Procalcitonin Increase Is Associated with the Development of Critical Care-Acquired Infections in COVID-19 ARDS
Abstract
:1. Introduction
Aims and Objectives
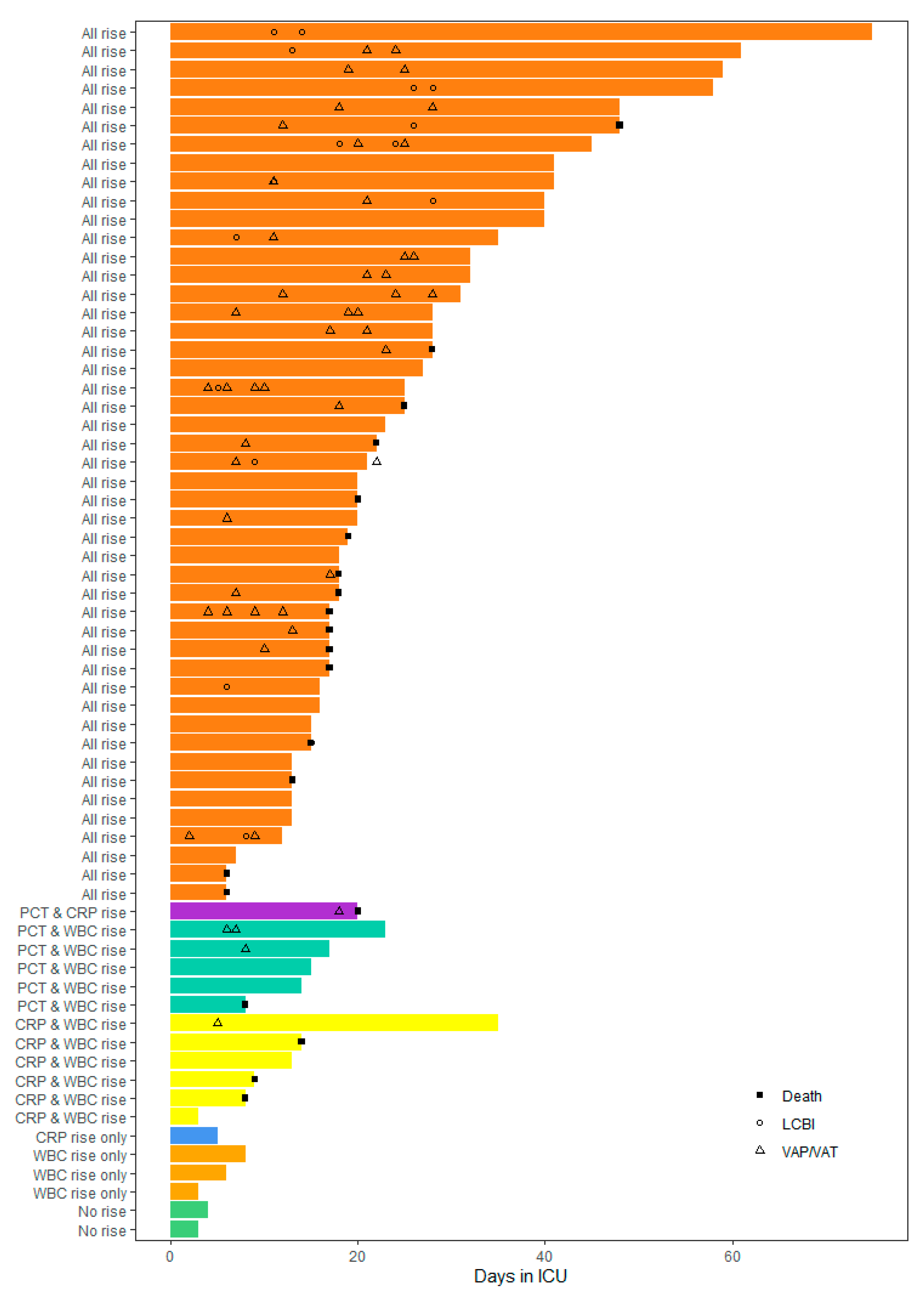
2. Results
3. Discussion
4. Materials and Methods
Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johns Hopkins Coronavirus Resource Center. COVID-19 Case Tracker. Available online: https://coronavirus.jhu.edu (accessed on 9 July 2021).
- Guan, W.; Ni, Z.; Hu, Y.; Liang, W.; Ou, C.; He, J.; Liu, L.; Shan, H.; Lei, C.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Self, W.H.; Balk, R.A.; Grijalva, C.G.; Williams, D.J.; Zhu, Y.; Anderson, E.J.; Waterer, G.W.; Courtney, D.M.; Bramley, A.M.; Trabue, C.; et al. Procalcitonin as a Marker of Etiology in Adults Hospitalized with Community-Acquired Pneumonia. Clin. Infect. Dis. 2017, 65, 183–190. [Google Scholar] [CrossRef]
- Harbarth, S.; Holeckova, K.; Froidevaux, C.; Pittet, D.; Ricou, B.; Grau, G.E.; Vadas, L.; Pugin, J. The Geneva Sepsis Network Diagnostic Value of Procalcitonin, Interleukin-6, and Interleukin-8 in Critically Ill Patients Admitted with Suspected Sepsis. Am. J. Respir. Crit. Care Med. 2001, 164, 396–402. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: A Retrospective Cohort Study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Vazzana, N.; Dipaola, F.; Ognibene, S. Procalcitonin and Secondary Bacterial Infections in COVID-19: Association with Disease Severity and Outcomes. Acta Clin. Belg. 2020, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Lansbury, L.; Lim, B.; Baskaran, V.; Lim, W.S. Co-Infections in People with COVID-19: A Systematic Review and Meta-Analysis. J. Infect. 2020, 81, 266–275. [Google Scholar] [CrossRef]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.-P.R.; Daneman, N. Bacterial Co-Infection and Secondary Infection in Patients with COVID-19: A Living Rapid Review and Meta-Analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef]
- Powell, N.; Howard, P.; Llewelyn, M.J.; Szakmany, T.; Albur, M.; Bond, S.E.; Euden, J.; Brookes-Howell, L.; Dark, P.; Hellyer, T.P.; et al. Use of Procalcitonin during the First Wave of COVID-19 in the Acute NHS Hospitals: A Retrospective Observational Study. Antibiotics 2021, 10, 516. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence. COVID-19 Rapid Guideline: Managing COVID-19. Available online: https://www.nice.org.uk/guidance/ng191/resources/fully-accessible-version-of-the-guideline-pdf-pdf-51035553326 (accessed on 13 July 2021).
- The ARDS Definition Task Force. Acute Respiratory Distress Syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef]
- van Berkel, M.; Kox, M.; Frenzel, T.; Pickkers, P.; Schouten, J. Biomarkers for Antimicrobial Stewardship: A Reappraisal in COVID-19 Times? Crit. Care 2020, 24, 600. [Google Scholar] [CrossRef]
- Pink, I.; Raupach, D.; Fuge, J.; Vonberg, R.-P.; Hoeper, M.M.; Welte, T.; Rademacher, J. C-Reactive Protein and Procalcitonin for Antimicrobial Stewardship in COVID-19. Infection 2021, 49, 935–943. [Google Scholar] [CrossRef]
- Maki, D.G.; Crnich, C.J.; Safdar, N. Nosocomial Infection in the Intensive Care Unit. In Critical Care Medicine; Elsevier: Amsterdam, The Netherlands, 2008; pp. 1003–1069. ISBN 978-0-323-04841-5. [Google Scholar]
- Westblade, L.F.; Simon, M.S.; Satlin, M.J. Bacterial Coinfections in Coronavirus Disease 2019. Trends Microbiol. 2021, 29, 930–941. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Scaravilli, V.; Mangioni, D.; Scudeller, L.; Alagna, L.; Bartoletti, M.; Bellani, G.; Biagioni, E.; Bonfanti, P.; Bottino, N.; et al. Hospital-Acquired Infections in Critically Ill Patients with COVID-19. Chest 2021, 160, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, M.; Misseri, G.; Catalisano, G.; Marino, C.; Ingoglia, G.; Alessi, M.; Consiglio, E.; Gregoretti, C.; Giarratano, A.; Cortegiani, A. Ventilator-Associated Pneumonia in Patients with COVID-19: A Systematic Review and Meta-Analysis. Antibiotics 2021, 10, 545. [Google Scholar] [CrossRef] [PubMed]
- Bardi, T.; Pintado, V.; Gomez-Rojo, M.; Escudero-Sanchez, R.; Azzam Lopez, A.; Diez-Remesal, Y.; Martinez Castro, N.; Ruiz-Garbajosa, P.; Pestaña, D. Nosocomial Infections Associated to COVID-19 in the Intensive Care Unit: Clinical Characteristics and Outcome. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Xiang, Y.; Fang, W.; Zheng, Y.; Li, B.; Hu, Y.; Lang, C.; Huang, D.; Sun, Q.; Xiong, Y.; et al. Clinical Features and Treatment of COVID-19 Patients in Northeast Chongqing. J. Med. Virol. 2020, 92, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Chu, H.; Hou, Y.; Chai, Y.; Shuai, H.; Lee, A.C.-Y.; Zhang, X.; Wang, Y.; Hu, B.; Huang, X.; et al. Attenuated Interferon and Proinflammatory Response in SARS-CoV-2–Infected Human Dendritic Cells Is Associated with Viral Antagonism of STAT1 Phosphorylation. J. Infect. Dis. 2020, 222, 734–745. [Google Scholar] [CrossRef]
- Merad, M.; Martin, J.C. Pathological Inflammation in Patients with COVID-19: A Key Role for Monocytes and Macrophages. Nat. Rev. Immunol. 2020, 20, 355–362. [Google Scholar] [CrossRef]
- Sinha, P.; Calfee, C.S.; Cherian, S.; Brealey, D.; Cutler, S.; King, C.; Killick, C.; Richards, O.; Cheema, Y.; Bailey, C.; et al. Prevalence of Phenotypes of Acute Respiratory Distress Syndrome in Critically Ill Patients with COVID-19: A Prospective Observational Study. Lancet Respir. Med. 2020, 8, 1209–1218. [Google Scholar] [CrossRef]
- Szakmany, T.; Tuckwell, W.; Harte, E.; Wetherall, N.; Ramachandran, S.; Price, S.; Breen, H.; Killick, C.; Cheema, Y.; King, C.; et al. Differences in Inflammatory Marker Kinetics between the First and Second Wave of COVID-19 Patients Admitted to the ICU: A Retrospective, Single-Center Study. JCM 2021, 10, 3290. [Google Scholar] [CrossRef]
- de Bruin, S.; Bos, L.D.; van Roon, M.A.; Tuip-de Boer, A.M.; Schuurman, A.R.; Koel-Simmelinck, M.J.A.; Bogaard, H.J.; Tuinman, P.R.; van Agtmael, M.A.; Hamann, J.; et al. Clinical Features and Prognostic Factors in Covid-19: A Prospective Cohort Study. EBioMedicine 2021, 67, 103378. [Google Scholar] [CrossRef]
- Theodorou, V.P.; Papaioannou, V.E.; Tripsianis, G.A.; Panopoulou, M.K.; Christophoridis, E.K.; Kouliatsis, G.A.; Gioka, T.M.; Maltezos, E.S.; Ktenidou-Kartali, S.I.; Pneumatikos, I.A. Procalcitonin and Procalcitonin Kinetics for Diagnosis and Prognosis of Intravascular Catheter-Related Bloodstream Infections in Selected Critically Ill Patients: A Prospective Observational Study. BMC Infect. Dis. 2012, 12, 247. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Gutiérrez, B.; Morales, I.; Pérez-Galera, S.; Fernández-Riejos, P.; Retamar, P.; de Cueto, M.; Pascual, Á.; Rodríguez-Baño, J. Predictive Value of the Kinetics of Procalcitonin and C-Reactive Protein for Early Clinical Stability in Patients with Bloodstream Infections Due to Gram-Negative Bacteria. Diagn. Microbiol. Infect. Dis. 2019, 93, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Cabral, L.; Afreixo, V.; Meireles, R.; Vaz, M.; Marques, M.; Tourais, I.; Chaves, C.; Almeida, L.; Paiva, J.A. Procalcitonin Kinetics after Burn Injury and Burn Surgery in Septic and Non-Septic Patients—A Retrospective Observational Study. BMC Anesth. 2018, 18, 122. [Google Scholar] [CrossRef] [PubMed]
- Trásy, D.; Tánczos, K.; Németh, M.; Hankovszky, P.; Lovas, A.; Mikor, A.; Hajdú, E.; Osztroluczki, A.; Fazakas, J.; Molnár, Z. Delta Procalcitonin is a Better Indicator of Infection Than Absolute Procalcitonin Values in Critically Ill Patients: A Prospective Observational Study. J. Immunol. Res. 2016, 2016, 3530752. [Google Scholar] [CrossRef]
- Trásy, D.; Tánczos, K.; Németh, M.; Hankovszky, P.; Lovas, A.; Mikor, A.; László, I.; Hajdú, E.; Osztroluczki, A.; Fazakas, J.; et al. Early Procalcitonin Kinetics and Appropriateness of Empirical Antimicrobial Therapy in Critically Ill Patients. J. Crit. Care 2016, 34, 50–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calderon, M.; Li, A.; Bazo-Alvarez, J.C.; Dennis, J.; Baker, K.F.; Schim van der Loeff, I.; Hanrath, A.T.; Capstick, R.; Payne, B.A.I.; Weiand, D.; et al. Evaluation of Procalcitonin-Guided Antimicrobial Stewardship in Patients Admitted to Hospital with COVID-19 Pneumonia. JAC-Antimicrob. Resist. 2021, 3, dlab133. [Google Scholar] [CrossRef]
- Russell, C.D.; Fairfield, C.J.; Drake, T.M.; Turtle, L.; Seaton, R.A.; Wootton, D.G.; Sigfrid, L.; Harrison, E.M.; Docherty, A.B.; de Silva, T.I.; et al. Co-Infections, Secondary Infections, and Antimicrobial Use in Patients Hospitalised with COVID-19 during the First Pandemic Wave from the ISARIC WHO CCP-UK Study: A Multicentre, Prospective Cohort Study. Lancet Microbe 2021, 2, e354–e365. [Google Scholar] [CrossRef]
- Kopczynska, M.; Sharif, B.; Unwin, H.; Lynch, J.; Forrester, A.; Zeicu, C.; Cleaver, S.; Kulikouskaya, S.; Chandy, T.; Ang, E.; et al. Real World Patterns of Antimicrobial Use and Microbiology Investigations in Patients with Sepsis Outside the Critical Care Unit: Secondary Analysis of Three Nation-Wide Point Prevalence Studies. JCM 2019, 8, 1337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNulty, P.; Szakmany, T.; Welsh Digital Data Collection Platform. Factors Influencing Clinician’s Coherence with Local Antimicrobial Guidelines in the Management of Sepsis. Anaesthesiol Intensive 2018, 50, 82–84. [Google Scholar] [CrossRef]
- Li, Y.; Guo, J.; Yang, H.; Li, H.; Shen, Y.; Zhang, D. Comparison of Culture-Negative and Culture-Positive Sepsis or Septic Shock: A Systematic Review and Meta-Analysis. Crit. Care 2021, 25, 167. [Google Scholar] [CrossRef]
- Hughes, S.; Mughal, N.; Moore, L.S.P. Procalcitonin to Guide Antibacterial Prescribing in Patients Hospitalised with COVID-19. Antibiotics 2021, 10, 1119. [Google Scholar] [CrossRef] [PubMed]
- Seaton, R.A.; Gibbons, C.L.; Cooper, L.; Malcolm, W.; McKinney, R.; Dundas, S.; Griffith, D.; Jeffreys, D.; Hamilton, K.; Choo-Kang, B.; et al. Survey of Antibiotic and Antifungal Prescribing in Patients with Suspected and Confirmed COVID-19 in Scottish Hospitals. J. Infect. 2020, 81, 952–960. [Google Scholar] [CrossRef] [PubMed]
- Schuetz, P.; Wirz, Y.; Sager, R.; Christ-Crain, M.; Stolz, D.; Tamm, M.; Bouadma, L.; Luyt, C.E.; Wolff, M.; Chastre, J.; et al. Procalcitonin to Initiate or Discontinue Antibiotics in Acute Respiratory Tract Infections. Cochrane Database Syst. Rev. 2017, 10, CD007498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gamberini, L.; Tonetti, T.; Spadaro, S.; Zani, G.; Mazzoli, C.A.; Capozzi, C.; Giampalma, E.; Bacchi Reggiani, M.L.; Bertellini, E.; Castelli, A.; et al. Factors Influencing Liberation from Mechanical Ventilation in Coronavirus Disease 2019: Multicenter Observational Study in Fifteen Italian ICUs. J. Intensive Care 2020, 8, 80. [Google Scholar] [CrossRef] [PubMed]
- Richards-Belle, A.; Orzechowska, I.; Gould, D.W.; Thomas, K.; Doidge, J.C.; Mouncey, P.R.; Christian, M.D.; Shankar-Hari, M.; Harrison, D.A.; Rowan, K.M.; et al. COVID-19 in Critical Care: Epidemiology of the First Epidemic Wave across England, Wales and Northern Ireland. Intensive Care Med. 2020, 46, 2035–2047. [Google Scholar] [CrossRef]
- Pugh, R.; Harrison, W.; Harris, S.; Roberts, H.; Scholey, G.; Szakmany, T.; WICSARG Investigators and WHAIP. Is HELICS the Right Way? Lack of Chest Radiography Limits Ventilator-Associated Pneumonia Surveillance in Wales. Front. Microbiol. 2016, 7, 1271. [Google Scholar] [CrossRef] [Green Version]
- Kooistra, E.J.; van Berkel, M.; van Kempen, N.F.; van Latum, C.R.M.; Bruse, N.; Frenzel, T.; van den Berg, M.J.W.; Schouten, J.A.; Kox, M.; Pickkers, P. Dexamethasone and Tocilizumab Treatment Considerably Reduces the Value of C-Reactive Protein and Procalcitonin to Detect Secondary Bacterial Infections in COVID-19 Patients. Crit. Care 2021, 25, 281. [Google Scholar] [CrossRef]
- RECOVERY Collaborative Group. Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- The RECOVERY Collaborative Group. Tocilizumab in Patients Admitted to Hospital with COVID-19 (RECOVERY): A Randomised, Controlled, Open-Label, Platform Trial. Lancet 2021, 397, 1637–1645. [Google Scholar] [CrossRef]
- The RECOVERY Collaborative Group. Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2020, 383, 2030–2040. [Google Scholar] [CrossRef] [PubMed]
- Horby, P.W.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Emberson, J.; Palfreeman, A.; Raw, J.; Elmahi, E.; Prudon, B.; et al. Lopinavir–Ritonavir in Patients Admitted to Hospital with COVID-19 (RECOVERY): A Randomised, Controlled, Open-Label, Platform Trial. Lancet 2020, 396, 1345–1352. [Google Scholar] [CrossRef]


| Variables | No PCT Rise | PCT Rise | Overall |
|---|---|---|---|
| (n = 12) | (n = 53) | (n = 65) | |
| Age (years) | |||
| Mean (SD) | 56.9 (10.6) | 55.8 (11.0) | 56.0 (10.8) |
| Median [Min, Max] | 56.5 [41.0, 76.0] | 57.0 [22.0, 77.0] | 57.0 [22.0, 77.0] |
| Sex | |||
| Female (n, %) | 4 (33.3%) | 18 (34.0%) | 22 (33.8%) |
| Male (n, %) | 8 (66.7%) | 35 (66.0%) | 43 (66.2%) |
| Outcome | |||
| Alive (n, %) | 9 (75.0%) | 35 (66.0%) | 44 (67.7%) |
| Deceased (n, %) | 3 (25.0%) | 18 (34.0%) | 21 (32.3%) |
| Comorbidities | |||
| Diabetes | 2 (16.7%) | 16 (30.2%) | 18 (27.7%) |
| Hypertension | 6 (50%) | 22 (41.5%) | 28 (43.1%) |
| Ischaemic heart disease | 0 (0%) | 3 (5.7%) | 3 (4.6%) |
| COPD | 0 (0%) | 1 (1.9%) | 1 (1.5%) |
| Asthma | 3 (25%) | 14 (26.42%) | 17 (26.2%) |
| Chronic renal disease | 0 (0%) | 2 (3.8%) | 2 (3.1%) |
| Other comorbidities | 0 (0%) | 10 (18.9%) | 10 (15.4%) |
| Ethnicity | |||
| Black (n, %) | 1 (3.3%) | 1 (2.9%) | 2 (3.1%) |
| Caucasian (n, %) | 21 (70.0%) | 26 (74.3%) | 47 (72.3%) |
| Indian Subcontinent (n, %) | 4 (13.3%) | 4 (11.4%) | 8 (12.3%) |
| Filipino (n, %) | 3 (10.0%) | 2 (5.7%) | 5 (7.7%) |
| Mixed other (n, %) | 1 (3.3%) | 2 (5.7%) | 3 (4.6%) |
| SOFA score on admission | |||
| Mean (SD) | 10.6 (4.35) | 9.74 (2.74) | 9.88 (3.03) |
| Median [Min, Max] | 10.0 [3.00, 16.0] | 10.0 [6.00, 15.0] | 10.0 [3.00, 16.0] |
| Length of ICU stay (days) | |||
| Mean (SD) | 9.25 (8.93) | 25.6 (15.2) | 22.6 (15.6) |
| Median [Min, Max] | 7.00 [3.00, 35.0] | 20.0 [6.00, 75.0] | 18.0 [3.00, 75.0] |
| Change in Biomarker Levels | No VAP/VAT or LCBI | VAP/VAT and/or LCBI | Overall | Multivariable OR (95%CI) | p-Value |
|---|---|---|---|---|---|
| (n = 32) | (n = 33) | (n = 65) | |||
| PCT | |||||
| No PCT rise | 11(34.4%) | 1 (3.0%) | 12 (18.5%) | ||
| PCT rise | 21 (65.6%) | 32 (97.0%) | 53 (81.5%) | 14.86 (2.20, 342.53) | p = 0.021 |
| CRP | |||||
| No CRP rise | 8 (25.0%) | 2 (6.1%) | 10 (15.4%) | ||
| CRP rise | 24 (75.0%) | 31 (93.9%) | 55 (84.6%) | 3.70 (0.62, 30.17) | p = 0.167 |
| WBC | |||||
| No WBC rise | 3 (9.4%) | 1 (3.0%) | 4 (6.2%) | ||
| WBC rise | 29 (90.6%) | 32 (97.0%) | 61 (93.8%) | 0.73 (0.02, 26.48) | p = 0.855 |
| Outcome | Definition | ||
|---|---|---|---|
| Healthcare-acquired laboratory-confirmed bloodstream infection (LCBI) | Patient has a recognised pathogen cultured from one or more blood cultures, and organism cultured from blood is not related to an infection at another site. | OR | Patient has at least one of the following signs or symptoms: fever (>38 °C), chills or hypotension; signs and symptoms and positive laboratory results are not related to an infection at another site and at least one of the following: (a) common skin contaminant (e.g., diphtheroids, Bacillus sp., Propionibacterium sp., coagulase-negative Staphylococci, or Micrococci) is cultured from two or more blood cultures drawn on separate occasions; (b) common skin contaminant is cultured from at least one blood culture from a patient with an intravascular line, and the physician institutes appropriate antimicrobial therapy; (c) positive antigen test on blood or urine (e.g., H. influenzae, S. pneumoniae, N. meningitidis, or Group B Streptococcus). |
| Ventilator-associated pneumonia/tracheobronchitis (VAP/VAT) | The diagnostic criteria for VAP include: a new infiltrate on chest X-ray associated with at least two of the following: body temperature ≥ 38.5 °C or < 36 °C; leukocyte count ≥ 10 × 109/L or < 1.5 × 109/L; and purulent tracheal aspirate or sputum. In addition, a microbiological confirmation is required for all patients (positive endotracheal aspirate culture ≥ 105 colony-forming units (cfu)/mL or positive bronchoalveolar lavage culture ≥ 104 cfu/mL). VAT is defined using the same criteria as for VAP, except the presence of new or progressive pulmonary infiltrate. | ||
| Procalcitonin (PCT) rise | An increase by at least 50% from previous value at any point in time. | ||
| C-reactive protein (CRP) rise | An increase by at least 50% from previous value at any point in time. | ||
| White blood cell count (WBC) rise | An increase by at least 20% from previous value at any point in time. | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Richards, O.; Pallmann, P.; King, C.; Cheema, Y.; Killick, C.; Thomas-Jones, E.; Harris, J.; Bailey, C.; Szakmany, T. Procalcitonin Increase Is Associated with the Development of Critical Care-Acquired Infections in COVID-19 ARDS. Antibiotics 2021, 10, 1425. https://doi.org/10.3390/antibiotics10111425
Richards O, Pallmann P, King C, Cheema Y, Killick C, Thomas-Jones E, Harris J, Bailey C, Szakmany T. Procalcitonin Increase Is Associated with the Development of Critical Care-Acquired Infections in COVID-19 ARDS. Antibiotics. 2021; 10(11):1425. https://doi.org/10.3390/antibiotics10111425
Chicago/Turabian StyleRichards, Owen, Philip Pallmann, Charles King, Yusuf Cheema, Charlotte Killick, Emma Thomas-Jones, Jessica Harris, Catherine Bailey, and Tamas Szakmany. 2021. "Procalcitonin Increase Is Associated with the Development of Critical Care-Acquired Infections in COVID-19 ARDS" Antibiotics 10, no. 11: 1425. https://doi.org/10.3390/antibiotics10111425
APA StyleRichards, O., Pallmann, P., King, C., Cheema, Y., Killick, C., Thomas-Jones, E., Harris, J., Bailey, C., & Szakmany, T. (2021). Procalcitonin Increase Is Associated with the Development of Critical Care-Acquired Infections in COVID-19 ARDS. Antibiotics, 10(11), 1425. https://doi.org/10.3390/antibiotics10111425







