Synergistic Effect of Combination Interventions for Methicillin-Resistant Staphylococcus aureus Transmission Control in Nursing Homes: A Computation Modelling Evaluation with Heterogeneous Contact Mixing
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
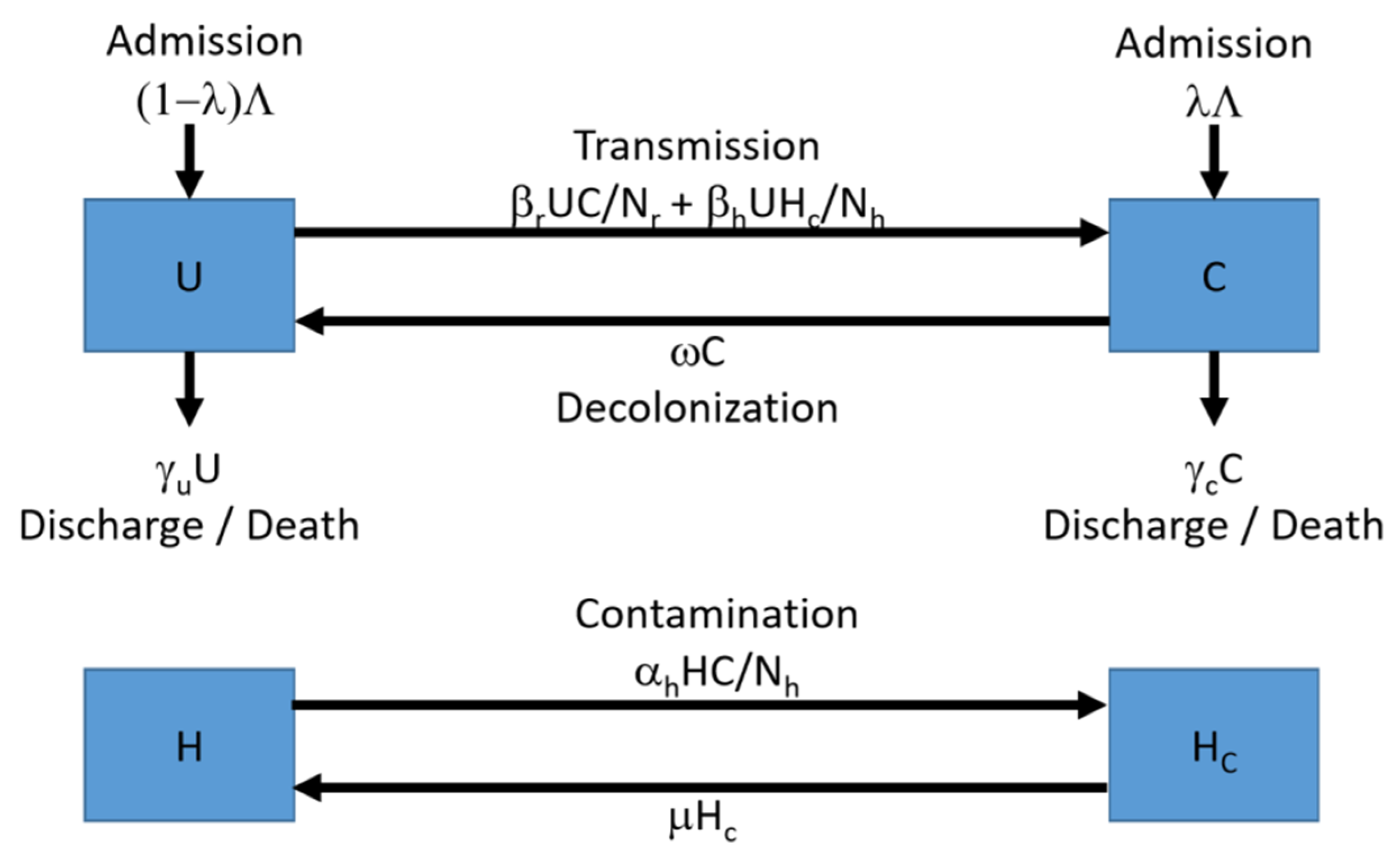
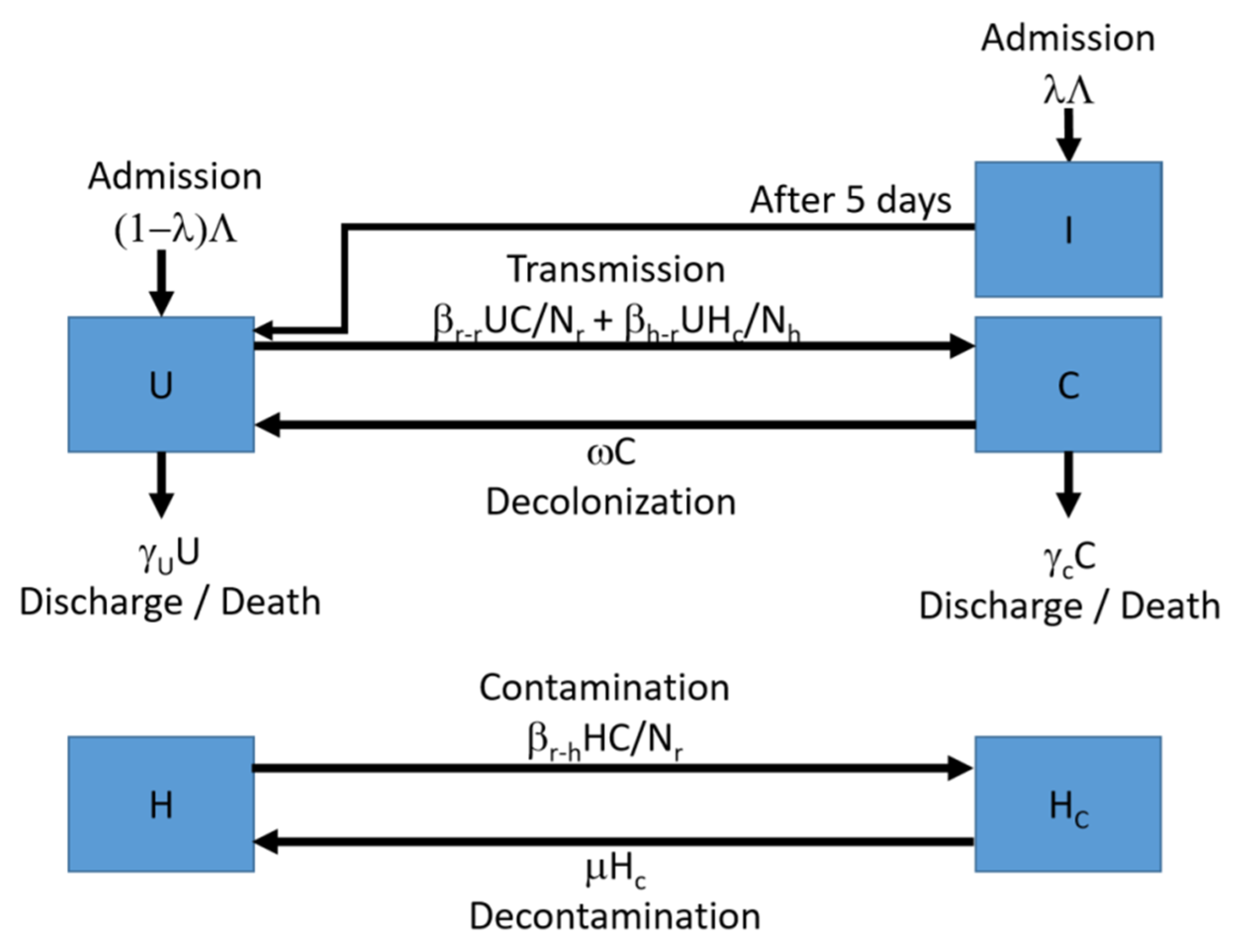
4.1. The Computational Model
4.2. Parameterization
4.2.1. Parameter Identification
4.2.2. Parameter Estimation
4.3. Simulations of Interventions
4.3.1. Intervention Scenario 1: Hand-Hygiene Compliance by HCWs
4.3.2. Intervention Scenario 2: Screening-and-Isolation Upon Admission
4.3.3. Intervention Scenario 3: Implementing Both Interventions at the Same Time
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- US Centers for Disease Control and Prevention. Biggest Threats and Data—Antibiotic/Antimicrobial Resistance (AR/AMR). Available online: https://www.cdc.gov/drugresistance/biggest_threats.html (accessed on 3 August 2020).
- Bradley, S.F. Methicillin-resistant Staphylococcus aureus in nursing homes. Epidemiology, prevention and management. Drugs Aging 1997, 10, 185–198. [Google Scholar] [CrossRef]
- O’Sullivan, N.R.; Keane, C.T. The prevalence of methicillin-resistant staphylococcus aureus among the residents of six nursing homes for the elderly. J. Hosp. Infect. 2000, 45, 322–329. [Google Scholar] [CrossRef]
- Wong, J.W.; Ip, M.; Tang, A.; Wei, V.W.; Wong, S.Y.; Riley, S.; Read, J.M.; Kwok, K.O. Prevalence and risk factors of community-associated methicillin-resistant Staphylococcus aureus carriage in Asia-Pacific region from 2000 to 2016: A systematic review and meta-analysis. Clin. Epidemiol. 2018, 10, 1489–1501. [Google Scholar] [CrossRef] [Green Version]
- Boucher, H.W.; Corey, G.R. Epidemiology of methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 2008, 46, S344–S349. [Google Scholar] [CrossRef] [Green Version]
- Chamchod, F.; Ruan, S. Modeling the spread of methicillin-resistant Staphylococcus aureus in nursing homes for elderly. PLoS ONE 2012, 7, e29757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Datta, R.; Huang, S.S. Risk of infection and death due to methicillin-resistant Staphylococcus aureus in long-term carriers. Clin. Infect. Dis. 2008, 47, 176–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawkins, G.; Stewart, S.; Blatchford, O.; Reilly, J. Should healthcare workers be screened routinely for meticillin-resistant Staphylococcus aureus? A review of the evidence. J. Hosp. Infect. 2011, 77, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Garazi, M.; Edwards, B.; Caccavale, D.; Auerbach, C.; Wolf-Klein, G. Nursing homes as reservoirs of MRSA: Myth or reality? J. Am. Med. Dir. Assoc. 2009, 10, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Mody, L.; Kauffman, C.A.; Donabedian, S.; Zervos, M.; Bradley, S.F. Epidemiology of Staphylococcus aureus colonization in nursing home residents. Clin. Infect. Dis. 2008, 46, 1368–1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynolds, C.; Quan, V.; Kim, D.; Peterson, E.; Dunn, J.; Whealon, M.; Terpstra, L.; Meyers, H.; Cheung, M.; Lee, B.; et al. Methicillinresistant Staphylococcus aureus (MRSA) carriage in 10 nursing homes in Orange County, California. Infect. Control Hosp. Epidemiol. 2011, 32, 91–93. [Google Scholar] [CrossRef]
- Bradley, S.F.; Terpenning, M.S.; Ramsey, M.A.; Zarins, L.T.; Jorgensen, K.A.; Sottile, W.S.; Schaberg, D.R.; Kauffman, C.A. Methicillinresistant Staphylococcus aureus: Colonization and infection in a long-term care facility. Ann. Intern. Med. 1991, 115, 417–422. [Google Scholar] [CrossRef]
- Sanford, M.D.; Widmer, A.F.; Bale, M.J.; Jones, R.N.; Wenzel, R.P. Efficient detection and long-term persistence of the carriage of methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 1994, 19, 1123–1128. [Google Scholar] [CrossRef]
- Hong Kong Centre for Health Protection. Methicillin-resistant Staphylococcus Aureus (MRSA) Infection. Available online: https://www.chp.gov.hk/en/healthtopics/content/24/10688.html (accessed on 3 August 2020).
- Kwok, K.O.; Read, J.M.; Tang, A.; Chen, H.; Riley, S.; Kam, K.M. A systematic review of transmission dynamic studies of methicillin-resistant Staphylococcus aureus in non-hospital residential facilities. BMC Infect. Dis. 2018, 18, 188. [Google Scholar] [CrossRef] [Green Version]
- Kwok, K.O.; Tang, A.; Chan, H.H.; Wong, S.Y.S.; Wei, W.I. A Longitudinal Contact Survey in Residential Care Homes for the Elderly in Hong Kong. Available online: https://www.cuhk.edu.hk/sphpc/kkokwok/manuscript_rche_contact.pdf (accessed on 23 February 2021).
- Hughes, C.; Tunney, M.; Bradley, M.C. Infection control strategies for preventing the transmission of methicillin-resistant Staphylococcus aureus (MRSA) in nursing homes for older people. Cochrane Database Syst. Rev. 2013, 11, CD006354. [Google Scholar]
- Baldwin, N.S.; Gilpin, D.F.; Tunney, M.M.; Kearney, M.P.; Crymble, L.; Cardwell, C.; Hughes, C.M. Cluster randomised controlled trial of an infection control education and training intervention programme focusing on meticillin-resistant Staphylococcus aureus in nursing homes for older people. J. Hosp. Infect. 2010, 76, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Hequet, D.; Rousson, V.; Blanc, D.S.; Bula, C.; Qalla-Widmer, L.; Masserey, E.; Zanetti, G.; Petignat, C. Universal screening and decolonization for control of MRSA in nursing homes: Follow-up of a cluster randomized controlled trial. J. Hosp. Infect. 2017, 96, 69–71. [Google Scholar] [CrossRef]
- Peterson, L.R.; Boehm, S.; Beaumont, J.L.; Patel, P.A.; Schora, D.M.; Peterson, K.E.; Burdsall, D.; Hines, C.; Fausone, M.; Robicsek, A.; et al. Reduction of Methicillin-Resistant Staphylococcus aureus (MRSA) Infection in Long Term Care is Possible While Maintaining Patient Socialization: A Prospective Randomized Clinical Trial. Am. J. Infect. Control 2017, 44, 1622–1627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ojanperä, H.; Kanste, O.I.; Syrjala, H. Hand-hygiene compliance by hospital staff and incidence of health-care-associated infections, Finland. Bull. World Health Organ. 2020, 98, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.Y.; Huang, Y.; Wei, W.I.; Wong, S.Y.S.; Kwok, K.O. Approaches to Multidrug-Resistant Organism Prevention and Control in Long-Term Care Facilities for Older People: A Systematic Review and Meta-Analysis. Available online: https://www.researchsquare.com/article/rs-207428/v1 (accessed on 23 February 2021).
- Huang, T.T.; Wu, S.C. Evaluation of a training programme on knowledge and compliance of nurse assistants’ hand hygiene in nursing homes. J. Hosp. Infect. 2010, 68, 164–170. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Health Organization Guidelines on Hand Hygiene in Health Care. First Global Patient Safety Challenge Clean Care is Safer Care. 2009. Available online: https://www.who.int/gpsc/5may/tools/en/ (accessed on 31 July 2020).
- Gould, D.J.; Moralejo, D.; Drey, N.; Chudleigh, J.H.; Taljaard, M. Interventions to improve hand hygiene compliance in patient care. Cochrane Database Syst. Rev. 2017, 9, CD005186. [Google Scholar] [CrossRef]
- Boyce, J.M.; Havill, N.L.; Kohan, C.; Dumigan, D.G.; Ligi, C.E. Do infection control measures work for methicillin-resistant Staphylococcus aureus? Infect. Control Hosp. Epidemiol. 2004, 25, 395–401. [Google Scholar] [CrossRef]
- Verbrugh, H.A. Value of screening and isolation for control of methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 2005, 41, 268–271. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.O.; Elramalli, A.K.; Amri, S.G.; Abuzweda, A.R.; Abouzeed, Y.M. Isolation and screening of methicillin-resistant Staphylococcus aureus from health care workers in Libyan hospitals. East. Mediterr. Health J. 2012, 18, 37–42. [Google Scholar] [CrossRef]
- Popiel, K.Y.; Miller, M.A. Evaluation of vancomycin-resistant enterococci (VRE)-associated morbidity following relaxation of VRE screening and isolation precautions in a tertiary care hospital. Infect. Control Hosp. Epidemiol. 2014, 35, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Cheng, V.C.; Tai, J.W.; Wong, Z.S.; Chen, J.H.; Pan, K.B.; Hai, Y.; Ng, W.-C.; Chow, D.M.K.; Yau, M.C.Y.; Chan, J.F.W.; et al. Transmission of methicillin-resistant Staphylococcus aureus in the long term care facilities in Hong Kong. BMC Infect. Dis. 2013, 13, 205. [Google Scholar] [CrossRef] [Green Version]
- Shenoy, E.S.; Paras, M.L.; Noubary, F.; Walensky, R.P.; Hooper, D.C. Natural history of colonization with methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE): A systematic review. BMC Infect. Dis. 2014, 14, 177. [Google Scholar] [CrossRef] [PubMed]
- Kwok, C.L.; Lee, C.K.M.; Lo, W.T.L.; Yip, P.S.F. The Contribution of Ageing to Hospitalisation Days in Hong Kong: A Decomposition Analysis. Int. J. Health Policy Manag. 2017, 6, 155–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pineles, L.; Morgan, D.J.; Lydecker, A.; Johnson, J.K.; Sorkin, J.D.; Langenberg, P.; Blanco, N.; Lesse, A.; Sellick, J.; Gupta, K.; et al. Transmission of methicillin-resistant Staphylococcus aureus to health care worker gowns and gloves during care of residents in Veterans Affairs nursing homes. Am. J. Infect. Control 2017, 45, 947–953. [Google Scholar] [CrossRef]
- Kwok, K.O.; Wei, W.I.; Yu, C.H.; Hsu, E.K.; Wong, S.Y.S.; Lee, S.S.; Chen, H. Prevalence of Methicillin-Resistant Staphylococcus Aureus in Residential Care Homes for the Elderly in Hong Kong. Available online: https://www.cuhk.edu.hk/sphpc/kkokwok/manuscript_rche_mrsa_prevalence.pdf (accessed on 23 February 2021).
- Chen, H.; Au, K.M.; Hsu, K.E.; Lai, C.K.; Myint, J.; Mak, Y.F.; Lee, S.Y.; Wong, T.Y.; Tsang, N.C. Multidrug-resistant organism carriage among residents from residential care homes for the elderly in Hong Kong: A prevalence survey with stratified cluster sampling. Hong Kong Med. J. 2018, 24, 350–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, V.C.C.; Chen, H.; Wong, S.C.; Chen, J.H.K.; Ng, W.C.; So, S.Y.C.; Chan, T.-C.; Wong, S.C.Y.; Ho, P.-L.; Mody, L.; et al. Role of Hand Hygiene Ambassador and Implementation of Directly Observed Hand Hygiene Among Residents in Residential Care Homes for the Elderly in Hong Kong. Infect. Control Hosp. Epidemiol. 2018, 39, 571–577. [Google Scholar] [CrossRef]



| Hand-Hygiene Compliance Level | Average Reduction in Prevalence (In Percentage Point) | Standard Deviation | 95% C.I. |
|---|---|---|---|
| 10% reduction in average contamination duration for HCWs | 1.38 | 0.92 | 1.30–1.46 |
| 20% reduction in average contamination duration for HCWs | 2.84 | 1.91 | 2.67–3.01 |
| 30% reduction in average contamination duration for HCWs | 4.37 | 2.93 | 4.10–4.62 |
| 40% reduction in average contamination duration for HCWs | 5.91 | 3.96 | 5.56–6.26 |
| 50% reduction in average contamination duration for HCWs | 7.44 | 4.93 | 7.01–7.88 |
| Hand-Hygiene Compliance Level | Average Reduction in Prevalence (In Percentage Point) | Standard Deviation | 95% C.I. |
|---|---|---|---|
| 10% reduction in average contamination duration for HCWs | 21.99 | 0.65 | 21.93–22.05 |
| 20% reduction in average contamination duration for HCWs | 25.34 | 2.60 | 25.11–25.57 |
| 30% reduction in average contamination duration for HCWs | 28.31 | 4.14 | 27.94–28.67 |
| 40% reduction in average contamination duration for HCWs | 30.00 | 4.49 | 29.60–30.40 |
| 50% reduction in average contamination duration for HCWs | 30.94 | 4.44 | 30.55–31.34 |
| Description | Symbol | Value |
|---|---|---|
| Residents-to-residents transmission rate | βr-r | ar-rpr-r |
| HCWs-to-residents transmission rate | βh-r | ah-rph-r |
| Residents-to-HCWs transmission rate | βr-h | ar-hpr-h |
| Description | Symbol |
|---|---|
| Average number of residents-to-residents contact | ar-r |
| Average number of HCWs-to-residents contact | ah-r |
| Average number of residents-to-HCWs contact | ar-h |
| Transmission probability via residents-to-residents contact | pr-r |
| Transmission probability via HCWs-to-residents contact | ph-r |
| Transmission probability via residents-to-HCWs contact | pr-h |
| Description | Symbol | Value | Reference |
|---|---|---|---|
| Number of residents | Nr | 75.10 | [16] |
| Number of HCWs | Nhcw | 8.61 | [16] |
| Probability of admission of colonized residents | Λ | 15.8% | [30] |
| Average duration of colonization (days) | 1/ω | 268.8 | [31] |
| Average length of stay for uncolonized residents (days) | 1/γu | 233.89 * | [30,32] |
| Average length of stay for colonized residents (days) | 1/γc | 233.89 * | [30,32] |
| Average contamination duration (hours) | 1/μ | 4.1 * | [16] |
| Average number of residents-to-residents contact | ar-r | 0.2 | [16] |
| Average number of HCWs-to-residents contact | ah-r | 1.2 | [16] |
| Average number of residents-to-HCWs contact | ar-h | 12.7 | [16] |
| Average number of HCWs-to-HCWs contact | ah-h | 0.6 | [16] |
| Transmission probability via residents-to-residents contact | pr-r | Not identified | |
| Transmission probability via HCWs-to-residents contact | ph-r | Not identified | |
| Transmission probability via residents-to-HCWs contact | pr-h | 20% | [33] |
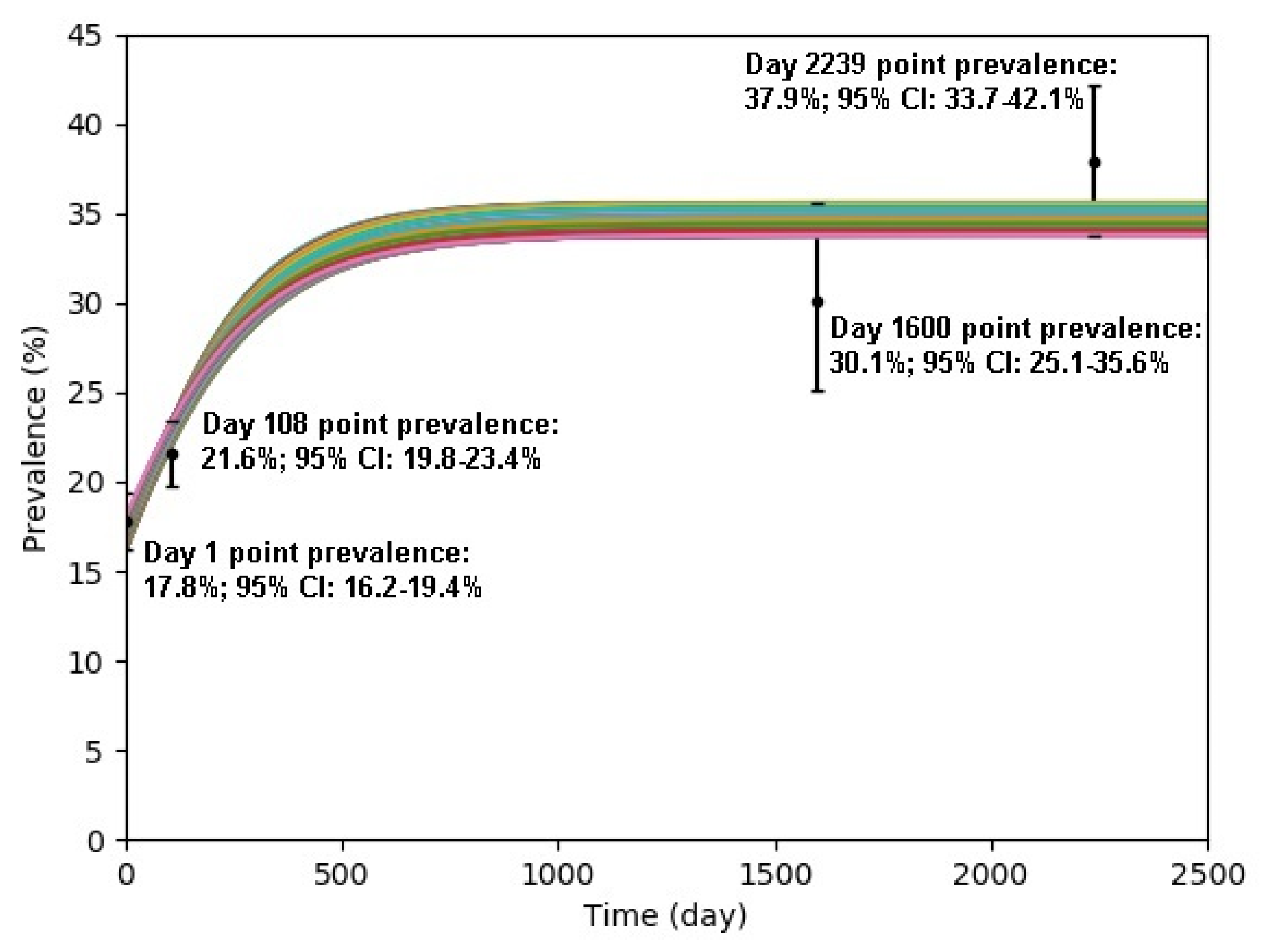
| Study Period | Mid-Date of Study Period | Day Count | Prevalence | 95% C.I. | Reference |
|---|---|---|---|---|---|
| 3 March–26 September 2011 | 14 June 2011 | 1 | 17.8% | 16.2–19.4% | [34] |
| 1 July–31 December 2011 | 30 September 2011 | 108 | 21.6% | 19.8–23.4% | [30] |
| 1 September–31 December 2015 | 31 October 2015 | 1600 | 30.1% | 25.1–35.6% | [35] |
| 1 July–31 August 2017 | 31 July 2015 | 2239 | 37.9% | 33.7–42.1% | [36] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, A.; Kwok, K.O.; Wei, V.W.I.; Chen, H.; Wong, S.Y.S.; Tam, W.W.S. Synergistic Effect of Combination Interventions for Methicillin-Resistant Staphylococcus aureus Transmission Control in Nursing Homes: A Computation Modelling Evaluation with Heterogeneous Contact Mixing. Antibiotics 2021, 10, 227. https://doi.org/10.3390/antibiotics10030227
Tang A, Kwok KO, Wei VWI, Chen H, Wong SYS, Tam WWS. Synergistic Effect of Combination Interventions for Methicillin-Resistant Staphylococcus aureus Transmission Control in Nursing Homes: A Computation Modelling Evaluation with Heterogeneous Contact Mixing. Antibiotics. 2021; 10(3):227. https://doi.org/10.3390/antibiotics10030227
Chicago/Turabian StyleTang, Arthur, Kin On Kwok, Vivian Wan In Wei, Hong Chen, Samuel Yeung Shan Wong, and Wilson Wai Sun Tam. 2021. "Synergistic Effect of Combination Interventions for Methicillin-Resistant Staphylococcus aureus Transmission Control in Nursing Homes: A Computation Modelling Evaluation with Heterogeneous Contact Mixing" Antibiotics 10, no. 3: 227. https://doi.org/10.3390/antibiotics10030227






