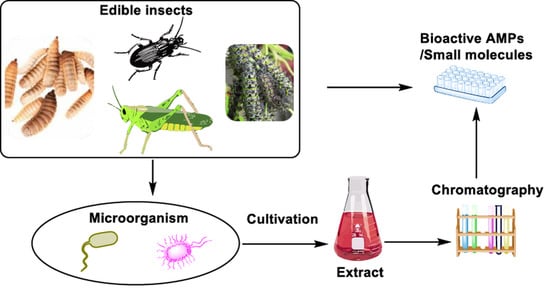
An Overview of Antimicrobial Compounds from African Edible Insects and Their Associated Microbiota
Abstract
:1. Introduction
2. Edible Insects in Africa and Their Microbiota
3. Multi-Drug Resistance (MDR)
4. Insects as Potential Antibiotic Producers
5. The Chemistry of Microorganisms from Selected Edible Insects
5.1. Black Soldier Fly Hermetia Illucens (Diptera: Stratiomyidae)
5.2. Termites (Isoptera)
5.3. Beetles (Coleoptera)
5.4. Locusts (Orthoptera: Acrididae)
5.5. Caterpillars (Lepidoptera)
5.6. Crickets (Orthoptera)
6. Future Perspectives
7. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Seabrooks, L.; Hu, L. Insects: An underrepresented resource for the discovery of biologically active natural products. Acta Pharm. Sin. B 2017, 7, 409–426. [Google Scholar] [CrossRef]
- Waglechner, N.; Wright, G.D. Antibiotic resistance: It’s bad, but why isn’t it worse? BMC Biol. 2017, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A.; Pirofski, L.A. Host-pathogen interactions: Redefining the basic concepts of virulence and pathogenicity. Infect. Immun. 1999, 67, 3703–3713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frey-Klett, P.; Burlinson, P.; Deveau, A.; Barret, M.; Tarkka, M.; Sarniguet, A. Bacterial-Fungal Interactions: Hyphens between Agricultural, Clinical, Environmental, and Food Microbiologists. Microbiol. Mol. Biol. Rev. 2011, 75, 583–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa-Neto, E.M. Entomotherapy, or the Medicinal Use of Insects. J. Ethnobiol. 2005, 25, 93–114. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Józefiak, D.; Józefiak, A.; Kierończyk, B.; Rawski, M.; Świątkiewicz, S.; Długosz, J.; Engberg, R.M. Insects—A Natural Nutrient Source for Poultry—A Review. Ann. Anim. Sci. 2016, 16, 297–313. [Google Scholar] [CrossRef] [Green Version]
- Ognik, K.; Kozłowski, K.; Stępniowska, A.; Listos, P.; Józefiak, D.; Zduńczyk, Z.; Jankowski, J. Antioxidant status and liver function of young Turkeys receiving a diet with full-fat insect meal from Hermetia illucens. Animals 2020, 10, 1339. [Google Scholar] [CrossRef]
- Krauze, M.; Cendrowska-Pinkosz, M.; Matuseviĉius, P.; Stępniowska, A.; Jurczak, P.; Ognik, K. The effect of administration of a phytobiotic containing cinnamon oil and citric acid on the metabolism, immunity, and growth performance of broiler chickens. Animals 2021, 11, 399. [Google Scholar] [CrossRef] [PubMed]
- Correa, Y.; Cabanillas, B.; Jullian, V.; Álvarez, D.; Castillo, D.; Dufloer, C.; Bustamante, B.; Roncal, E.; Neyra, E.; Sheen, P.; et al. Identification and characterization of compounds from Chrysosporium multifidum, a fungus with moderate antimicrobial activity isolated from Hermetia illucens gut microbiota. PLoS ONE 2019, 14, e0218837. [Google Scholar] [CrossRef]
- Yi, H.Y.; Chowdhury, M.; Huang, Y.D.; Yu, X.Q. Insect antimicrobial peptides and their applications. Appl. Microbiol. Biotechnol. 2014, 98, 5807–5822. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Xiang, Q.; Zhang, Q.; Huang, Y.; Su, Z. Overview on the recent study of antimicrobial peptides: Origins, functions, relative mechanisms and application. Peptides 2012, 37, 207–215. [Google Scholar] [CrossRef]
- Moretta, A.; Salvia, R.; Scieuzo, C.; Di Somma, A.; Vogel, H.; Pucci, P.; Sgambato, A.; Wolff, M.; Falabella, P. A bioinformatic study of antimicrobial peptides identified in the Black Soldier Fly (BSF) Hermetia illucens (Diptera: Stratiomyidae). Sci. Rep. 2020, 10, 16875. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Patočka, J.; Kuča, K. Insect Antimicrobial Peptides, a Mini Review. Toxins 2018, 10, 461. [Google Scholar] [CrossRef]
- Park, S.I.; Kim, J.W.; Yoe, S.M. Purification and characterization of a novel antibacterial peptide from black soldier fly (Hermetia illucens) larvae. Dev. Comp. Immunol. 2015, 52, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-I.; Yoe, S.M. A novel cecropin-like peptide from black soldier fly, Hermetia illucens: Isolation, structural and functional characterization. Entomol. Res. 2017, 47, 115–124. [Google Scholar] [CrossRef]
- Gould, I.M.; Bal, A.M. New antibiotic agents in the pipeline and how they can help overcome microbial resistance. Virulence 2013, 4, 185–191. [Google Scholar] [CrossRef] [Green Version]
- Wright, G.D. Something old, something new: Revisiting natural products in Antibiotic drug discovery. Can. J. Microbiol. 2014, 60, 147–154. [Google Scholar] [CrossRef]
- Jideani, A.I.O.; Netshiheni, R.K. Selected Edible Insects and Their Products in Traditional Medicine, Food and Pharmaceutical Industries in Africa: Utilisation and Prospects. In Future Foods; InTech: London, UK, 2017. [Google Scholar]
- Yang, H. Nonvesicular sterol transport: Two protein families and a sterol sensor? Trends Cell Biol. 2006, 16, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Rumpold, B.A.; Schlüter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.; Roy, S. Edible Insects: Future of Human Food - A Review. Int. Lett. Nat. Sci. 2014, 26, 1–11. [Google Scholar] [CrossRef]
- Flores, D.R.; Casados, L.E.; Velasco, S.F.; Ramírez, A.C.; Velázquez, G. Comparative study of composition, antioxidant and antimicrobial activity of two adult edible insects from Tenebrionidae family. BMC Chem. 2020, 14, 55. [Google Scholar] [CrossRef]
- Mutungi, C.; Irungu, F.G.; Nduko, J.; Mutua, F.; Affognon, H.; Nakimbugwe, D.; Ekesi, S.; Fiaboe, K.K.M. Postharvest processes of edible insects in Africa: A review of processing methods, and the implications for nutrition, safety and new products development. Crit. Rev. Food Sci. Nutr. 2019, 59, 276–298. [Google Scholar] [CrossRef] [Green Version]
- Klunder, H.C.; Wolkers-Rooijackers, J.; Korpela, J.M.; Nout, M.J.R. Microbiological aspects of processing and storage of edible insects. Food Control 2012, 26, 628–631. [Google Scholar] [CrossRef]
- Amadi, E.N.; Kiin-Kabari, D.B. Nutritional Composition and Microbiology of Some Edible Insects Commonly Eaten in Africa, Hurdles and Future Prospects: A Critical Review. J. Food Microbiol. Saf. Hyg. 2016, 1, 107. [Google Scholar] [CrossRef] [Green Version]
- Tanga, C.M.; Waweru, J.W.; Tola, Y.H.; Onyoni, A.A.; Khamis, F.M.; Ekesi, S.; Paredes, J.C. Organic Waste Substrates Induce Important Shifts in Gut Microbiota of Black Soldier Fly (Hermetia illucens L.): Coexistence of Conserved, Variable, and Potential Pathogenic Microbes. Front. Microbiol. 2021, 12, 1–24. [Google Scholar] [CrossRef]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad Bugs, No Drugs: No ESKAPE! An Update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikaido, H. Multidrug Resistance in Bacteria. Annu. Rev. Biochem. 2009, 78, 119–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanwar, J.; Das, S.; Fatima, Z.; Hameed, S. Multidrug resistance: An emerging crisis. Interdiscip. Perspect. Infect. Dis. 2014, 2014, 541340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavine, M.D.; Strand, M.R. Insect hemocytes and their role in immunity. Insect Biochem. Mol. Biol. 2002, 32, 1295–1309. [Google Scholar] [CrossRef]
- Kanost, M.R.; Jiang, H.; Yu, X.Q. Innate immune responses of a lepidopteran insect, Manduca sexta. Immunol. Rev. 2004, 198, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Ekengren, S.; Hultmark, D. Drosophila cecropin as an antifungal agent. Insect Biochem. Mol. Biol. 1999, 29, 965–972. [Google Scholar] [CrossRef]
- Abdallah, E.M.; Ali, A.M.; Rauf, A. Insects as producers of antimicrobial polypeptides: A short review. GSC Biol. Pharm. Sci. 2020, 2020, 102–107. [Google Scholar] [CrossRef]
- Hoffmann, J.A.; Reichhart, J.M. Drosophila innate immunity: An evolutionary perspective. Nat. Immunol. 2002, 3, 121–126. [Google Scholar] [CrossRef]
- Bulet, P.; Hetru, C.; Dimarcq, J.L.; Hoffmann, D. Antimicrobial peptides in insects; structure and function. Dev. Comp. Immunol. 1999, 23, 329–344. [Google Scholar] [CrossRef]
- Rees, J.A.; Moniatte, M.; Bulet, P. Novel antibacterial peptides isolated from a European bumblebee, Bombus pascuorum (Hymenoptera, apoidea). Insect Biochem. Mol. Biol. 1997, 27, 413–422. [Google Scholar] [CrossRef]
- Rahnamaeian, M.; Cytryńska, M.; Zdybicka-Barabas, A.; Dobslaff, K.; Wiesner, J.; Twyman, R.M.; Zuchner, T.; Sadd, B.M.; Regoes, R.R.; Schmid-Hempel, P.; et al. Insect antimicrobial peptides show potentiating functional interactions against Gram-negative bacteria. Proc. R. Soc. B 2015, 282, 20150293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pöppel, A.K.; Vogel, H.; Wiesner, J.; Vilcinskas, A. Antimicrobial peptides expressed in medicinal maggots of the blow fly Lucilia sericata show combinatorial activity against bacteria. Antimicrob. Agents Chemother. 2015, 59, 2508–2514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thevissen, K.; Warnecke, D.C.; François, I.E.J.A.; Leipelt, M.; Heinz, E.; Ott, C.; Zähringer, U.; Thomma, B.P.H.J.; Ferket, K.K.A.; Cammue, B.P.A. Defensins from Insects and Plants Interact with Fungal Glucosylceramides. J. Biol. Chem. 2004, 279, 3900–3905. [Google Scholar] [CrossRef] [Green Version]
- Lei, J.; Sun, L.C.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q.Y. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019, 11, 3919–3931. [Google Scholar]
- Boccazzi, I.V.; Ottoboni, M.; Martin, E.; Comandatore, F.; Vallone, L.; Spranghers, T.; Eeckhout, M.; Mereghetti, V.; Pinotti, L.; Epis, S. A survey of the mycobiota associated with larvae of the black soldier fly (Hermetia illucens) reared for feed production. PLoS ONE 2017, 12, e0182533. [Google Scholar] [CrossRef] [Green Version]
- Müller, A.; Wolf, D.; Gutzeit, H.O. The black soldier fly, Hermetia illucens—A promising source for sustainable production of proteins, lipids and bioactive substances. Z. Naturforsch. Sect. C J. Biosci. 2017, 72, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.H.; Jiang, M. Evaluation of antibacterial activity of hexanedioic acid isolated from Hermetia illucens larvae. J. Appl. Biomed. 2014, 12, 179–189. [Google Scholar] [CrossRef]
- Raheem, N.; Straus, S.K. Mechanisms of Action for Antimicrobial Peptides with Antibacterial and Antibiofilm Functions. Front. Microbiol. 2019, 10, 2866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbari, R.; Hakemi Vala, M.; Hashemi, A.; Aghazadeh, H.; Sabatier, J.M.; Bagheri, K.P. Action mechanism of melittin-derived antimicrobial peptides, MDP1 and MDP2, de novo designed against multidrug resistant bacteria. Amino Acids 2018, 50, 1231–1243. [Google Scholar] [CrossRef]
- Park, S.-I.; Chang, B.S.; Yoe, S.M. Detection of antimicrobial substances from larvae of the black soldier fly, Hermetia illucens (Diptera: Stratiomyidae). Entomol. Res. 2014, 44, 58–64. [Google Scholar] [CrossRef]
- Choi, W.-H.; Yun, J.-H.; Chu, J.-P.; Chu, K.-B. Antibacterial effect of extracts of Hermetia illucens (Diptera: Stratiomyidae) larvae against Gram-negative bacteria. Entomol. Res. 2012, 42, 219–226. [Google Scholar] [CrossRef]
- Elhag, O.; Zhou, D.; Song, Q.; Soomro, A.A.; Cai, M.; Zheng, L.; Yu, Z.; Zhang, J. Screening, Expression, Purification and Functional Characterization of Novel Antimicrobial Peptide Genes from Hermetia illucens (L.). PLoS ONE 2017, 12, e0169582. [Google Scholar] [CrossRef]
- Vasconcellos, A.; Moura, F.M.D.S. Wood litter consumption by three species of Nasutitermes termites in an area of the Atlantic Coastal Forest in northeastern Brazil. J. Insect Sci. 2010, 10, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Schäfer, A.; Konrad, R.; Kuhnigk, T.; Kämpfer, P.; Hertel, H.; König, H. Hemicellulose-degrading bacteria and yeasts from the termite gut. J. Appl. Bacteriol. 1996, 80, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, D.; Vijayarani, K.; Kumanan, K. 6S rRNA typing of cellulolytic bacteria from the termite Odontotermes formosanus. J. Vet. Anim. Sci. Res. 2014, 43, 359–368. [Google Scholar]
- Wenzel, M.; Schonig, I.; Berchtold, M.; Kampfer, P.; Konig, H. Aerobic and facultatively anaerobic cellulolytic bacteria from the gut of the termite Zootermopsis angusticollis. J. Appl. Microbiol. 2002, 92, 32–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butera, G.; Ferraro, C.; Alonzo, G.; Colazza, S.; Quatrini, P. The gut microbiota of the wood-feeding termite Reticulitermes lucifugus (Isoptera; Rhinotermitidae). Ann. Microbiol. 2016, 66, 253–260. [Google Scholar] [CrossRef] [Green Version]
- Borji, M.; Rahimi, S.; Ghorbani, G.R.; Vandyousefi, J.; Fazaeli, H. Isolation and identification of some bacteria from termites gut capable in degrading straw lignin and polysaccharides. J. Vet. Res. 2003, 58, 249–256. [Google Scholar]
- Fröhlich, J.; Koustiane, C.; Kämpfer, P.; Rosselló-Mora, R.; Valens, M.; Berchtold, M.; Kuhnigk, T.; Hertel, H.; Maheshwari, D.K.; König, H. Occurrence of rhizobia in the gut of the higher termite Nasutitermes nigriceps. Syst. Appl. Microbiol. 2007, 30, 68–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harazono, K.; Yamashita, N.; Shinzato, N.; Watanabe, Y.; Fukatsu, T.; Kurane, R. Isolation and Characterization of Aromatics-degrading Microorganisms from the Gut of the Lower Termite Coptotermes formosanus. Biosci. Biotechnol. Biochem. 2003, 67, 889–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Dexter, S.; Boopathy, R. Biodegradation of phenol by Acinetobacter tandoii isolated from the gut of the termite. Environ. Sci. Pollut. Res. 2019, 26, 34067–34072. [Google Scholar] [CrossRef] [PubMed]
- Tsegaye, B.; Balomajumder, C.; Roy, P. Isolation and Characterization of Novel Lignolytic, Cellulolytic, and Hemicellulolytic Bacteria from Wood-Feeding Termite Cryptotermes brevis. Int. Microbiol. 2019, 22, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Konig, H. Bacillus species in the intestine of termites and other soil invertebrates. J. Appl. Microbiol. 2006, 101, 620–627. [Google Scholar] [CrossRef]
- Breznak, J.A. Intestinal Microbiota of Termites and other Xylophagous Insects. Annu. Rev. Microbiol. 1982, 36, 323. [Google Scholar] [CrossRef]
- Eutick, M.L.; O’Brien, R.W.; Slaytor, M. Bacteria from the Gut of Australian Termites. Appl. Environ. Microbiol. 1978, 35, 823–828. [Google Scholar] [CrossRef] [Green Version]
- Adams, L.; Boopathy, R. Isolation and characterization of enteric bacteria from the hindgut of Formosan termite. Bioresour. Technol. 2005, 96, 1592–1598. [Google Scholar] [CrossRef]
- Chevrette, M.G.; Carlson, C.M.; Ortega, H.E.; Thomas, C.; Ananiev, G.E.; Barns, K.J.; Book, A.J.; Cagnazzo, J.; Carlos, C.; Flanigan, W.; et al. The antimicrobial potential of Streptomyces from insect microbiomes. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Shao, M.; Yin, C.; Mao, Z.; Shi, J.; Yu, X.; Wang, Y.; Sun, F.; Zhang, Y. Diversity, Bacterial Symbionts, and Antimicrobial Potential of Termite-Associated Fungi. Front. Microbiol. 2020, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.-F.; Wu, J.; Li, S.; Li, Q.; Jin, L.-P.; Yin, C.-P.; Zhang, Y.-L. Antibacterial Potential of Termite-Associated Streptomyces sp. ACS Omega 2021, 6, 4329–4334. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Ramadhar, T.R.; Beemelmanns, C.; Cao, S.; Poulsen, M.; Currie, C.R.; Clardy, J. Natalamycin A, an ansamycin from a termite-associated Streptomyces sp. Chem. Sci. 2014, 5, 4333–4338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.R.; Lee, D.; Benndorf, R.; Jung, W.H.; Beemelmanns, C.; Kang, K.S.; Kim, K.H. Termisoflavones A–C, Isoflavonoid Glycosides from Termite-Associated Streptomyces sp. RB1. J. Nat. Prod. 2016, 79, 3072–3078. [Google Scholar] [CrossRef]
- Wyche, T.P.; Ruzzini, A.C.; Beemelmanns, C.; Kim, K.H.; Klassen, J.L.; Cao, S.; Poulsen, M.; Bugni, T.S.; Currie, C.R.; Clardy, J. Linear Peptides Are the Major Products of a Biosynthetic Pathway That Encodes for Cyclic Depsipeptides. Org. Lett. 2017, 19, 1772–1775. [Google Scholar] [CrossRef] [PubMed]
- Benndorf, R.; Guo, H.; Sommerwerk, E.; Weigel, C.; Garcia-Altares, M.; Martin, K.; Hu, H.; Küfner, M.; de Beer, Z.W.; Poulsen, M.; et al. Natural products from actinobacteria associated with fungus-growing termites. Antibiotics 2018, 7, 83. [Google Scholar] [CrossRef] [Green Version]
- Beemelmanns, C.; Ramadhar, T.R.; Kim, K.H.; Klassen, J.L.; Cao, S.; Wyche, T.P.; Hou, Y.; Poulsen, M.; Bugni, T.S.; Currie, C.R.; et al. Macrotermycins A-D, Glycosylated Macrolactams from a Termite-Associated Amycolatopsis sp. M39. Org. Lett. 2017, 19, 1000–1003. [Google Scholar] [CrossRef]
- Guo, H.; Benndorf, R.; Leichnitz, D.; Klassen, J.L.; Vollmers, J.; Görls, H.; Steinacker, M.; Weigel, C.; Dahse, H.M.; Kaster, A.K.; et al. Isolation, Biosynthesis and Chemical Modifications of Rubterolones A–F: Rare Tropolone Alkaloids from Actinomadura sp. 5-2. Chem. A Eur. J. 2017, 23, 9338–9345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayasuriya, H.; Ball, R.G.; Zink, D.L.; Smith, J.L.; Goetz, M.A.; Jenkins, R.G.; Nallin-Omstead, M.; Silverman, K.C.; Bills, G.F.; Lingham, R.B.; et al. Barceloneic acid A, a new farnesyl-protein transferase inhibitor from a Phoma species. J. Nat. Prod. 1995, 58, 986–991. [Google Scholar] [CrossRef] [PubMed]
- Supong, K.; Thawai, C.; Choowong, W.; Kittiwongwattana, C.; Thanaboripat, D.; Laosinwattana, C.; Koohakan, P.; Parinthawong, N.; Pittayakhajonwut, P. Antimicrobial compounds from endophytic Streptomyces sp. BCC72023 isolated from rice (Oryza sativa L.). Res. Microbiol. 2016, 167, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Klassen, J.L.; Lee, S.R.; Poulsen, M.; Beemelmanns, C.; Kim, K.H. Efomycins K and L from a termite-associated Streptomyces sp. M56 and their putative biosynthetic origin. Front. Microbiol. 2019, 10, 1739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Bonin, A.; Buchmann, B.; Bader, B.; Rausch, A.; Venstrom, K.; Schäfer, M.; Gründemann, S.; Günther, J.; Zorn, L.; Nubbemeyer, R.; et al. Efomycine M: An inhibitor of selectins? Nat. Med. 2006, 12, 873. [Google Scholar] [CrossRef] [PubMed]
- Frobel, K.; Miller, H.; Bischoff, E.; Salcher, O.; de Jong, A.; Berschauer, F.; Scheer, M. Efomycin G and it’s use as yield Promoter in Animals. U.S. Patent 49,27,810, 22 May 1990. [Google Scholar]
- Wu, C.; Tan, Y.; Gan, M.; Wang, Y.; Guan, Y.; Hu, X.; Zhou, H.; Shang, X.; You, X.; Yang, Z.; et al. Identification of elaiophylin derivatives from the marine-derived actinomycete Streptomyces sp. 7-145 using PCR-based screening. J. Nat. Prod. 2013, 76, 2153–2157. [Google Scholar] [CrossRef]
- Pimentel-Elardo, S.M.; Sørensen, D.; Ho, L.; Ziko, M.; Bueler, S.A.; Lu, S.; Tao, J.; Moser, A.; Lee, R.; Agard, D.; et al. Activity-Independent Discovery of Secondary Metabolites Using Chemical Elicitation and Cheminformatic Inference. ACS Chem. Biol. 2015, 10, 2616–2623. [Google Scholar] [CrossRef] [Green Version]
- Kretschmer, A.; Dorgerloh, M.; Deeg, M.; Hagenmaier, H. The structures of novel insecticidal macrolides: Bafilomycins D and E, and oxohygrolidin. Agric. Biol. Chem. 1985, 49, 2509–2511. [Google Scholar] [CrossRef]
- Liu, X.F.; Xiang, L.; Zhou, Q.; Carralot, J.P.; Prunotto, M.; Niederfellner, G.; Pastan, I. Actinomycin D enhances killing of cancer cells by immunotoxin RG7787 through activation of the extrinsic pathway of apoptosis. Proc. Natl. Acad. Sci. USA 2016, 113, 10666–10671. [Google Scholar] [CrossRef] [Green Version]
- Koba, M.; Konopa, J. Actinomycin D and its mechanisms of action. Postep. Hig. Med. Dosw. 2005, 59, 290–298. [Google Scholar]
- Guo, H.; Benndorf, R.; König, S.; Leichnitz, D.; Weigel, C.; Peschel, G.; Berthel, P.; Kaiser, M.; Steinbeck, C.; Werz, O.; et al. Expanding the Rubterolone Family: Intrinsic Reactivity and Directed Diversification of PKS-derived Pyrans. Chem. A Eur. J. 2018, 24, 11319–11324. [Google Scholar] [CrossRef] [PubMed]
- Carr, G.; Poulsen, M.; Klassen, J.L.; Hou, Y.; Wyche, T.P.; Bugni, T.S.; Currie, C.R.; Clardy, J. Microtermolides A and B from termite-associated Streptomyces sp. and structural revision of vinylamycin. Org. Lett. 2012, 14, 2822–2825. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Kreuzenbeck, N.B.; Otani, S.; Garcia-Altares, M.; Dahse, H.M.; Weigel, C.; Aanen, D.K.; Hertweck, C.; Poulsen, M.; Beemelmanns, C. Pseudoxylallemycins A–F, Cyclic Tetrapeptides with Rare Allenyl Modifications Isolated from Pseudoxylaria sp. X802: A Competitor of Fungus-Growing Termite Cultivars. Org. Lett. 2016, 18, 3338–3341. [Google Scholar] [CrossRef]
- Lee, S.R.; Lee, D.; Yu, J.S.; Benndorf, R.; Lee, S.; Lee, D.-S.; Huh, J.; de Beer, Z.W.; Kim, Y.H.; Beemelmanns, C.; et al. Natalenamides A–C, Cyclic Tripeptides from the Termite-Associated Actinomadura sp. RB99. Molecules 2018, 23, 3003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blodgett, J.A.V.; Oh, D.C.; Cao, S.; Currie, C.R.; Kolter, R.; Clardy, J. Common biosynthetic origins for polycyclic tetramate macrolactams from phylogenetically diverse bacteria. Proc. Natl. Acad. Sci. USA 2010, 107, 11692–11697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, J.J.; Oh, D.C.; Yuceer, M.C.; Klepzig, K.D.; Clardy, J.; Currie, C.R. Bacterial protection of beetle-fungus mutualism. Science 2008, 322, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakashima, T.; Iizuka, T.; Ogura, K.; Maeda, M.; Tanaka, T. Isolation of some microorganisms associated with five species of Ambrosia beetles and two kinds of antibiotics produced by Xv-3 strain in these isolates. J. Fac. Agric. Hokkaido Univ. 1982, 61, 60–72. [Google Scholar]
- Kellner, R.L.L.; Dettner, K. Allocation of pederin during lifetime of Paederus rove beetles (Coleoptera: Staphylinidae): Evidence for polymorphism of hemolymph toxin. J. Chem. Ecol. 1995, 21, 1719–1733. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Ko, H.; Bang, H.S.; Park, S.H.; Kim, D.G.; Kwon, H.C.; Kim, S.Y.; Shin, J.; Oh, D.C. Coprismycins A and B, neuroprotective phenylpyridines from the dung beetle-associated bacterium, Streptomyces sp. Bioorganic Med. Chem. Lett. 2011, 21, 5715–5718. [Google Scholar] [CrossRef]
- Park, S.H.; Moon, K.; Bang, H.S.; Kim, S.H.; Kim, D.G.; Oh, K.B.; Shin, J.; Oh, D.C. Tripartilactam, a cyclobutane-bearing tricyclic lactam from a Streptomyces sp. in a dung beetle’s brood ball. Org. Lett. 2012, 14, 1258–1261. [Google Scholar] [CrossRef]
- Kim, S.H.; Kwon, S.H.; Park, S.H.; Lee, J.K.; Bang, H.S.; Nam, S.J.; Kwon, H.C.; Shin, J.; Oh, D.C. Tripartin, a histone demethylase inhibitor from a bacterium associated with a dung beetle larva. Org. Lett. 2013, 15, 1834–1837. [Google Scholar] [CrossRef]
- Um, S.; Park, S.H.; Kim, J.; Park, H.J.; Ko, K.; Bang, H.S.; Lee, S.K.; Shin, J.; Oh, D.C. Coprisamides A and B, new branched cyclic peptides from a gut bacterium of the dung beetle Copris tripartitus. Org. Lett. 2015, 17, 1272–1275. [Google Scholar] [CrossRef]
- Um, S.; Bach, D.H.; Shin, B.; Ahn, C.H.; Kim, S.H.; Bang, H.S.; Oh, K.B.; Lee, S.K.; Shin, J.; Oh, D.C. Naphthoquinone-Oxindole Alkaloids, Coprisidins A and B, from a Gut-Associated Bacterium in the Dung Beetle, Copris tripartitus. Org. Lett. 2016, 18, 5792–5795. [Google Scholar] [CrossRef] [PubMed]
- An, J.S.; Hong, S.H.; Somers, E.; Lee, J.; Kim, B.Y.; Woo, D.; Kim, S.W.; Hong, H.J.; Jo, S.-I.; Shin, J.; et al. Lenzimycins A and B, Metabolites With Antibacterial Properties From Brevibacillus sp. Associated With the Dung Beetle Onthophagus lenzii. Front. Microbiol. 2020, 11, 599911. [Google Scholar] [CrossRef]
- Lu, J.; Sun, Q.; Tu, Z.C.; Lv, Q.; Shui, P.X.; Cheng, Y.X. Identification of N-acetyldopamine dimers from the dung beetle Catharsius molossus and their COX-1 and COX-2 inhibitory activities. Molecules 2015, 20, 15589–15596. [Google Scholar] [CrossRef] [Green Version]
- Mori, N.; Yoshinaga, N.; Sawada, Y.; Fukui, M.; Shimoda, M.; Fujisaki, K.; Nishida, R.; Kuwahara, Y. Identification of Volicitin-related Compounds from the Regurgitant of Lepidopteran Caterpillars. Biosci. Biotechnol. Biochem. 2003, 67, 1168–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theodore, C.M.; King, J.B.; You, J.; Cichewicz, R.H. Production of cytotoxic glidobactins/luminmycins by Photorhabdus asymbiotica in liquid media and live crickets. J. Nat. Prod. 2012, 75, 2007–2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Widdick, D.A.; Dodd, H.M.; Barraille, P.; White, J.; Stein, T.H.; Chater, K.F.; Gasson, M.J.; Bibb, M.J. Cloning and engineering of the cinnamycin biosynthetic gene cluster from Streptomyces cinnamoneus cinnamoneus DSM 40005. Proc. Natl. Acad. Sci. USA 2003, 100, 4316–4321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ökesli, A.; Cooper, L.E.; Fogle, E.J.; Van Der Donk, W.A. Nine post-translational modifications during the biosynthesis of cinnamycin. J. Am. Chem. Soc. 2011, 133, 13753–13760. [Google Scholar] [CrossRef]
- Repka, L.M.; Chekan, J.R.; Nair, S.K.; Van Der Donk, W.A. Mechanistic Understanding of Lanthipeptide Biosynthetic Enzymes. Chem. Rev. 2017, 117, 5457–5520. [Google Scholar] [CrossRef]
- Zhang, N.; Suh, S.O.; Blackwell, M. Microorganisms in the gut of beetles: Evidence from molecular cloning. J. Invertebr. Pathol. 2003, 84, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Stone, W.E.; Wolfe, M.L. Response of understory vegetation to variable tree mortality following a mountain pine beetle epidemic in lodgepole pine stands in northern Utah. Vegetatio 1996, 122, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Beemelmanns, C.; Guo, H.; Rischer, M.; Poulsen, M. Natural products from microbes associated with insects. Beilstein J. Org. Chem. 2016, 12, 314–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziganshina, E.E.; Mohammed, W.S.; Shagimardanova, E.I.; Vankov, P.Y.; Gogoleva, N.E.; Ziganshin, A.M. Fungal, bacterial, and archaeal diversity in the digestive tract of several beetle larvae (coleoptera). Biomed Res. Int. 2018, 2018, 6765438. [Google Scholar] [CrossRef] [PubMed]
- Lavy, O.; Gophna, U.; Gefen, E.; Ayali, A. Locust Bacterial Symbionts: An Update. Insects 2020, 11, 655. [Google Scholar] [CrossRef]
- Dillon, R.; Charnley, K. Mutualism between the desert locust Schistocerca gregaria and its gut microbiota. Res. Microbiol. 2002, 153, 503–509. [Google Scholar] [CrossRef]
- Payne, D.W.; Davidson, L.M. Cellulose digestion in the locust, Schistocerra gregaria. J. Entomol. Ser. A Gen. Entomol. 2009, 48, 213–215. [Google Scholar] [CrossRef]
- Stevenson, J.P. The normal bacterial flora of the alimentary canal of laboratory stocks of the desert locust, Schistocerca gregaria. J. Invertebr. Pathol. 1966, 8, 205–211. [Google Scholar] [CrossRef]
- Cheseto, X.; Kuate, S.P.; Tchouassi, D.P.; Ndung’u, M.; Teal, P.E.A.; Torto, B. Potential of the Desert Locust Schistocerca gregaria (Orthoptera: Acrididae) as an Unconventional Source of Dietary and Therapeutic Sterols. PLoS ONE 2015, 10, e0127171. [Google Scholar] [CrossRef]
- Yen, A.L. Conservation of Lepidoptera used as human food and medicine. Curr. Opin. Insect Sci. 2015, 12, 102–108. [Google Scholar] [CrossRef]
- Ngute, A.S.K.; Dongmo, M.A.K.; Effa, J.A.M.; Ambombo Onguene, E.M.; Fomekong Lontchi, J.; Cuni-Sanchez, A. Edible caterpillars in central Cameroon: Host plants, value, harvesting, and availability. For. Trees Livelihoods 2020, 29, 16–33. [Google Scholar] [CrossRef]
- Hammer, T.J.; Janzen, D.H.; Hallwachs, W.; Jaffe, S.P.; Fierer, N. Caterpillars lack a resident gut microbiome. Proc. Natl. Acad. Sci. USA 2017, 114, 9641–9646. [Google Scholar] [CrossRef] [Green Version]
- Sawada, Y.; Yoshinaga, N.; Fujisaki, K.; Nishida, R.; Kuwahara, Y.; Mori, N. Absolute Configuration of Volicitin from the Regurgitant of Lepidopteran Caterpillars and Biological Activity of Volicitin-Related Compounds. Biosci. Biotechnol. Biochem. 2014, 70, 2185–2190. [Google Scholar] [CrossRef]
- Pemberton, R.W. Insects and other arthropods used as drugs in Korean traditional medicine. J. Ethnopharmacol. 1999, 65, 207–216. [Google Scholar] [CrossRef]
- Alves, R.R.N.; Alves, H.N. The faunal drugstore: Animal-based remedies used in traditional medicines in Latin America. J. Ethnobiol. Ethnomed. 2011, 7, 1–43. [Google Scholar] [CrossRef] [Green Version]
- Ulrich, R.G.; Buthala, D.A.; Klug, M.J. Microbiota Associated with the Gastrointestinal Tract of the Common House Cricket, Acheta domestica. Appl. Environ. Microbiol. 1981, 41, 246–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodrich-Blair, H.; Clarke, D.J. Mutualism and pathogenesis in Xenorhabdus and Photorhabdus: Two roads to the same destination. Mol. Microbiol. 2007, 64, 260–268. [Google Scholar] [CrossRef]
- Amatuni, A.; Renata, H. Identification of a lysine 4-hydroxylase from the glidobactin biosynthesis and evaluation of its biocatalytic potential. Org. Biomol. Chem. 2019, 17, 1736–1739. [Google Scholar] [CrossRef]



| Termite Family/Genera | Related Microbe Genera | Source | Ref |
|---|---|---|---|
| Formosan termite: Coptotermes formosanus | Serratia marcescens, Enterobacter aerogens, Enterobacter cloacae, and Citrobacter farmeri. | Hindgut | [63] |
| Odontotermes formosanus | Bacillus sp., Citrobacter freundii, Pseudomonas aeruginosa Salmonella entrica, Enterococcus casseliflavus, Staphylococcus gallinarum, and Serratia marcescens. | Gut | [52] |
| Mastotermes darwiniensis | Streptococcus sp. | Gut | [62] |
| Cryptotermes primus | Streptococcus sp. | Gut | [62] |
| Rhinotermitidae species: (Heterotermes ferox, Coptotermes acinaciformis, C. lacteus, and Schedorhinotermes intermedius intermedius) | Enterobacter sp. | Gut | [62] |
| Termitidae species (Nasutitermes exitiosus, N. graveolus, N. walkeri) | Staphylococcus sp. | Gut | [62] |
| Insect | Origin, Producer Organism | Compounds | Biological Activity | Ref. |
|---|---|---|---|---|
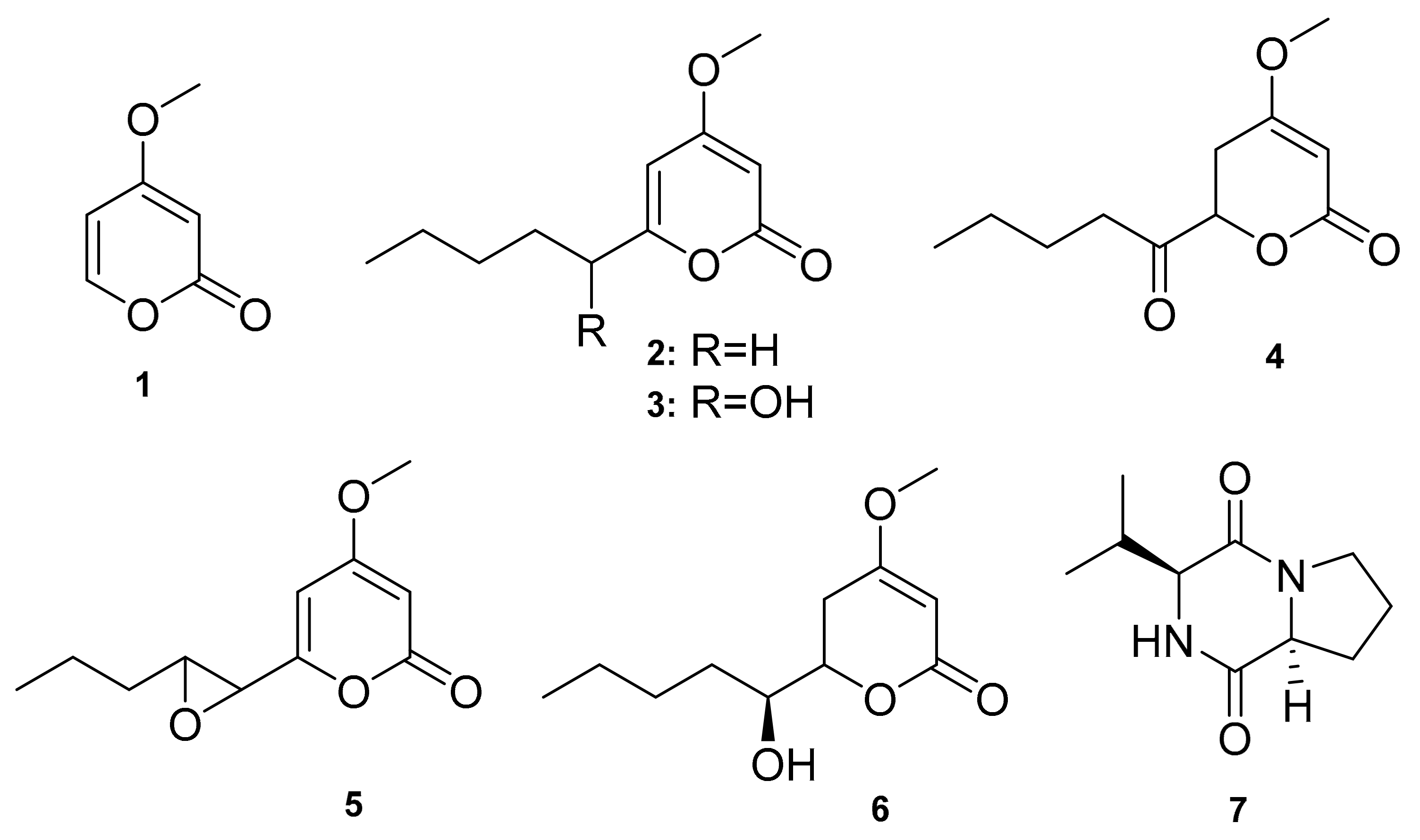
| Black Soldier Fly | Chrysosporium multifidum | Pyrone derivatives (1–6), Diketopiperazine (7) | Antibacterial | [10] |
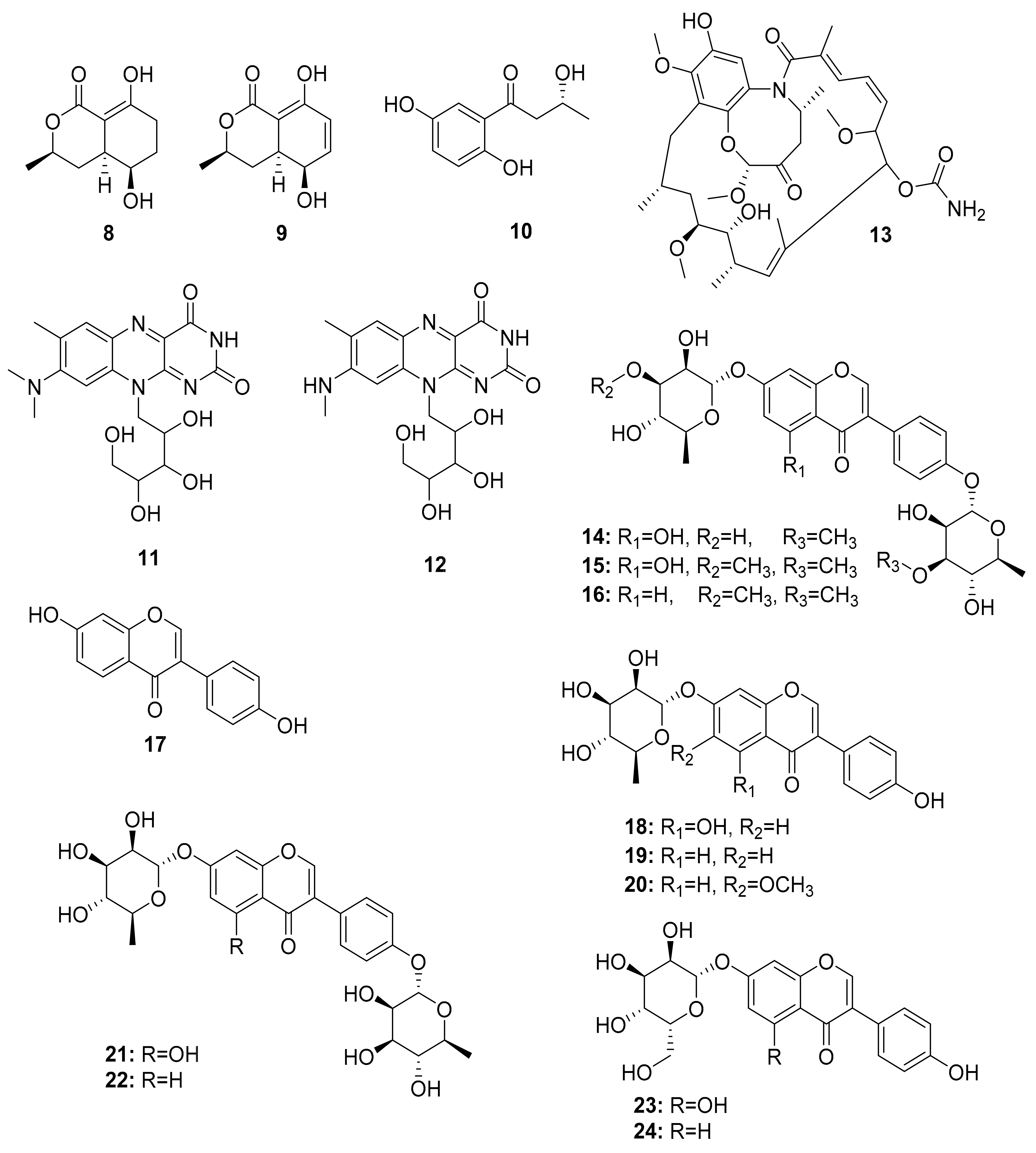
| Termites | Pleosporales sp. BYCDW4 | 5-Hydroxyramulosin (8), biatriosporin M (9) | Antifungal - | [65] |
| Microdiplodia sp. BYCDW8 | 1-(2,5-Dihydroxyphenyl)-3-hydroxybutan-1-one (10) | Antibacterial | [65] | |
| Streptomyces davaonensis YH01 | Roseoflavin (11), 8-methylamino-8-demethyl-d-riboflavin (12) | Antibacterial | [66] | |
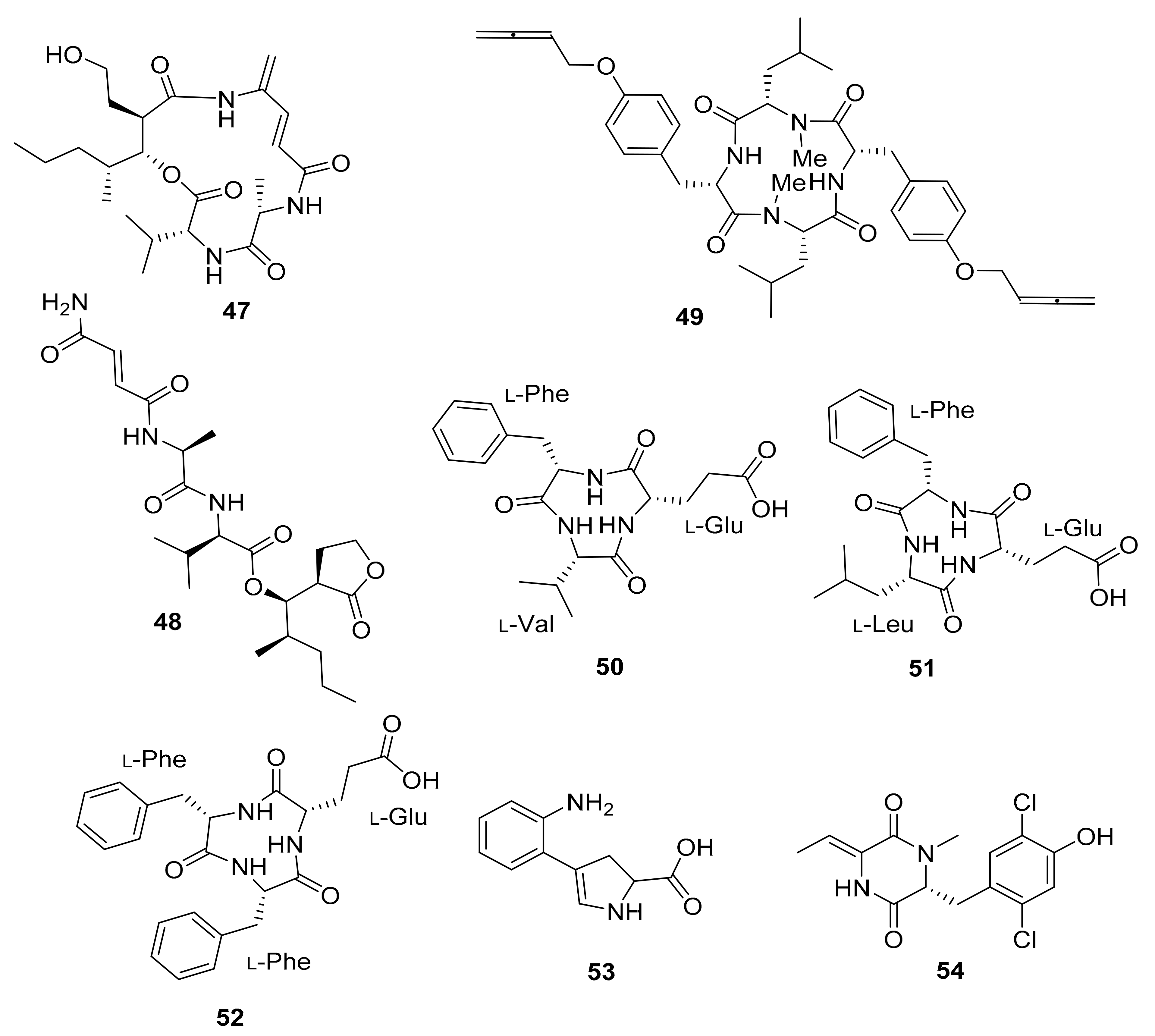
| Streptomyces sp. M56 | Natalamycin (13), Efomycins K (40), and L (41), Efomycin M (42), Efomycin G (43), Elaiophylin (44), 11-O-methylelaiophylin (45), 11,11′-O-dimethylelaiophylin (46) | Antifungal Antifungal Selectin inhibitor | [67,74,75,76,77,78,79,80] | |
| Streptomyces sp. RB1 | Termisoflavones A–C (14–16), Isoflavanoids (17–24) | Cisplatin-induced cytotoxity | [68] | |
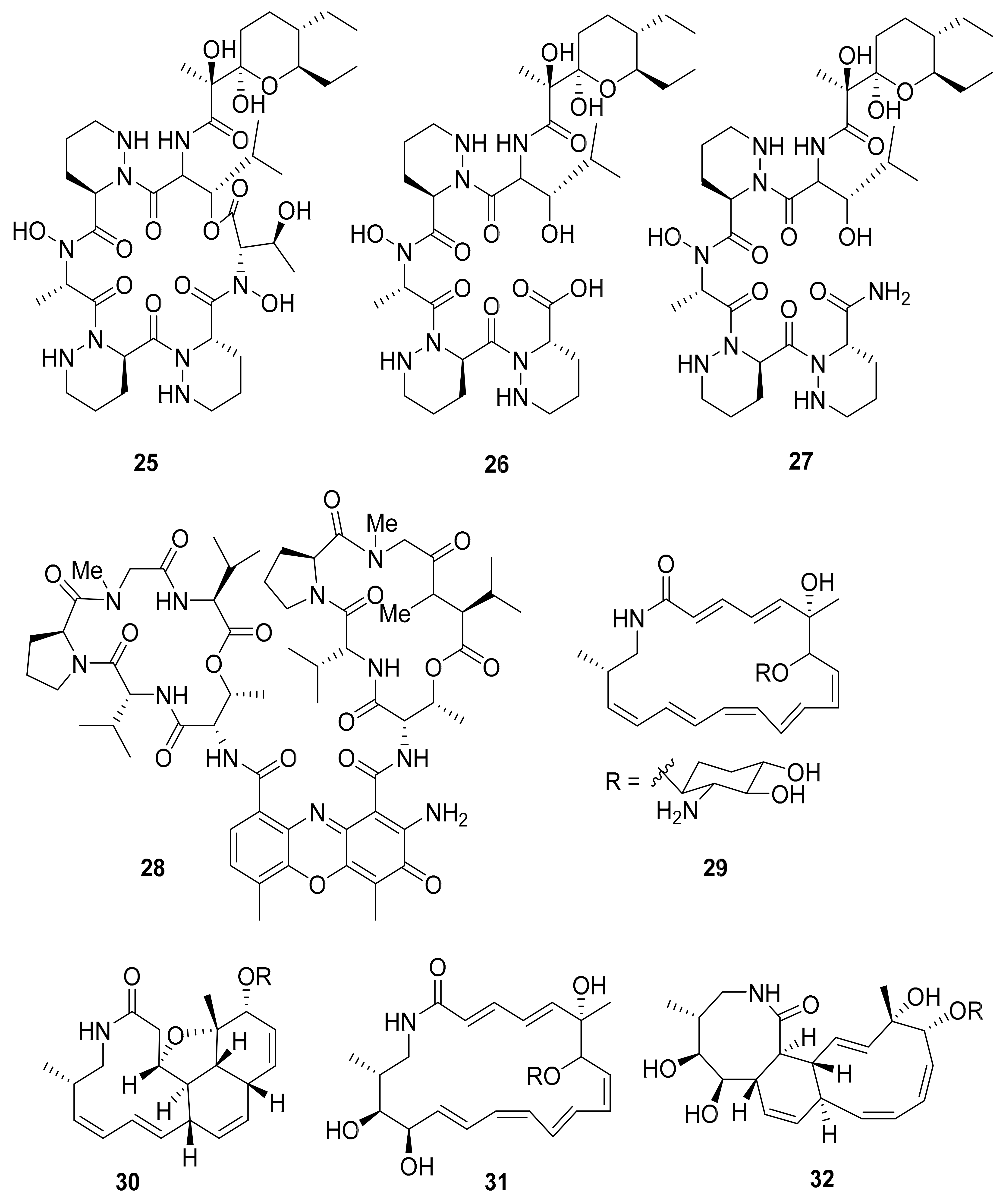
| Streptomyces sp. M41 | Dentigerumycins B–D (25–27) | - | [69] | |
| Streptomyces sp. RB94 | Actinomycin D (28) | Actibacterial, antitumor | [70,81,82] | |
| Amycolatopsis sp. M39 | Macrotermycin A–D (29–32) | Antibacterial, antifungal | [71] | |
| Actinomadura sp. RB29/5-2 | Rubterolone A–F (33–38) | anti-inflammatory activity | [70,72,83] | |
| Co-culture: Streptomyces sp. RB108 with Pleosporales sp. | Barceloneic acid A (39) | Farnesyl-protein transferase inhibitor | [70,73] | |
| Streptomyces sp. MspM5 | Microtermolide A–B (47–48) | - | [84] | |
| Pseudoxylaria sp. X802 | Pseudoxyallemycin B (49) | Antibacterial | [85] | |
| Actinomadura sp. RB99 | Natalenamides A–C (50–52) | Cytotoxic, anti-inflammatory activity | [86] | |
| Co-culture: Actinomadura sp. RB29 and Trichoderma | Banegasine (53), Cyclo(NMe-l-3,5-dichlorotyrosine-Dhb (54) | Antifungal | [70] | |
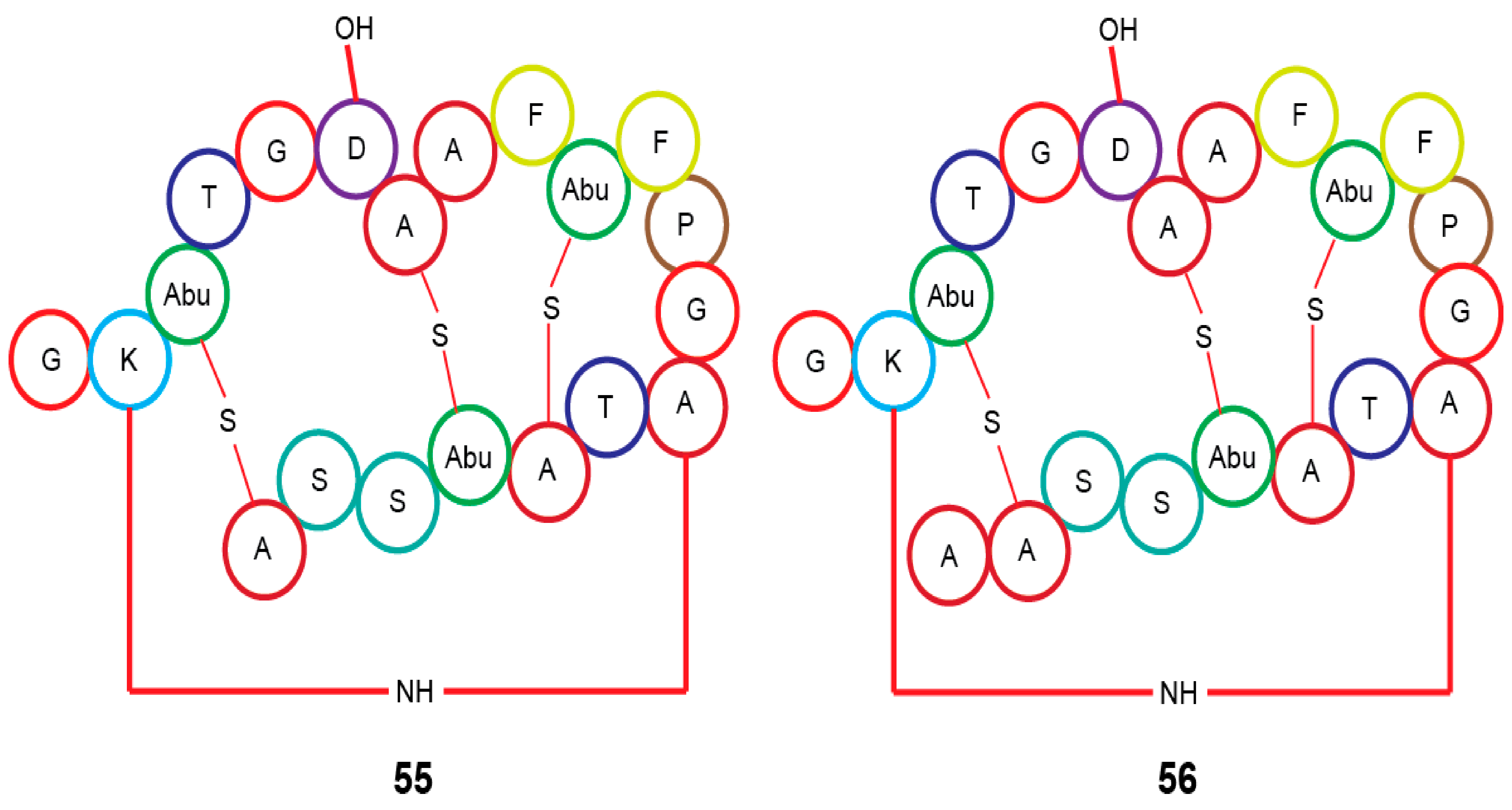
| Actinomadura sp. RB29 | Rubrominin A–B (55–56) | - | [70] | |
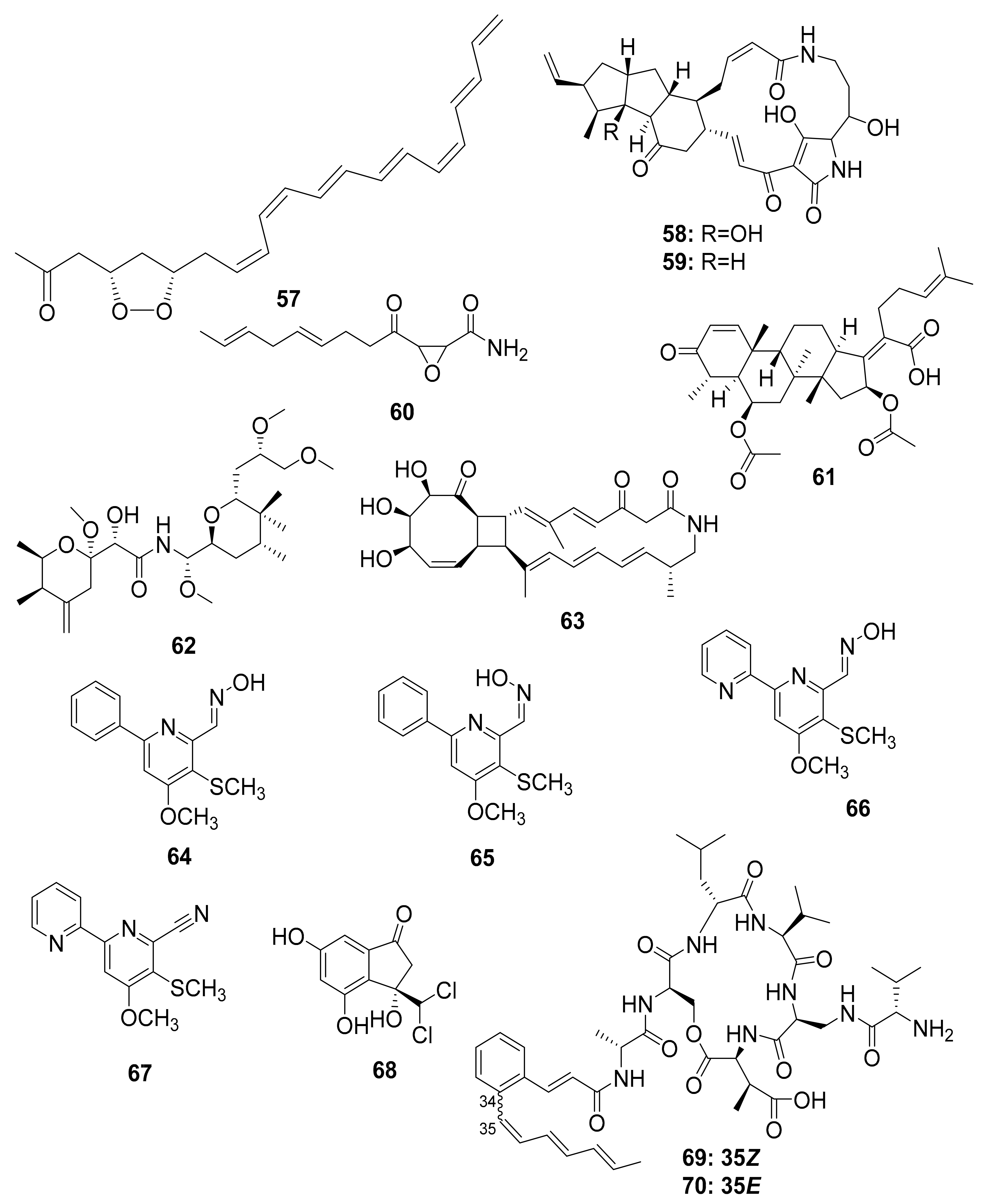
| Beetles | Pine beetles, Streptomyces sp. | Mycangimycin (57), Frontalamide A (58), and Frontalamide B (59) | Antimalarial | [87,88] |
| Ambrosia beetle, Fusarium sp. | Cerulenin (60), Helvolic acid (61) | Antifungal | [89] | |
| Rove beetle, Pseudomonas sp. [49,50,51,52] | Pederin (62) | Anticancer | [90] | |
| Dung beetle | Tripartilactam (63) | Na+/K+ ATPase inhibitor | [91,92,93,94,95] | |
| Actinobacteria | ||||
| Coprismycin A–B (64–65) | Neuroprotective effects | |||
| Collismycin A (66) | ||||
| SF2738D (67) | ||||
| Tripartin (68) | Histone H3 lysine 9 demethylase KDM4 inhibitor | |||
| Streptomyces sp. | Coprisamides A–B (69–70) | Quinone reductase inducer | ||
| Coprisidin A (71) | Na+/K+ ATPase inhibitor | |||
| Coprisidin B (72) | NAD(P)H:quinone oxidoreductase 1 inducer | |||
| Dung beetle, Brevibacillus sp. PTH23 | Lenzimycins A–B (73–74) | Antibacterial | [96] | |
| Catharsius molossus | Molossusamides A–C (75–77) | - | [97] | |
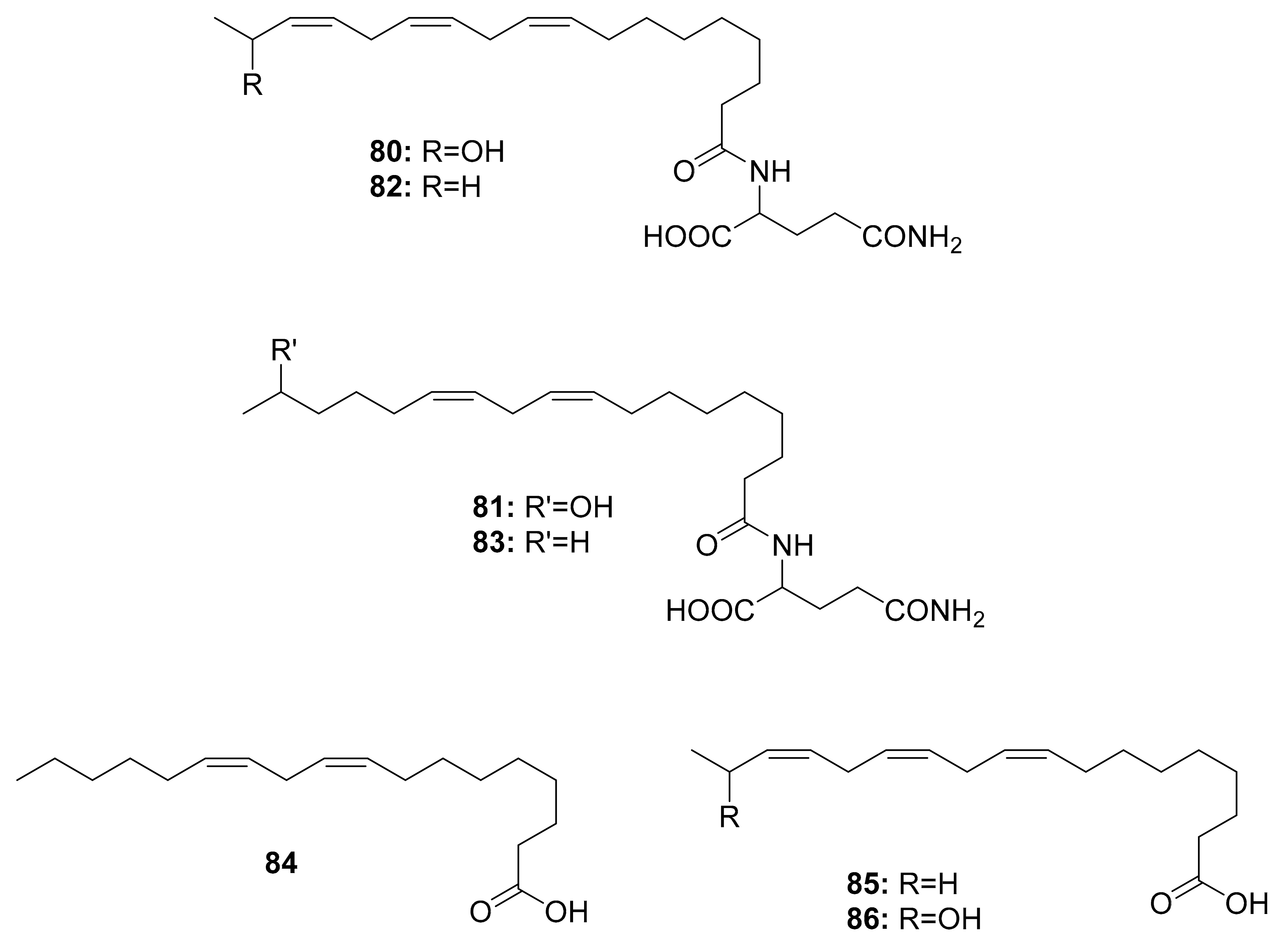
| Caterpillars | Helicoverpa armigera, Mythimna separata, Spodoptera litura, and Agrius convolvuli | Volicitin (80) | - | [98] |
| N-(17-hydroxy-linoleoyl)-l-glutamine (81), | ||||
| N-linolenoyl-l-glutamine (82), | ||||
| N-linoleoyl-l-glutamine (83), | ||||
| linolenic acid (85), and 17-Hydroxylinolenic acid (86) | ||||
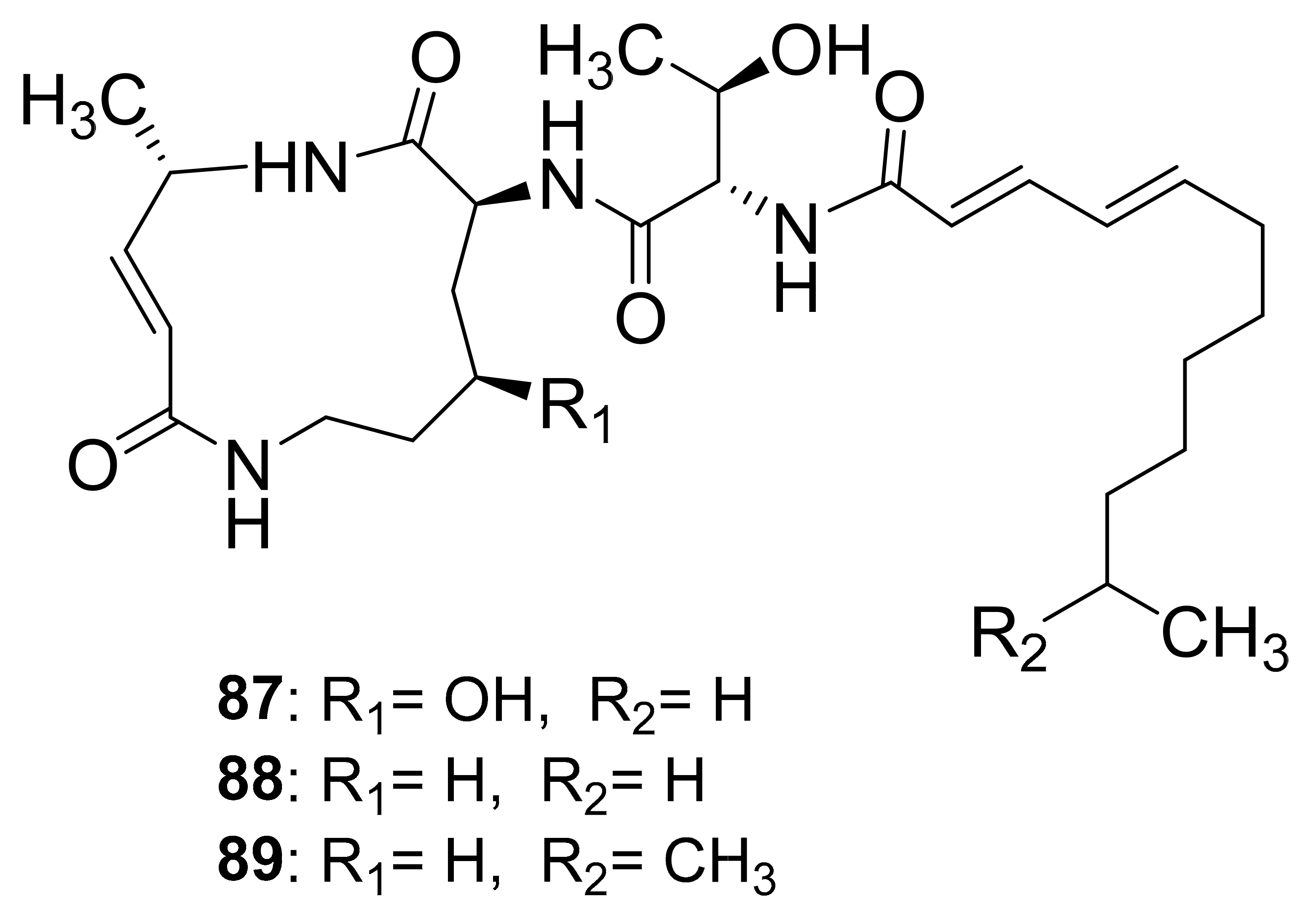
| Crickets | Infected with Photorhabdus asymbiotica | Glidobactin A (87) | - | [99] |
| Luminmycin A (88) | - | |||
| Luminmycin D (89) | Cytotoxic, Proteasome inhibitor |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mudalungu, C.M.; Tanga, C.M.; Kelemu, S.; Torto, B. An Overview of Antimicrobial Compounds from African Edible Insects and Their Associated Microbiota. Antibiotics 2021, 10, 621. https://doi.org/10.3390/antibiotics10060621
Mudalungu CM, Tanga CM, Kelemu S, Torto B. An Overview of Antimicrobial Compounds from African Edible Insects and Their Associated Microbiota. Antibiotics. 2021; 10(6):621. https://doi.org/10.3390/antibiotics10060621
Chicago/Turabian StyleMudalungu, Cynthia M., Chrysantus M. Tanga, Segenet Kelemu, and Baldwyn Torto. 2021. "An Overview of Antimicrobial Compounds from African Edible Insects and Their Associated Microbiota" Antibiotics 10, no. 6: 621. https://doi.org/10.3390/antibiotics10060621
APA StyleMudalungu, C. M., Tanga, C. M., Kelemu, S., & Torto, B. (2021). An Overview of Antimicrobial Compounds from African Edible Insects and Their Associated Microbiota. Antibiotics, 10(6), 621. https://doi.org/10.3390/antibiotics10060621