoo, A.E.; Babalola, O.O. Metagenomic Insights into the Bacterial Community Structure and Functional Potentials in the Rhizosphere Soil of Maize Plants. J. Plant Interact. 2021, 16, 258–269. [Google Scholar] [CrossRef]
- Västermark, A.; Saier, M.H., Jr. The involvement of transport proteins in transcriptional and metabolic regulation. Curr. Opin. Microbiol. 2014, 18, 8–15. [Google Scholar] [CrossRef]
- Douzi, B.; Brunet, Y.R.; Spinelli, S.; Lensi, V.; Legrand, P.; Blangy, S.; Kumar, A.; Journet, L.; Cascales, E.; Cambillau, C. Structure and Specificity of the Type VI Secretion System ClpV-TssC Interaction in Enteroaggregative Escherichia Coli. Sci. Rep. 2016, 6, 34405. [Google Scholar] [CrossRef] [PubMed]
- Velichko, N.S.; Kokoulin, M.S.; Sigida, E.N.; Kuchur, P.D.; Komissarov, A.S.; Kovtunov, E.A.; Fedonenko, Y.P. Struc-tural and Genetic Characterization of the Colitose-Containing O-Specific Polysaccharide from the Lipopolysaccharide of Herbaspirillum Frisingense GSF30T. Int. J. Biol. Macromol. 2020, 161, 891–897. [Google Scholar] [CrossRef]
- Antunes, V.D.C.; Freitag, D.; Serrato, R.V. Differential Exopolysaccharide Production and Composition by Her-baspirillum Strains from Diverse Ecological Environments. Arch. Microbiol. 2021, 203, 3883–3892. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Wang, Y.; Walsh, T.R.; Yi, L.-X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of Plasmid-Mediated Colistin Resistance Mechanism MCR-1 in Animals and Human Beings in China: A Microbiological and Molecular Biological Study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Yong, D.; Toleman, M.; Giske, C.G.; Cho, H.S.; Sundman, K.; Lee, K.; Walsh, T.R. Characterization of a New Metallo-β-Lactamase Gene, bla NDM-1, and a Novel Erythromycin Esterase Gene Carried on a Unique Genetic Structure in Klebsiella pneumoniae Sequence Type 14 from India. Antimicrob. Agents Chemother. 2009, 53, 5046–5054. [Google Scholar] [CrossRef]
- Kirchhof, G.; Eckert, B.; Stoffels, M.; Baldani, J.; Reis, V.M.; Hartmann, A. Herbaspirillum frisingense sp. nov., a new nitrogen-fixing bacterial species that occurs in C4-fibre plants. Int. J. Syst. Evol. Microbiol. 2001, 51, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Faoro, H.; Oliveira, W.K.; Weiss, V.A.; Tadra-Sfeir, M.Z.; Cardoso, R.L.; Balsanelli, E.; Brusamarello-Santos, L.C.C.; Camilios-Neto, D.; Cruz, L.M.; Raittz, R.T.; et al. Genome comparison between clinical and environmental strains of Herbaspirillum seropedicae reveals a potential new emerging bacterium adapted to human hosts. BMC Genom. 2019, 20, 630. [Google Scholar] [CrossRef]
- Balsanelli, E.; Serrato, R.V.; De Baura, V.A.; Sassaki, G.; Yates, M.G.; Rigo, L.U.; Pedrosa, F.O.; De Souza, E.M.; Monteiro, R.A. Herbaspirillum Seropedicae RfbB and RfbC Genes Are Required for Maize Colonization. Environ. Microbiol. 2010, 12, 2233–2244. [Google Scholar] [PubMed]
- Park, S.; Nahm, M. L-Rhamnose Is Often an Important Part of Immunodominant Epitope for Pneumococcal Serotype 23F Polysaccharide Antibodies in Human Sera Immunized with PPV23. PLoS ONE 2013, 8, e83810. [Google Scholar] [CrossRef][Green Version]
- Oyelaran, O.; McShane, L.M.; Dodd, L.; Gildersleeve, J.C. Profiling Human Serum Antibodies with a Carbohydrate Antigen Microarray. J. Proteome Res. 2009, 8, 4301–4310. [Google Scholar] [CrossRef] [PubMed]
- Giraud, M.-F.; Naismith, J.H. The rhamnose pathway. Curr. Opin. Struct. Biol. 2000, 10, 687–696. [Google Scholar] [CrossRef]
- Ho, B.; Dong, T.; Mekalanos, J.J. A View to a Kill: The Bacterial Type VI Secretion System. Cell Host Microbe 2014, 15, 9–21. [Google Scholar] [CrossRef]
- Cianfanelli, F.R.; Diniz, J.A.; Guo, M.; De Cesare, V.; Trost, M.; Coulthurst, S.J. VgrG and PAAR Proteins Define Distinct Versions of a Functional Type VI Secretion System. PLoS Pathog. 2016, 12, e1005735. [Google Scholar] [CrossRef]
- Tuleski, T.R.; Kimball, J.; do Amaral, F.P.; Pereira, T.P.; Tadra-Sfeir, M.Z.; de Oliveira Pedrosa, F.; Maltempi de Souza, E.; Balint-Kurti, P.; Monteiro, R.A.; Stacey, G. Herbaspirillum Rubrisubalbicans as a Phytopathogenic Model to Study the Immune System of Sorghum Bicolor. Mol. Plant-Microbe Interact. 2020, 33, 235–246. [Google Scholar] [CrossRef]
- Arellano-Reynoso, B.; Lapaque, N.; Salcedo, S.; Briones, G.; Ciocchini, A.E.; Ugalde, R.; Moreno, E.; Moriyon, I.; Gorvel, J.-P. Cyclic β-1,2-glucan is a brucella virulence factor required for intracellular survival. Nat. Immunol. 2005, 6, 618–625. [Google Scholar] [CrossRef]
- Spiers, A.J.; Bohannon, J.; Gehrig, S.M.; Rainey, P.B. Biofilm Formation at the Air-Liquid Interface by the Pseudo-monas Fluorescens SBW25 Wrinkly Spreader Requires an Acetylated Form of Cellulose: Biofilm Formation by P. Fluo-rescens SBW25. Mol. Microbiol. 2002, 50, 15–27. [Google Scholar] [CrossRef]
- O’Neill, J. Review on Antimicrobial Resistance: Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. Rev. Antimicrob. Resist. Tackling Drug-Resist. Infect. Glob. Final. Rep. Recomm. 2016, 10–16. Available online: https://apo.org.au/node/63983https://apo.org.au/sites/default/files/resource-files/2016-05/apo-nid63983.pdf (accessed on 26 October 2021).
- Oliveira, W.K.; Ferrarini, M.; Morello, L.G.; Faoro, H. Resistome Analysis of Bloodstream Infection Bacterial Ge-nomes Reveals a Specific Set of Proteins Involved in Antibiotic Resistance and Drug Efflux. NAR Genomics Bioinforma. 2020, 2, lqaa055. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Nishino, K.; Yamaguchi, A. Novel Macrolide-Specific ABC-Type Efflux Transporter in Escherichia coli. J. Bacteriol. 2001, 183, 5639–5644. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Wang-Kan, X.; Neuberger, A.; Van Veen, H.W.; Pos, K.M.; Piddock, L.J.V.; Luisi, B.F. Multidrug efflux pumps: Structure, function and regulation. Nat. Rev. Microbiol. 2018, 16, 523–539. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Bloise, I.; Guedez-López, G.V.; Tejedor-Rodríguez, M.; Romero-Gómez, M.P.; García-Rodríguez, J.; Mingorance, J.; Cendejas-Bueno, E. Bloodstream infection due to Herbaspirillum sp.: Case series and review of literature. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 779–785. [Google Scholar] [CrossRef]
- Chen, Q.-L.; Cui, H.-L.; Su, J.-Q.; Penuelas, J.; Zhu, Y.-G. Antibiotic Resistomes in Plant Microbiomes. Trends Plant Sci. 2019, 24, 530–541. [Google Scholar] [CrossRef]
- Bezanson, G.; Macinnis, R.; Potter, G.; Hughes, T. Presence and Potential for Horizontal Transfer of Antibiotic Re-sistance in Oxidase-Positive Bacteria Populating Raw Salad Vegetables. Int. J. Food Microbiol. 2008, 127, 37–42. [Google Scholar] [CrossRef]
- Verraes, C.; Van Boxstael, S.; Van Meervenne, E.; Van Coillie, E.; Butaye, P.; Catry, B.; De Schaetzen, M.-A.; Van Huffel, X.; Imberechts, H.; Dierick, K.; et al. Antimicrobial Resistance in the Food Chain: A Review. Int. J. Environ. Res. Public Health 2013, 10, 2643–2669. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; An, X.; Li, H.; Su, J.; Ma, Y.; Zhu, Y.-G. Long-Term Field Application of Sewage Sludge Increases the Abun-dance of Antibiotic Resistance Genes in Soil. Environ. Int. 2016, 92–93, 1–10. [Google Scholar] [CrossRef]
- Udikovic-Kolic, N.; Wichmann, F.; Broderick, N.; Handelsman, J. Bloom of resident antibiotic-resistant bacteria in soil following manure fertilization. Proc. Natl. Acad. Sci. USA 2014, 111, 15202–15207. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Chen, Q.; Chen, S.; Zhu, Y.-G. Does Organically Produced Lettuce Harbor Higher Abundance of Antibiotic Resistance Genes than Conventionally Produced? Environ. Int. 2017, 98, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Berg, G.; Eberl, L.; Hartmann, A. The rhizosphere as a reservoir for opportunistic human pathogenic bacteria. Environ. Microbiol. 2005, 7, 1673–1685. [Google Scholar] [CrossRef] [PubMed]
- Olszewski, J.; Galvão, C.W.; Lipuma, J.J.; Paludo, K.S. Environmental and clinical isolates of Herbaspirillum induce pulmonary infection in mice and its secretome is cytotoxic to human lung cells. J. Med. Microbiol. 2021, 70, 001343. [Google Scholar] [CrossRef]
- Klassen, G.; Pedrosa, F.O.; Souza, E.M.; Funayama, S.; Rigo, L.U. Effect of Nitrogen Compounds on Nitrogenase Activity in Herbaspirillum Seropedicae SMR1. Can. J. Microbiol. 1997, 43, 887–891. [Google Scholar] [CrossRef]
- Pedrosa, F.O.; Monteiro, R.A.; Wassem, R.; Cruz, L.M.; Ayub, R.A.; Colauto, N.B.; Fernandez, M.A.; Fungaro, M.H.P.; Grisard, E.C.; Hungria, M.; et al. Genome of Herbaspirillum Seropedicae Strain SmR1, a Specialized Diazotrophic Endo-phyte of Tropical Grasses. PLoS Genet. 2011, 7, e1002064. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef]
- Carver, T.J.; Rutherford, K.M.; Berriman, M.; Rajandream, M.-A.; Barrell, B.G.; Parkhill, J. ACT: The Artemis Com-parison Tool. Bioinformatics 2005, 21, 3422–3423. [Google Scholar] [CrossRef] [PubMed]
- Piro, V.C.; Faoro, H.; Weiss, V.; Steffens, M.B.; Pedrosa, F.; Souza, E.M.; Raittz, R.T. FGAP: An automated gap closing tool. BMC Res. Notes 2014, 7, 371. [Google Scholar] [CrossRef] [PubMed]
- Guizelini, D.; Raittz, R.T.; Cruz, L.M.; Souza, E.M.; Steffens, M.B.R.; Pedrosa, F.O. GFinisher: A New Strategy to Re-fine and Finish Bacterial Genome Assemblies. Sci. Rep. 2016, 6, 34963. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid Prokaryotic Genome Annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Pritchard, L.; Glover, R.H.; Humphris, S.; Elphinstone, J.G.; Toth, I.K. Genomics and taxonomy in diagnostics for food security: Soft-rotting enterobacterial plant pathogens. Anal. Methods 2016, 8, 12–24. [Google Scholar] [CrossRef]
- Alikhan, N.-F.; Petty, N.K.; Ben Zakour, N.L.; Beatson, S.A. BLAST Ring Image Generator (BRIG): Simple prokaryote genome comparisons. BMC Genom. 2011, 12, 402. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Punta, M.; Coggill, P.C.; Eberhardt, R.; Mistry, J.; Tate, J.; Boursnell, C.; Pang, N.; Forslund, S.K.; Ceric, G.; Clements, J.; et al. The Pfam protein families database. Nucleic Acids Res. 2011, 40, D290–D301. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.C.; Geyik, H.; Ramos, R.T.; de Sá, P.H.; Barbosa, E.G.; Baumbach, J.; Figueiredo, H.C.; Miyoshi, A.; Tauch, A.; Silva, A.; et al. GIPSy: Genomic island prediction software. J. Biotechnol. 2016, 232, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Vernikos, G.S.; Parkhill, J. Interpolated Variable Order Motifs for Identification of Horizontally Acquired DNA: Re-visiting the Salmonella Pathogenicity Islands. Bioinformatics 2006, 22, 2196–2203. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, J.; Yu, J.; Yao, Z.; Sun, L.; Shen, Y.; Jin, Q. VFDB: A reference database for bacterial virulence factors. Nucleic Acids Res. 2005, 33, D325–D328. [Google Scholar] [CrossRef] [PubMed]
- Urban, M.; Cuzick, A.; Seager, J.; Wood, V.; Rutherford, K.; Venkatesh, S.Y.; De Silva, N.; Martinez, M.C.; Pedro, H.; Yates, A.D.; et al. PHI-base: The pathogen–host interactions database. Nucleic Acids Res. 2019, 48, D613–D620. [Google Scholar] [CrossRef] [PubMed]
- Arndt, D.; Grant, J.R.; Marcu, A.; Sajed, T.; Pon, A.; Liang, Y.; Wishart, D.S. PHASTER: A better, faster version of the PHAST phage search tool. Nucleic Acids Res. 2016, 44, W16–W21. [Google Scholar] [CrossRef] [PubMed]
- Eddy, S.R. Accelerated Profile HMM Searches. PLoS Comput. Biol. 2011, 7, e1002195. [Google Scholar] [CrossRef] [PubMed]
- Gibson, M.K.; Forsberg, K.J.; Dantas, G. Improved Annotation of Antibiotic Resistance Determinants Reveals Micro-bial Resistomes Cluster by Ecology. ISME J. 2015, 9, 207–216. [Google Scholar] [CrossRef] [PubMed]
- The UniProt Consortium. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, W.K.; Ávila, H.L.; Tadra, M.Z.; Cardoso, R.L.; Fadel-Pichet, C.M.T.; de Souza, E.M.; de Oliveira Pedrosa, F.; Faoro, H. High Genomic Identity between Clinical and Environmental Strains of Herbaspirillum frisingense Suggests Pre-Adaptation to Different Hosts and Intrinsic Resistance to Multiple Drugs. Antibiotics 2021, 10, 1409. https://doi.org/10.3390/antibiotics10111409
Oliveira WK, Ávila HL, Tadra MZ, Cardoso RL, Fadel-Pichet CMT, de Souza EM, de Oliveira Pedrosa F, Faoro H. High Genomic Identity between Clinical and Environmental Strains of Herbaspirillum frisingense Suggests Pre-Adaptation to Different Hosts and Intrinsic Resistance to Multiple Drugs. Antibiotics. 2021; 10(11):1409. https://doi.org/10.3390/antibiotics10111409
Chicago/Turabian StyleOliveira, Willian Klassen, Hugo Leonardo Ávila, Michelle Zibeti Tadra, Rodrigo Luiz Cardoso, Cyntia Maria Teles Fadel-Pichet, Emanuel Maltempi de Souza, Fábio de Oliveira Pedrosa, and Helisson Faoro. 2021. "High Genomic Identity between Clinical and Environmental Strains of Herbaspirillum frisingense Suggests Pre-Adaptation to Different Hosts and Intrinsic Resistance to Multiple Drugs" Antibiotics 10, no. 11: 1409. https://doi.org/10.3390/antibiotics10111409
APA StyleOliveira, W. K., Ávila, H. L., Tadra, M. Z., Cardoso, R. L., Fadel-Pichet, C. M. T., de Souza, E. M., de Oliveira Pedrosa, F., & Faoro, H. (2021). High Genomic Identity between Clinical and Environmental Strains of Herbaspirillum frisingense Suggests Pre-Adaptation to Different Hosts and Intrinsic Resistance to Multiple Drugs. Antibiotics, 10(11), 1409. https://doi.org/10.3390/antibiotics10111409






