Assessing the Impact of Heat Treatment of Food on Antimicrobial Resistance Genes and Their Potential Uptake by Other Bacteria—A Critical Review
Abstract
:1. Introduction
2. Results
3. Discussion
3.1. Heat Resistance of AMR Bacteria
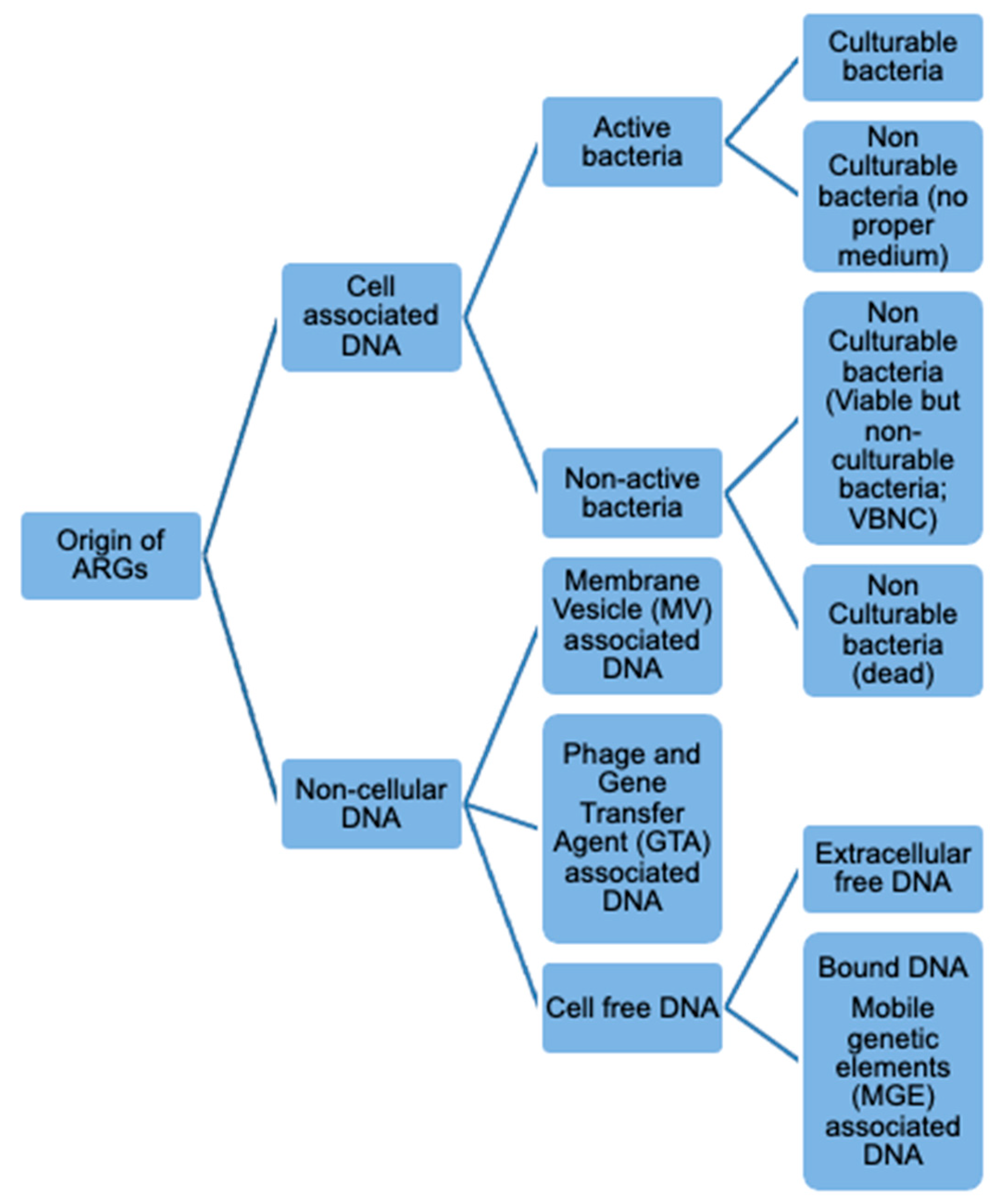
3.2. Fate of ARGs in Heat-Treated Food
3.3. Transfer of ARGs in the Human Gut from Heat-Treated Food
4. Materials and Methods
- (1)
- The publication did not address the impact of heat treatments on AMR bacteria or genes;
- (2)
- The publication was in a language other than English;
- (3)
- The publication measured irrelevant interventions (no heat treatment), outcomes, or populations or samples.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- O’Neill, J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. 2014. Available online: https://amr-review.org/ (accessed on 14 May 2021).
- Verraes, C.; Van Boxstael, S.; Van Meervenne, E.; Van Coillie, E.; Butaye, P.; Catry, B.; Schaetzen, M.A.; Huffel, X.V.; Imberechts, H.; Dierick, K.; et al. Antimicrobial resistance in the food chain: A review. Int. J. Environ. Res. Public Health 2013, 10, 2643–2669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munita, J.M.; Arias, C.A. Mechanisms of antibiotic resistance. Microbiol Spectr. 2016, 4, 4.2.15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bengtsson-Palme, J. Antibiotic resistance in the food supply chain: Where can sequencing and metagenomics aid risk assessment? Curr. Opin. Food Sci. 2017, 14, 66–71. [Google Scholar] [CrossRef]
- Woegerbauer, M.; Bellanger, X.; Merlin, C. Cell-Free DNA: An underestimated source of antibiotic resistance gene dissemination at the interface between human activities and downstream environments in the context of wastewater reuse. Front. Microbiol. 2020, 11, 671. [Google Scholar] [CrossRef] [Green Version]
- Bennett, P.M. Plasmid encoded antibiotic resistance: Acquisition and transfer of antibiotic resistance genes in bacteria. Br. J. Pharmacol. 2008, 153, S347–S357. [Google Scholar] [CrossRef] [Green Version]
- Rossi, F.; Rizzotti, L.; Felis, G.E.; Torriani, S. Horizontal gene transfer among microorganisms in food: Current knowledge and future perspectives. Food Microbiol. 2014, 42, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.P.; Brockhurst, M.A.; Harrison, E. Sampling the mobile gene pool: Innovation via horizontal gene transfer in bacteria. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160424. [Google Scholar] [CrossRef] [Green Version]
- McMahon, M.A.S.; Xu, J.; Moore, J.E.; Blair, I.S.; McDowell, D.A. Environmental stress and antibiotic resistance in food-related pathogens. Appl. Environ. Microbiol. 2007, 73, 211–217. [Google Scholar] [CrossRef] [Green Version]
- Le Devendec, L.; Jouy, E.; Kempf, I. Evaluation of resistance gene transfer from heat-treated Escherichia coli. Int. J. Food Microbiol. 2018, 270, 39–43. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, F.; Mercanoglu Taban, B. A state-of-art review on multi-drug resistant pathogens in foods of animal origin: Risk factors and mitigation strategies. Front. Microbiol. 2019, 10, 2091. [Google Scholar] [CrossRef] [Green Version]
- Lorenz, M.G.; Wackernagel, W. Bacterial gene transfer by natural genetic transformation in the environment. Microbiol. Rev. 1994, 58, 563–602. [Google Scholar] [CrossRef] [PubMed]
- Griffith, F. The significance of pneumococcal types. Epidemiol. Infect. 1928, 27, 113–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huddleston, J.R. Horizontal gene transfer in the human gastrointestinal tract: Potential spread of antibiotic resistance genes. Infect. Drug Resist. 2014, 7, 167. [Google Scholar] [CrossRef] [Green Version]
- Von Wintersdorff, C.J.; Penders, J.; Van Niekerk, J.M.; Mills, N.D.; Majumder, S.; Van Alphen, L.B.; Savelkoul, P.H.M.; Wolffs, P.F. Dissemination of antimicrobial resistance in microbial ecosystems through horizontal gene transfer. Front. Microbiol. 2016, 7, 173. [Google Scholar] [CrossRef] [Green Version]
- Kharazmi, M.; Bauer, T.; Hammes, W.P.; Hertel, C. Effect of food processing on the fate of DNA with regard to degradation and transformation capability in Bacillus subtilis. Syst. Appl. Microbiol. 2003, 26, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Bennett, P.M.; Livesey, C.T.; Nathwani, D.; Reeves, D.S.; Saunders, J.R.; Wise, R. An assessment of the risks associated with the use of antibiotic resistance genes in genetically modified plants: Report of the Working Party of the British Society for Antimicrobial Chemotherapy. J. Antimicrob. Chemother. 2004, 53, 418–431. [Google Scholar] [CrossRef] [PubMed]
- Colavecchio, A.; Cadieux, B.; Lo, A.; Goodridge, L.D. Bacteriophages contribute to the spread of antibiotic resistance genes among foodborne pathogens of the Enterobacteriaceae family–a review. Front. Microbiol. 2017, 8, 1108. [Google Scholar] [CrossRef] [Green Version]
- Jebri, S.; Rahmani, F.; Hmaied, F. Bacteriophages as antibiotic resistance genes carriers in agro-food systems. J. Appl. Microbiol. 2021, 130, 688–698. [Google Scholar] [CrossRef]
- Gómez-Gómez, C.; Blanco-Picazo, P.; Brown-Jaque, M.; Quirós, P.; Rodríguez-Rubio, L.; Cerdà-Cuellar, M.; Muniesa, M. Infectious phage particles packaging antibiotic resistance genes found in meat products and chicken feces. Sci. Rep. 2019, 9, 13281. [Google Scholar] [CrossRef]
- Lee, H.; Ku, H.J.; Lee, D.H.; Kim, Y.T.; Shin, H.; Ryu, S.; Lee, J.H. Characterization and genomic study of the novel bacteriophage HY01 infecting both Escherichia coli O157: H7 and Shigella flexneri: Potential as a biocontrol agent in food. PLoS ONE 2016, 11, e0168985. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, H.; Radford, D.; Kropinski, A.M.; Lim, L.T.; Balamurugan, S. Thermal-stability and reconstitution ability of Listeria phages P100 and A511. Front. Microbiol. 2017, 8, 2375. [Google Scholar] [CrossRef] [PubMed]
- Sommer, J.; Trautner, C.; Witte, A.K.; Fister, S.; Schoder, D.; Rossmanith, P.; Mester, P.J. Don’t shut the stable door after the phage has bolted—The importance of bacteriophage inactivation in food environments. Viruses 2019, 11, 468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, J.; Mahony, J.; Bonestroo, M.; Nauta, A.; van Sinderen, D. Impact of thermal and biocidal treatments on lactococcal 936-type phages. Int. Dairy J. 2014, 34, 56–61. [Google Scholar] [CrossRef]
- Caruana, J.C.; Walper, S.A. Bacterial membrane vesicles as mediators of microbe–microbe and microbe–host community interactions. Front. Microbiol. 2020, 11, 432. [Google Scholar] [CrossRef] [Green Version]
- Toyofuku, M.; Nomura, N.; Eberl, L. Types and origins of bacterial membrane vesicles. Nat. Rev. Microbiol. 2019, 17, 13–24. [Google Scholar] [CrossRef]
- Uddin, M.J.; Dawan, J.; Jeon, G.; Yu, T.; He, X.; Ahn, J. The role of bacterial membrane vesicles in the dissemination of antibiotic resistance and as promising carriers for therapeutic agent delivery. Microorganisms 2020, 8, 670. [Google Scholar] [CrossRef]
- Kulkarni, H.M.; Nagaraj, R.; Jagannadham, M.V. Protective role of E. coli outer membrane vesicles against antibiotics. Microbiol. Res. 2015, 181, 1–7. [Google Scholar] [CrossRef]
- Wagner, T.; Joshi, B.; Janice, J.; Askarian, F.; Škalko-Basnet, N.; Hagestad, O.C.; Mekhkif, A.; Wai, S.N.; Hegstad, K.; Johannessen, M. Enterococcus faecium produces membrane vesicles containing virulence factors and antimicrobial resistance related proteins. J. Proteom. 2018, 187, 28–38. [Google Scholar] [CrossRef] [PubMed]
- James, C.; Dixon, R.A.; Talbot, L.; James, S.J.; Williams, N.; Onarinde, B.A. Assessing the Impact of Heat Treatment on Antimicrobial Resistance Genes and Their Potential Uptake by Other ‘Live’ Bacteria; Food Standards Agency (FSA) Project Number FS307036; Food Standards Agency: London, UK, 2021. [CrossRef]
- Friedman, M. Antibiotic-resistant bacteria: Prevalence in food and inactivation by food-compatible compounds and plant extracts. J. Agric. Food Chem. 2015, 63, 3805–3822. [Google Scholar] [CrossRef]
- Report of the Scientific Committee of the Food Safety Authority of Ireland. Potential for Transmission of Antimicrobial Resistance in the Food Chain; Food Safety Authority of Ireland: Dublin, Ireland, 2015.
- Zhang, Y.; Wang, B. Comparison of the efficacy of commercial antimicrobial interventions for reducing antibiotic resistant and susceptible beef-associated Salmonella and Escherichia coli strains. J. Verbrauch. Lebensm. 2018, 13, 3–23. [Google Scholar] [CrossRef]
- Liao, X.; Ma, Y.; Daliri, E.B.M.; Koseki, S.; Wei, S.; Liu, D.; Ye, X.; Chen, S.; Ding, T. Interplay of antibiotic resistance and food-associated stress tolerance in foodborne pathogens. Trends Food Sci. Technol. 2020, 95, 97–106. [Google Scholar] [CrossRef]
- Bennani, H.; Mateus, A.; Mays, N.; Eastmure, E.; Stärk, K.D.; Häsler, B. Overview of evidence of antimicrobial use and antimicrobial resistance in the food chain. Antibiotics 2020, 9, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutchison, M.L.; Corry, J.E.L.; Madden, R.H. A Review of the Impact of Food Processing on Antimicrobial Resistant Bacteria in Secondary Processed Meats and Meat Products; Project Number FS301059; Food Standards Agency: London, UK, 2020. [CrossRef]
- Woode, B.K.; Daliri, F.; Daliri, E.B.M. Correlation between food processing-associated stress tolerance and antimicrobial resistance in food pathogens. J. Food Hyg. Saf. 2020, 35, 103–108. [Google Scholar] [CrossRef]
- Jiang, X.; Yu, T.; Wu, N.; Meng, H.; Shi, L. Detection of qnr, aac(6 ‘)-Ib-cr and qepA genes in Escherichia coli isolated from cooked meat products in Henan, China. Int. J. Food Microbiol. 2014, 187, 22–25. [Google Scholar] [CrossRef]
- Li, L.; Ye, L.; Yu, L.; Zhou, C.; Meng, H. Characterization of extended spectrum beta-lactamase producing enterobacteria and methicillin-resistant Staphylococcus aureus isolated from raw pork and cooked pork products in South China. J. Food Sci. 2016, 81, M1773–M1777. [Google Scholar] [CrossRef]
- Yu, T.; Zhang, J.; Jiang, X.; Wu, J.; Dai, Z.; Wu, Z.; Liang, Y.; Wang, X. Characterization and horizontal transfer of class 1 integrons in Escherichia coli isolates from cooked meat products. J. Infect. Dev. Ctries 2016, 10, 68–73. [Google Scholar] [CrossRef]
- Jiang, X.; Yu, T.; Liu, L.; Li, Y.; Zhang, K.; Wang, H.; Shi, L. Examination of Quaternary Ammonium Compound resistance in Proteus mirabilis isolated from cooked meat products in China. Front. Microbiol. 2017, 8, 2417. [Google Scholar] [CrossRef]
- Yu, T.; Jiang, X.; Liang, Y.; Zhu, Y.; Tian, J.; Ying, H.; Wang, X.; Shi, L. Characterization and horizontal transfer of antimicrobial resistance genes and integrons in bacteria isolated from cooked meat products in China. J. Food Prot. 2017, 80, 2048–2055. [Google Scholar] [CrossRef]
- Taher, E.M.; Hemmatzadeh, F.; Aly, S.A.; Elesswy, H.A.; Petrovski, K.R. Molecular characterization of antimicrobial resistance genes on farms and in commercial milk with emphasis on the effect of currently practiced heat treatments on viable but nonculturable formation. J. Dairy Sci. 2020, 103, 9936–9945. [Google Scholar] [CrossRef]
- Stopforth, J.D.; Suhalim, R.; Kottapalli, B.; Hill, W.E.; Samadpour, M. Thermal inactivation D-and z-values of multidrug-resistant and non–multidrug-resistant Salmonella serotypes and survival in ground beef exposed to consumer-style cooking. J. Food Prot. 2008, 71, 509–515. [Google Scholar] [CrossRef]
- Bacon, R.T.; Ransom, J.R.; Sofos, J.N.; Kendall, P.A.; Belk, K.E.; Smith, G.C. Thermal inactivation of susceptible and multiantimicrobial-resistant Salmonella strains grown in the absence or presence of glucose. Appl. Environ. Microbiol. 2003, 69, 4123–4128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doherty, A.M.; McMahon, C.M.M.; Sheridan, J.J.; Blair, I.S.; McDowell, D.A.; Hegarty, T. Thermal resistance of Yersinia enterocolitica and Listeria monocytogenes in meat and potato substrates. J. Food Saf. 1998, 18, 69–83. [Google Scholar] [CrossRef]
- Dombroski, C.S.; Jaykus, L.A.; Green, D.P.; Farkas, B.E. Use of mutant strain for evaluating processing strategies to inactivate Vibrio vulnificus in oysters. J. Food Prot. 1999, 62, 592–600. [Google Scholar] [CrossRef]
- Duffy, G.; Walsh, C.; Blair, I.S.; McDowell, D.A. Survival of antibiotic resistant and antibiotic sensitive strains of E. coli O157 and E. coli O26 in food matrices. Int. J. Food Microbiol. 2006, 109, 179–186. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.S.; Beuchat, L.R. Survival of multidrug-resistant Salmonella typhimurium DT104 in egg powders as affected by water activity and temperature. Int. J. Food Microbiol. 1999, 49, 1–8. [Google Scholar] [CrossRef]
- Jung, Y.S.; Beuchat, L.R. Sensitivity of multidrug-resistant Salmonella typhimurium DT104 to organic acids and thermal inactivation in liquid egg products. Food Microbiol. 2000, 17, 63–71. [Google Scholar] [CrossRef]
- Walsh, D.; Sheridan, J.J.; Duffy, G.; Blair, I.S.; McDowell, D.A.; Harrington, D. Thermal resistance of wild-type and antibiotic-resistant Listeria monocytogenes in meat and potato substrates. J. Appl. Microbiol. 2001, 90, 555–560. [Google Scholar] [CrossRef]
- Walsh, C.; Duffy, G.; Sheridan, J.J.; Fanning, S.; Blair, I.S.; McDowell, D.A. Thermal resistance of antibiotic-resistant and antibiotic-sensitive Salmonella spp. on chicken meat. J. Food Saf. 2005, 25, 288–302. [Google Scholar] [CrossRef]
- McKay, A.M. Antimicrobial resistance and heat sensitivity of oxacillin-resistant, mecA-positive Staphylococcus spp. from unpasteurized milk. J. Food Prot. 2008, 71, 186–190. [Google Scholar] [CrossRef]
- Lianou, A.; Koutsoumanis, K.P. Evaluation of the strain variability of Salmonella enterica acid and heat resistance. Food Microbiol. 2013, 34, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Maserati, A.; Diez-Gonzalez, F.; Sampedro, F. Does antibiotic resistance influence shiga-toxigenic Escherichia coli O26 and O103 survival to stress environments? Food Control. 2016, 68, 330–336. [Google Scholar] [CrossRef] [Green Version]
- Rincón, A.M.; Singh, R.K. Effect of radio frequency heating on nalidixic acid-adapted shiga toxin-producing and non-pathogenic Escherichia coli strains in buffer. Food Bioprocess. Technol. 2016, 9, 1535–1541. [Google Scholar] [CrossRef]
- Komora, N.; Bruschi, C.; Rui, M.; Ferreira, V.; Teixeira, P. Survival of Listeria monocytogenes with different antibiotic resistance patterns to food-associated stresses. Int. J. Food Microbiol. 2017, 245, 79–87. [Google Scholar] [CrossRef]
- Ma, Y.; Lan, G.; Li, C.; Cambaza, E.M.; Liu, D.; Ye, X.; Chen, S.; Ding, T. Stress tolerance of Staphylococcus aureus with different antibiotic resistance profiles. Microb. Pathog. 2019, 133, 103549. [Google Scholar] [CrossRef]
- Xu, A.; Chuang, S.; Scullen, O.J.; Huang, L.; Sheen, S.; Sheen, L.Y.; Johnson, J.R.; Sommers, C.H. Thermal inactivation of extraintestinal pathogenic Escherichia coli suspended in ground chicken meat. Food Control. 2019, 104, 269–277. [Google Scholar] [CrossRef]
- Yehia, H.M.; Al-Masoud, A.H.; Alarjani, K.M.; Alamri, M.S. Prevalence of methicillin-resistant (mecA gene) and heat-resistant Staphylococcus aureus strains in pasteurized camel milk. J. Dairy Sci. 2020, 103, 5947–5963. [Google Scholar] [CrossRef]
- Sarjit, A.; Ravensdale, J.T.; Coorey, R.; Fegan, N.; Dykes, G.A. Survival of Salmonella on red meat in response to dry heat. J. Food Prot. 2021, 84, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.K.; Yanamala, S.; Francisco, M.S.; Loneragan, G.H.; Miller, M.F.; Brashears, M.M. Reduction of multidrug-resistant and drug-susceptible Salmonella in ground beef and freshly harvested beef briskets after exposure to commonly used industry antimicrobial interventions. J. Food Prot. 2010, 73, 1231–1237. [Google Scholar] [CrossRef]
- Foegeding, P.M.; Stanley, N.W. Listeria innocua transformed with an antibiotic resistance plasmid as a thermal-resistance indicator for Listeria monocytogenes. J. Food Prot. 1991, 54, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Geornaras, I.; Belk, K.E.; Smith, G.C.; Sofos, J.N. Inactivation of Escherichia coli O157: H7 in moisture-enhanced nonintact beef by pan-broiling or roasting with various cooking appliances set at different temperatures. J. Food Sci. 2011, 76, M64–M71. [Google Scholar] [CrossRef]
- Luchansky, J.B.; Porto-Fett, A.C.; Shoyer, B.A.; Thippareddi, H.; Amaya, J.R.; Lemler, M. Thermal inactivation of Escherichia coli O157: H7 and non-O157 Shiga toxin–producing Escherichia coli cells in mechanically tenderized veal. J. Food Prot. 2014, 77, 1201–1206. [Google Scholar] [CrossRef]
- Capita, R.; Alonso-Calleja, C. Antibiotic-resistant bacteria: A challenge for the food industry. Crit. Rev. Food Sci. Nutr. 2013, 53, 11–48. [Google Scholar] [CrossRef]
- Ferri, M.; Ranucci, E.; Romagnoli, P.; Giaccone, V. Antimicrobial resistance: A global emerging threat to public health systems. Crit. Rev. Food Sci. Nutr. 2017, 57, 2857–2876. [Google Scholar] [CrossRef] [PubMed]
- Wesche, A.M.; Gurtler, J.B.; Marks, B.P.; Ryser, E.T. Stress, sublethal injury, resuscitation, and virulence of bacterial foodborne pathogens. J. Food Prot. 2009, 72, 1121–1138. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.D. Lethal effects of heat on bacterial physiology and structure. Sci. Prog. 2003, 86, 115–137. [Google Scholar] [CrossRef]
- Mackey, B.M.; Miles, C.A.; Parsons, S.E.; Seymour, D.A. Thermal denaturation of whole cells and cell components of Escherichia coli examined by differential scanning calorimetry. Microbiology 1991, 137, 2361–2374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohácsi-Farkas, C.; Farkas, J.; Meszaros, L.; Reichart, O.; Andrassy, E. Thermal denaturation of bacterial cells examined by differential scanning calorimetry. J. Therm. Anal. Calorim. 1999, 57, 409–414. [Google Scholar] [CrossRef]
- Wang, X.; Lim, H.J.; Son, A. Characterization of denaturation and renaturation of DNA for DNA hybridization. Environ. Health Toxicol. 2014, 29, e2014007. [Google Scholar] [CrossRef]
- Ducey, T.F.; Collins, J.C.; Ro, K.S.; Woodbury, B.L.; Griffin, D.D. Hydrothermal carbonization of livestock mortality for the reduction of pathogens and microbially-derived DNA. Front. Environ. Sci. Eng. 2017, 11, 1–8. [Google Scholar] [CrossRef]
- Koncan, R.; García-Albiach, R.; Bravo, D.; Cantón, R.; Baquero, F.; Cornaglia, G.; del Campo, R. The fate of antibiotic resistance genes in cooked meat. Int. J. Antimicrob. Agents 2007, 29, S130. [Google Scholar] [CrossRef]
- Taher, E.M.; Hemmatzadeh, F.; Aly, S.A.; Elesswy, H.A.; Petrovski, K.R. Survival of staphylococci and transmissibility of their antimicrobial resistance genes in milk after heat treatments. LWT 2020, 109584. [Google Scholar] [CrossRef]
- Masters, C.I.; Miles, C.A.; Mackey, B.M. Survival and biological activity of heat damaged DNA. Lett. Appl. Microbiol. 1998, 27, 279–282. [Google Scholar] [CrossRef]
- Sparo, M.; Delpech, G.; García Allende, N. Impact on public health of the spread of high-level resistance to gentamicin and vancomycin in enterococci. Front. Microbiol. 2018, 9, 3073. [Google Scholar] [CrossRef] [Green Version]
- Lewis, R.J.; Baldwin, A.; O’Neill, T.; Alloush, H.A.; Nelson, S.M.; Dowman, T.; Salisbury, V. Use of Salmonella enterica serovar Typhimurium DT104 expressing lux genes to assess, in real time and in situ, heat inactivation and recovery on a range of contaminated food surfaces. J. Food Eng. 2006, 76, 41–48. [Google Scholar] [CrossRef]
- De Jonge, R. Predictable and unpredictable survival of foodborne pathogens during non-isothermal heating. Int. J. Food Microbiol. 2019, 291, 151–160. [Google Scholar] [CrossRef]
- Gunasekera, T.S.; Sørensen, A.; Attfield, P.V.; Sørensen, S.J.; Veal, D.A. Inducible gene expression by nonculturable bacteria in milk after pasteurization. Appl. Environ. Microbiol. 2002, 68, 1988–1993. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Lee, E.Y.; Kim, S.H.; Kim, D.K.; Park, K.S.; Kim, K.P.; Kim, Y.K.; Roh, T.Y.; Gho, Y.S. Staphylococcus aureus extracellular vesicles carry biologically active β-lactamase. Antimicrob. Agents Chemother. 2013, 57, 2589–2595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aubry-Damon, H.; Grenet, K.; Sall-Ndiaye, P.; Che, D.; Cordeiro, E.; Bougnoux, M.E.; Rigaud, E.; Le Strat, Y.; Lemanissier, V.; Armand-Lefèvre, L.; et al. Antimicrobial resistance in commensal flora of pig farmers. Emerg. Infect. Dis. 2004, 10, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Hart, W.S.; Heuzenroeder, M.W.; Barton, M.D. A study of the transfer of tetracycline resistance genes between Escherichia coli in the intestinal tract of a mouse and a chicken model. J. Vet. Med. B Infect. Dis Vet. Public Health 2006, 53, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Ramchandani, M.; Manges, A.R.; DebRoy, C.; Smith, S.P.; Johnson, J.R.; Riley, L.W. Possible animal origin of human-associated, multidrug-resistant, uropathogenic Escherichia coli. Clin. Infect. Dis. 2005, 40, 251–257. [Google Scholar] [CrossRef]
- Schjørring, S.; Krogfelt, K.A. Assessment of bacterial antibiotic resistance transfer in the gut. Int. J. Microbiol. 2011, 2011, 312956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broaders, E.; Gahan, C.G.; Marchesi, J.R. Mobile genetic elements of the human gastrointestinal tract: Potential for spread of antibiotic resistance genes. Gut Microbes 2013, 4, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.; Matthias, T.; Aminov, R. Potential effects of horizontal gene exchange in the human gut. Front. Immunol. 2017, 8, 1630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McInnes, R.S.; McCallum, G.E.; Lamberte, L.E.; van Schaik, W. Horizontal transfer of antibiotic resistance genes in the human gut microbiome. Curr. Opin. Microbiol. 2020, 53, 35–43. [Google Scholar] [CrossRef]
- Buffie, C.G.; Pamer, E.G. Microbiota-mediated colonization resistance against intestinal pathogens. Nat. Rev. Immunol. 2013, 13, 790–801. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, H.; Suzuki, E.; Maeda, S. Horizontal plasmid transfer by transformation in Escherichia coli: Environmental factors and possible mechanisms. Front. Microbiol. 2018, 9, 2365. [Google Scholar] [CrossRef] [Green Version]
| Evaluation Temperatures (°C) | Medium | Species and Strains | Enhanced Thermal Resistance | Stated Antimicrobial Resistance Profiles (Antimicrobial or Class) | Reference |
|---|---|---|---|---|---|
| 50–60 | Minced beef and potato | Y. enterocolitica | No | Nalidixic acid | [46] |
| 47 | Oysters | Vibrio vulnificus | No | Nalidixic acid | [47] |
| 54, 82 | Egg white powder | S. Typhimurium DT104 | No | NS | [49] |
| 51, 53, 55, 57, 59, 61 | Liquid whole egg, egg yolk, egg white, whole egg + 10% salt, egg yolk + 10% salt | S. Typhimurium DT104 | No | NS, but strains of DT104 quoted as being resistant to ampicillin, chloramphenicol streptomycin, sulfonamides, tetracyclines | [50] |
| 55 | Minced beef and potato | L. monocytogenes | No | Streptomycin | [51] |
| 55, 57, 59, 61 | Tryptic soy broth (TSB) | Salmonella spp. serovars Saint-Paul, Anatum, Mbandaka, Agona, Reading, Typhimurium (DT104) | No | Ampicillin, chloramphenicol, streptomycin, sulfonamides, and tetracycline, amoxicillin-clavulanic acid, ampicillin-sulbactam, gentamicin, trimethoprim-sulfamethoxazole Depending on serotype or strain | [45] |
| 55 | Chicken pieces | S. Typhimurium DT104 | Yes | Ampicillin, streptomycin, sulfonamides, chloramphenicol, tetracyclines | [52] |
| 55 | Chicken pieces | S. Enteritidis, S. Typhimurium | No | Nalidixic acid, streptomycin | [52] |
| 55 | Minced beef | E. coli O157:H7, O26 | No | Ampicillin, kanamycin, streptomycin, trimethoprim, nalidixic acid, rifampicin, sulfonamides, chloramphenicol, tetracycline, minocycline, doxycycline Depending on serotype or strain | [48] |
| 55, 60, 65, 70 | Tryptic soy broth (TSB) | Salmonella spp. serovars Montevideo, Typhimurium, Anatum, Muenster, Newport, Mbandaka, Dublin Reading, Agona, Give | No | Ampicillin, chloramphenicol, streptomycin, sulfonamides, tetracycline, amoxicillin–clavulanic acid, kanamycin, sulfamethoxazole-trimethoprim, gentamicin Depending on serotype or strain | [44] |
| 56 | Whole milk | mecA- carrying Staphylococcus spp. strains (S. epidermidis, haemolyticus, lentus) | No | Tetracycline, kanamycin, spectinomycin, erythromycin, trimethoprim, sulfamethoxazole-trimethoprim Depending on serotype or strain | [53] |
| 57 | Tryptic soy broth (TSB-G) | 60 Salmonella spp. serovars including: Typhimurium (18 strains), Enteritidis (10 strains), Newport (9 strains), Heidelberg (8 strains), Montevideo (4 strains), Senftenberg (4 strains), Agona (3 strains), Infantis (3 strains) and Derby (1 strain). | No | NS | [54] |
| 60, 61, 62.5 | Tryptic soy broth (TSB) | E. coli (STEC) serotypes O26 and O103 | No | Ampicillin, penicillin, ceftiofur, spectinomycin, oxytetracycline, clindamycin, sulfadimethoxime, tiamulin, tilmicosin, tetracycline Depending on serotype or strain | [55] |
| 55, 60, 65 (Radio Frequency heating) | Phosphate buffer saline (PBS) | E. coli (STEC) serotypes O157:H7, O26:H11, O11 | No | Nalidixic acid | [56] |
| 58 | Ringer’s solution | L. monocytogenes | No | Erythromycin, ciprofloxacin, nitrofurantoin | [57] |
| 63 | Saline solution | S. aureus | No | Ciprofloxacin, chloramphenicol, erythromycin, penicillin, sulfamethoxazole, clindamycin, tetracycline, oxacillin, cefoxitin, gentamicin, ciprofloxacin Depending on serotype or strain | [58] |
| 55, 60, 65 | Minced chicken | Extraintestinal pathogenic E. coli (ExPEC) | No | Aminoglycosides, macrolides, sulfonamides, trimethoprim, tetracycline, beta-lactams, cefotaxime, phenicol, aminoglycosides, streptomycin Depending on serotype or strain | [59] |
| 85, 95 | BHI medium | MRSA S. aureus (ATCC 29,737, control) | Yes | Cefoxitin, cefadroxil, cephalothin, colistin, polymyxin, aminoglycosides, streptomycin, amikacin, kanamycin:cyclic peptides, bacitracin, tetracycline: sulfonamide, sulfamethoxazole, nalidixic acid:fluoroquinolone, ciprofloxacin:oxazolidone, linezolid:macrobid | [60] |
| Reference | [74] | [10] | [75] | [76] |
|---|---|---|---|---|
| Mimic | Cooking—boiled (20 min), grilled (10 min), microwaved (5 min, 900 W), or autoclaved (20 min, 121 °C) | General heat treatments | Milk pasteurization (sterilization) | Non-food autoclaving |
| Medium | Chicken, beef, pork | Saline | Milk and elution buffer | Distilled water and in presence of salt |
| Evaluation temperatures (°C) | Not Stated | 40, 50,60, 70, 80, 90, 100 | 63.5, 121 | 121, 135 |
| Species | E. faecalis | E. coli | S. aureus, S. sciuri | Plasmid (pUC18) |
| Antimicrobial Resistance Genes (ARGs) present | aac(6′)-Ie-aph(2″)-Ia | blaCTX-M-1, blaCMY-2, tetA, strA | blaZ, mecC, tetK | NS |
| Stated antimicrobial resistance profiles | Aminoglycosides, except to streptomycin(predicted profile, not tested) | Cephalosporins, tetracycline, streptomycin | Penicillin, methicillin, tetracycline | Ampicillin |
| Recipient species | E. faecalis | E. coli | S. aureus | E. coli |
| ARGs detected post treatment from non-culturable samples | YES | YES | YES | - |
| Transformation demonstrated | NO | YES 70 °C for 30 min | YES 63.5 °C for 30 min | YES 121 °C for 15 min |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
James, C.; Dixon, R.; Talbot, L.; James, S.J.; Williams, N.; Onarinde, B.A. Assessing the Impact of Heat Treatment of Food on Antimicrobial Resistance Genes and Their Potential Uptake by Other Bacteria—A Critical Review. Antibiotics 2021, 10, 1440. https://doi.org/10.3390/antibiotics10121440
James C, Dixon R, Talbot L, James SJ, Williams N, Onarinde BA. Assessing the Impact of Heat Treatment of Food on Antimicrobial Resistance Genes and Their Potential Uptake by Other Bacteria—A Critical Review. Antibiotics. 2021; 10(12):1440. https://doi.org/10.3390/antibiotics10121440
Chicago/Turabian StyleJames, Christian, Ronald Dixon, Luke Talbot, Stephen J. James, Nicola Williams, and Bukola A. Onarinde. 2021. "Assessing the Impact of Heat Treatment of Food on Antimicrobial Resistance Genes and Their Potential Uptake by Other Bacteria—A Critical Review" Antibiotics 10, no. 12: 1440. https://doi.org/10.3390/antibiotics10121440
APA StyleJames, C., Dixon, R., Talbot, L., James, S. J., Williams, N., & Onarinde, B. A. (2021). Assessing the Impact of Heat Treatment of Food on Antimicrobial Resistance Genes and Their Potential Uptake by Other Bacteria—A Critical Review. Antibiotics, 10(12), 1440. https://doi.org/10.3390/antibiotics10121440








