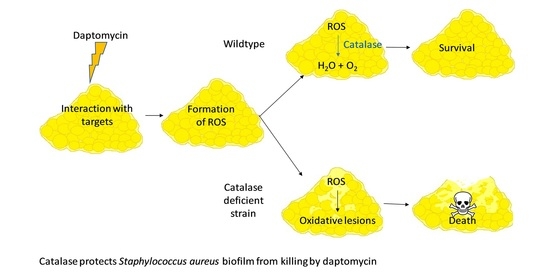
Catalase Protects Biofilm of Staphylococcus aureus against Daptomycin Activity
Abstract
:1. Introduction
2. Results
2.1. Catalase Protects S. aureus Biofilm against Daptomycin
2.2. Thiourea Protects S. aureus Biofilm against Daptomycin
2.3. Reduced Survival and Increased ROS Accumulation in Biofilm of a Resistant Clinical Isolate Requires High Concentrations of Daptomycin
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Strains and Susceptibility Studies
5.2. Studies of Survival and Reactive Oxygen Species
5.3. Whole-Genome Sequencing
5.4. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Del Pozo, J.L.; Patel, R. The challenge of treating biofilm-associated bacterial infections. Clin. Pharm. Ther. 2007, 82, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S.; Costerton, J.W. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef]
- Archer, N.K.; Mazaitis, M.J.; Costerton, J.W.; Leid, J.G.; Powers, M.E.; Shirtliff, M.E. Staphylococcus aureus biofilms: Properties, regulation, and roles in human disease. Virulence 2011, 2, 445–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolpen, M.; Appeldorff, C.F.; Brandt, S.; Mousavi, N.; Kragh, K.N.; Aydogan, S.; Uppal, H.A.; Bjarnsholt, T.; Ciofu, O.; Høiby, N.; et al. Increased bactericidal activity of colistin on Pseudomonas aeruginosa biofilms in anaerobic conditions. Pathog. Dis. 2016, 74, ftv086. [Google Scholar] [CrossRef] [Green Version]
- Steenbergen, J.N.; Alder, J.; Thorne, G.M.; Tally, F.P. Daptomycin: A lipopeptide antibiotic for the treatment of serious gram-positive infections. J. Antimicrob. Chemother. 2005, 55, 283–288. [Google Scholar] [CrossRef] [Green Version]
- Stewart, P.S.; Davison, W.M.; Steenbergen, J.N. Daptomycin rapidly penetrates a Staphylococcus epidermidis biofilm. Antimicrob. Agents Chemother. 2009, 53, 3505–3507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rybak, M.J. The efficacy and safety of daptomycin: First in a new class of antibiotics for gram-positive bacteria. Clin. Microbiol. Infect. 2006, 12, 24–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heidary, M.; Khosravi, A.D.; Khoshnood, S.; Nasiri, M.J.; Soleimani, S.; Goudarzi, M. Daptomycin. J. Antimicrob. Chemother. 2018, 73, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; Wenzel, M.; Strahl, H.; Grein, F.; Saaki, T.N.V.; Kohl, B.; Siersma, T.; Bandow, J.E.; Sahl, H.G.; Schneider, T.; et al. Daptomycin inhibits cell envelope synthesis by interfering with fluid membrane microdomains. Proc. Natl. Acad. Sci. USA 2016, 113, 7077–7086. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Liu, X.; Qu, Y.; Wang, X.; Li, L.; Zhao, X. Inhibitors of reactive oxygen species accumulation delay and/or reduce the lethality of several antistaphylococcal agents. Antimicrob. Agents Chemother. 2012, 56, 6048–6050. [Google Scholar] [CrossRef] [Green Version]
- Kohanski, M.A.; Dwyer, D.J.; Hayete, B.; Lawrence, C.A.; Collins, J.J. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 2007, 130, 797–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Hong, Y.; Drlica, K. Moving forward with reactive oxygen species involvement in antimicrobial lethality. J. Antimicrob. Chemother. 2015, 70, 639–642. [Google Scholar] [CrossRef]
- Van, A.H.; Coenye, T. The role of reactive oxygen species in antibiotic-mediated killing of bacteria. Trends Microbiol. 2017, 25, 456–466. [Google Scholar] [CrossRef]
- Keren, I.; Wu, Y.; Inocencio, J.; Mulcahy, L.R.; Lewis, K. Killing by bactericidal antibiotics does not depend on reactive oxygen species. Science 2013, 339, 1213–1216. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Imlay, J.A. Cell death from antibiotics without the involvement of reactive oxygen species. Science 2013, 339, 1210–1213. [Google Scholar] [CrossRef] [Green Version]
- Brochmann, R.P.; Toft, A.; Ciofu, O.; Briales, A.; Kolpen, M.; Hempel, C.; Bjarnsholt, T.; Høiby, N.; Jensen, P.Ø. Bactericidal effect of colistin on planktonic Pseudomonas aeruginosa is independent of hydroxyl radical formation. Int. J. Antimicrob. Agents 2014, 43, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Hassett, D.J.; Imlay, J.A. Bactericidal antibiotics and oxidative stress: A radical proposal. ACS Chem. Biol. 2007, 2, 708–710. [Google Scholar] [CrossRef] [Green Version]
- Wasil, M.; Halliwell, B.; Grootveld, M.; Moorhouse, C.P.; Hutchison, D.C.; Baum, H. The specificity of thiourea, dimethylthiourea and dimethyl sulphoxide as scavengers of hydroxyl radicals. Their protection of alpha 1-antiproteinase against inactivation by hypochlorous acid. Biochem. J. 1987, 243, 867–870. [Google Scholar] [CrossRef]
- Jensen, P.Ø.; Briales, A.; Brochmann, R.P.; Wang, H.; Kragh, K.N.; Kolpen, M.; Hempel, C.; Bjarnsholt, T.; Høiby, N.; Ciofu, O. Formation of hydroxyl radicals contributes to the bactericidal activity of ciprofloxacin against pseudomonas aeruginosa biofilms. Pathog. Dis. 2014, 70, 440–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, M.; Kawada, H.; Uchida, H.; Takagi, Y.; Obata, S.; Eda, R.; Hanaki, H.; Kitasato, H. Single nucleotide polymorphism leads to daptomycin resistance causing amino acid substitution-T345I in MprF of clinically isolated MRSA strains. PLoS ONE 2021, 16, e0245732. [Google Scholar] [CrossRef]
- Tran, T.T.; Munita, J.M.; Arias, C.A. Mechanisms of drug resistance: Daptomycin resistance. Ann. N. Y. Acad. Sci. 2015, 1354, 32–53. [Google Scholar] [CrossRef] [Green Version]
- Fowler, V.G.; Boucher, H.W.; Corey, G.R.; Abrutyn, E.; Karchmer, A.W.; Rupp, M.E.; Levine, D.P.; Chambers, H.F.; Tally, F.P.; Vigliani, G.A.; et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N. Engl. J. Med. 2006, 355, 653–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mascio, C.T.M.; Alder, J.D.; Silverman, J.A. Bactericidal action of daptomycin against stationary-phase and nondividing Staphylococcus aureus cells. Antimicrob. Agents Chemother. 2007, 51, 4255–4260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E.; et al. Clinical practice guidelines by the infectious diseases society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 2011, 52, 18–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Repine, J.E.; Fox, R.B.; Berger, E. Hydrogen peroxide kills Staphylococcus aureus by reacting with staphylococcal iron to form hydroxyl radical. J. Biol. Chem. 1981, 256, 7094–7096. [Google Scholar] [CrossRef]
- Cosgrove, K.; Coutts, G.; Jonsson, I.M.; Tarkowski, A.; Kokai-Kun, J.F.; Mond, J.J.; Foster, S.J. Catalase (KatA) and alkyl hydroperoxide reductase (AhpC) have compensatory roles in peroxide stress resistance and are required for survival, persistence, and nasal colonization in Staphylococcus aureus. J. Bacteriol. 2007, 189, 1025–1035. [Google Scholar] [CrossRef] [Green Version]
- Painter, K.L.; Strange, E.; Parkhill, J.; Bamford, K.B.; Armstrong-James, D.; Edwards, A.M. Staphylococcus aureus adapts to oxidative stress by producing H2O2-resistant small-colony variants via the SOS response. Infect. Immun. 2015, 83, 1830–1844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, J.R.; Cockerill, F.R.; Bradford, P.A.; Eliopoulos, G.M.; Hindler, J.A.; Jenkins, S.G.; Lewis, J.S.; Limbargo, B.; Miller, L.A.; Nicolau, D.P.; et al. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 10th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015; p. 35. [Google Scholar]
- GitHub MigleSur/BacDist: Snakemake Pipeline for Bacterial SNP Distance and Phylogeny Analysis. Available online: https://github.com/MigleSur/BacDist (accessed on 24 April 2021).




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Haj, C.; Lichtenberg, M.; Nielsen, K.L.; Bjarnsholt, T.; Jensen, P.Ø. Catalase Protects Biofilm of Staphylococcus aureus against Daptomycin Activity. Antibiotics 2021, 10, 511. https://doi.org/10.3390/antibiotics10050511
El Haj C, Lichtenberg M, Nielsen KL, Bjarnsholt T, Jensen PØ. Catalase Protects Biofilm of Staphylococcus aureus against Daptomycin Activity. Antibiotics. 2021; 10(5):511. https://doi.org/10.3390/antibiotics10050511
Chicago/Turabian StyleEl Haj, Cristina, Mads Lichtenberg, Karen Leth Nielsen, Thomas Bjarnsholt, and Peter Østrup Jensen. 2021. "Catalase Protects Biofilm of Staphylococcus aureus against Daptomycin Activity" Antibiotics 10, no. 5: 511. https://doi.org/10.3390/antibiotics10050511