Influence of Nutrient Media Compared to Human Synovial Fluid on the Antibiotic Susceptibility and Biofilm Gene Expression of Coagulase-Negative Staphylococci In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain Selection
2.2. Antibiotics Used
2.3. Nutrient Media
2.4. Gene Expression Analysis
2.5. Primers for Biofilm Genes Used in RT-qPCR
2.6. Initial Biofilm Formation
2.7. Determination of the Minimal Inhibitory Concentration (MIC)
2.8. Determination of the Biofilm Inhibitory Concentration (BIC)
2.9. Antibiotic Susceptibility Tests with Different Nutrient Media
2.10. Evaluation of Antibiotic Activity in Different Nutrient Media by Measuring Biofilm Recovery Capacity
2.11. Evaluation of Antibiotic Activity in Different Nutrient Media by Measuring Bacterial Viability
2.12. Isolation of Bacterial RNA from Biofilm Grown in Different Nutrient Media on Stainless Steel Discs
2.13. Synthesis of cDNA from Bacterial RNA
2.14. Investigation of the Influence of Different Nutrient Media on the Bacterial Biofilm Gene Expression Using Real-Time Quantitative Polymerase Chain Reaction (Real-Time qPCR)
2.15. Data Analysis
3. Results
3.1. Identification of the MIC and BIC
3.2. Recovery of Biofilm Cells after Treatment with Different Antibiotics Using Different Nutrient Media
3.3. Bacterial Viability after Treatment with Different Antibiotics Using Different Nutrient Media
3.4. Impact of Different Nutrient Media and Glucose on the Biofilm Growth
3.5. Impact of Different Nutrient Media and Glucose on the Biofilm Cell Viability
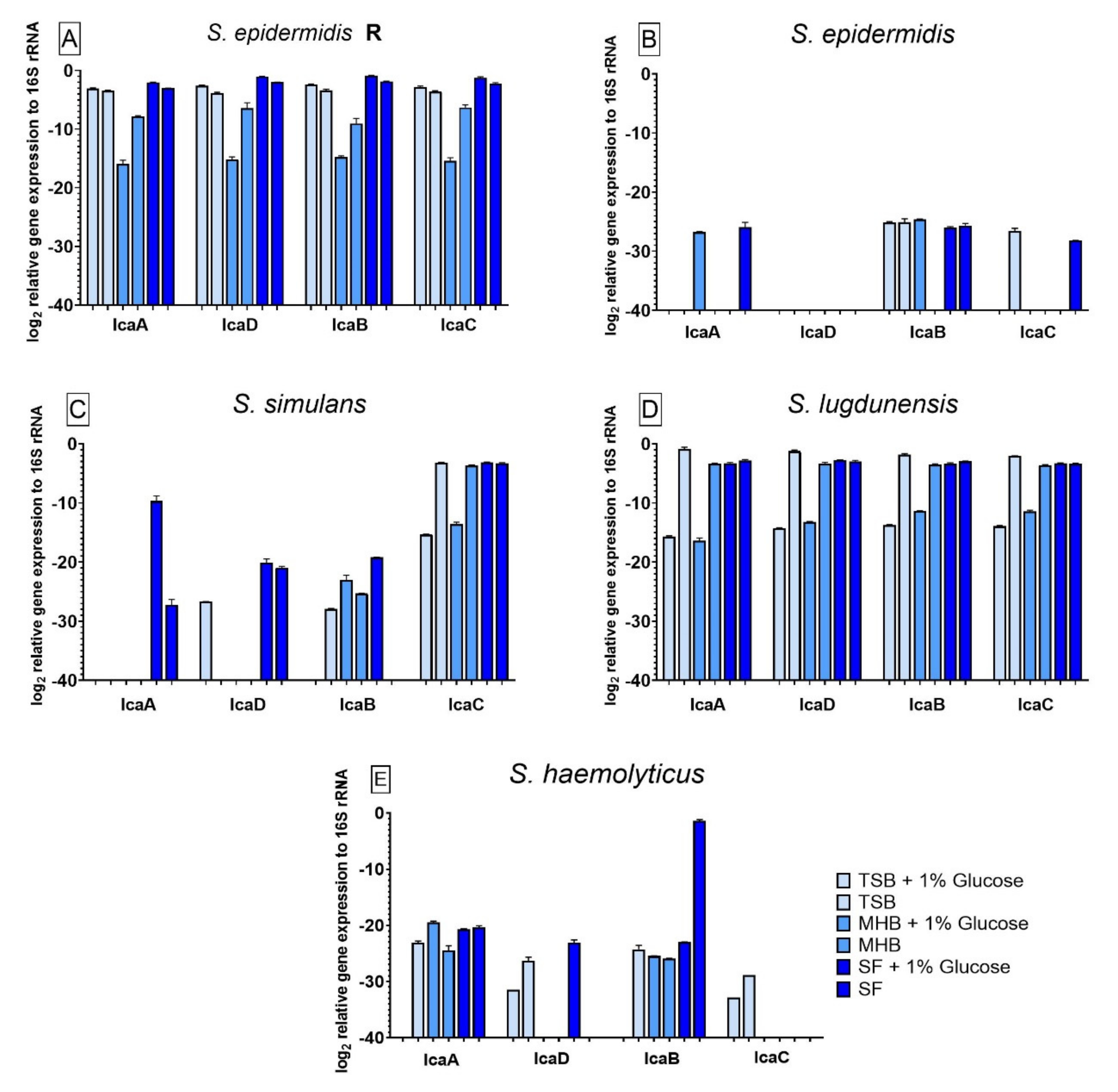
3.6. Influence of Different Nutrient Media and Glucose-Enriched Nutrient Media on the Bacterial icaADBC Gene Expression Compared to Biofilm Grown in SF
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lourtet-Hascoët, J.; Bicart-See, A.; Félicé, M.; Giordano, G.; Bonnet, E. Staphylococcus lugdunensis, a serious pathogen in periprosthetic joint infections: Comparison to Staphylococcus aureus and Staphylococcus epidermidis. Int. J. Infect. Dis. 2016, 51, 56–61. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.D.; Pavlou, G.; Mújica-Mota, R.E.; Toms, A.D. Wales: A comparative analysis with projections for the United States. A study The epidemiology of revision total knee and hip arthroplasty in England and Wales. Bone Jt. J. 2015, 97-B, 1076–1081. [Google Scholar] [CrossRef] [Green Version]
- Leitner, L.; Türk, S.; Heidinger, M.; Stöckl, B.; Posch, F.; Maurer-Ertl, W.; Leithner, A.; Sadoghi, P. Trends and Economic Impact of Hip and Knee Arthroplasty in Central Europe: Findings from the Austrian National Database. Sci. Rep. 2018, 8, 4707. [Google Scholar] [CrossRef] [Green Version]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting microbial biofilms: Current and prospective therapeutic strategies. Nat. Rev. Microbiol. 2018, 15, 740–755. [Google Scholar] [CrossRef]
- Coraça-Hubér, D.C.; Fille, M.; Hausdorfer, J.; Pfaller, K.; Nogler, M. Evaluation of MBEC™-HTP biofilm model for studies of implant associated infections. J. Orthop. Res. 2012, 30, 1176–1180. [Google Scholar] [CrossRef]
- Nodzo, S.R.; Frisch, N.B. The Use of Antibiograms in Orthopedic Surgery. Curr. Rev. Musculoskelet. Med. 2018, 11, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, Y.; Wu, H.; Høiby, N.; Molin, S.; Song, Z. Current understanding of multi-species biofilms. Int. J. Oral Sci. 2011, 3, 74–81. [Google Scholar] [CrossRef]
- Young, K.D. The Selective Value of Bacterial Shape. Microbiol. Mol. Biol. Rev. 2006, 70, 660–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Dong, Y.; Wang, G.; Xu, X.; Zhou, G. Effect of growth media on gene expression levels in Salmonella Typhimurium biofilm formed on stainless steel surface. Food Control. 2016, 59, 546–552. [Google Scholar] [CrossRef]
- Ammann, C.G.; Neuhauser, D.; Eberl, C.; Nogler, M.; Coraça-Huber, D. Tolerance towards gentamicin is a function of nutrient concentration in biofilms of patient-isolated Staphylococcus epidermidis. Folia Microbiol. 2018, 63, 299–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weerasekera, M.M.; Wijesinghe, G.K.; Jayarathna, T.A.; Gunasekara, C.P.; Fernando, N.; Kottegoda, N.; Samaranayake, L.P. Culture media profoundly affect Candida albicans and Candida tropicalis growth, adhesion and biofilm development. Memórias do Instituto Oswaldo Cruz 2016, 111, 697–702. [Google Scholar] [CrossRef] [Green Version]
- Mohamad, M.; Deabate, L.; Belaieff, W.; Bouvet, C.; Zingg, M.; Kuczma, P.; Suva, D.; Uckay, I. Prosthetic Joint Infections Due to Coagulase-Negative Staphylococci. Int. J. Infect. 2015, 3, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Rohde, H.; Burandt, E.C.; Siemssen, N.; Frommelt, L.; Burdelski, C.; Wurster, S.; Scherpe, S.; Davies, A.P.; Harris, L.; Horstkotte, M.A.; et al. Polysaccharide intercellular adhesin or protein factors in biofilm accumulation of Staphylococcus epidermidis and Staphylococcus aureus isolated from prosthetic hip and knee joint infections. Biomaterials 2007, 28, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-H.; Naber, K.; Hacker, J.; Ziebuhr, W. Detection of the icaADBC gene cluster and biofilm formation in Staphylococcus epidermidis isolates from catheter-related urinary tract infections. Int. J. Antimicrob. Agents 2002, 19, 570–575. [Google Scholar] [CrossRef]
- Ziebuhr, W.; Heilmann, C.; Götz, F.; Meyer, P.; Wilms, K.; Straube, E.; Hacker, J. Detection of the intercellular adhesion gene cluster (ica) and phase variation in Staphylococcus epidermidis blood culture strains and mucosal isolates. Infect. Immun. 1997, 65, 890–896. [Google Scholar] [CrossRef] [Green Version]
- Galdbart, J.; Allignet, J.; Tung, H.; Rydèn, C.; El Solh, N. Screening forStaphylococcus epidermidisMarkers Discriminating between Skin-Flora Strains and Those Responsible for Infections of Joint Prostheses. J. Infect. Dis. 2000, 182, 351–355. [Google Scholar] [CrossRef] [Green Version]
- Frebourg, N.B.; Lefebvre, S.; Baert, S.; Lemeland, J.-F. PCR-Based Assay for Discrimination between Invasive and Contaminating Staphylococcus epidermidis Strains. J. Clin. Microbiol. 2000, 38, 877–880. [Google Scholar] [CrossRef] [Green Version]
- Kung, M.S.; Markantonis, J.; Nelson, S.D.; Campbell, P. The Synovial Lining and Synovial Fluid Properties after Joint Arthroplasty. Lubricants 2015, 3, 394–412. [Google Scholar] [CrossRef] [Green Version]
- Gilbertie, J.M.; Schnabel, L.V.; Hickok, N.J.; Jacob, M.E.; Conlon, B.; Shapiro, I.M.; Parvizi, J.; Schaer, T.P. Equine or porcine synovial fluid as a novel ex vivo model for the study of bacterial free-floating biofilms that form in human joint infections. PLoS ONE 2019, 14, e0221012. [Google Scholar] [CrossRef] [Green Version]
- Thing, M.; Mertz, N.; Ågårdh, L.; Larsen, S.; Østergaard, J.; Larsen, C. Simulated synovial fluids for in vitro drug and prodrug release testing of depot injectables intended for joint injection. J. Drug Deliv. Sci. Technol. 2019, 49, 169–176. [Google Scholar] [CrossRef]
- Bortel, E.L.; Charbonnier, B.; Heuberger, R. Development of a Synthetic Synovial Fluid for Tribological Testing. Lubricants 2015, 3, 664–686. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef] [Green Version]
- Lüthje, P.; Von Köckritz-Blickwede, M.; Schwarz, S. Identification and characterization of nine novel types of small staphylococcal plasmids carrying the lincosamide nucleotidyltransferase gene lnu(A). J. Antimicrob. Chemother. 2007, 59, 600–606. [Google Scholar] [CrossRef] [Green Version]
- Arciola, C.R.; Baldassarri, L.; Montanaro, L. Presence of icaA and icaD Genes and Slime Production in a Collection of Staphylococcal Strains from Catheter-Associated Infections. J. Clin. Microbiol. 2001, 39, 2151–2156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milisavljevic, V.; Tran, L.P.; Batmalle, C.; Bootsma, H.J. Benzyl alcohol and ethanol can enhance the pathogenic potential of clinical Staphylococcus epidermidis strains. Am. J. Infect. Control. 2008, 36, 552–558. [Google Scholar] [CrossRef]
- Freitas, A.I.; de Lopes, N.; Oliveira, F.; Brás, S.; França, Â.; Vasconcelos, C.; Vilanova, M.; Cerca, N. Comparative analysis between biofilm formation and gene expression inStaphylococcus epidermidisisolates. Future Microbiol. 2018, 13, 415–427. [Google Scholar] [CrossRef]
- Agarwal, A.; Jain, A. Glucose & sodium chloride induced biofilm production & ica operon in clinical isolates of staphylococci. Indian J. Med. Res. 2013, 138, 262–266. [Google Scholar] [PubMed]
- Pinto, E.; Lago, M.; Branco, L.; Vale-Silva, L.A.; Pinheiro, M.D. Evaluation of Etest Performed in Mueller–Hinton Agar Supplemented with Glucose for Antifungal Susceptibility Testing of Clinical Isolates of Filamentous Fungi. Mycopathologia 2014, 177, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Lade, H.; Park, J.H.; Chung, S.H.; Kim, I.H.; Kim, J.-M.; Joo, H.-S.; Kim, J.-S. Biofilm Formation by Staphylococcus aureus Clinical Isolates is Differentially Affected by Glucose and Sodium Chloride Supplemented Culture Media. J. Clin. Med. 2019, 8, 1853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayak, R.; Khan, S.A.; Watson, R.H.; Cerniglia, C.E. Influence of growth media on vancomycin resistance of En-terococcus isolates and correlation with resistance gene determinants. FEMS Microbiol. Lett. 2002, 214, 159–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibberson, C.B.; Parlet, C.P.; Kwiecinski, J.; Crosby, H.A.; Meyerholz, D.K.; Horswill, A.R. Hyaluronan Modulation Impacts Staphylococcus aureus Biofilm Infection. Infect. Immun. 2016, 84, 1917–1929. [Google Scholar] [CrossRef] [Green Version]
- Brannan, S.R.; Jerrard, D.A. Synovial fluid analysis. J. Emerg. Med. 2006, 30, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Tamer, T.M. Hyaluronan and synovial joint: Function, distribution and healing. Interdiscip. Toxicol. 2013, 6, 111–125. [Google Scholar] [CrossRef]
- Jaradat, Z.W.; Bhunia, A.K. Glucose and Nutrient Concentrations Affect the Expression of a 104-Kilodalton Listeria Adhesion Protein in Listeria monocytogenes. Appl. Environ. Microbiol. 2002, 68, 4876–4883. [Google Scholar] [CrossRef] [Green Version]
- Stumvoll, M.; Goldstein, B.J.; van Haeften, T.W. Type 2 diabetes: Principles of pathogenesis and therapy Patho-physiology of hyperglycaemia. Lancet 2005, 365, 1333–1346. [Google Scholar] [CrossRef]
- Eka, A.; Chen, A.F. Patient-related medical risk factors for periprosthetic joint infection of the hip and knee. Ann. Transl. Med. 2015, 3. [Google Scholar] [CrossRef]
- Waldrop, R.; McLaren, A.; Calara, F.; McLemore, R. Biofilm Growth Has a Threshold Response to Glucose in Vitro. Clin. Orthop. Relat. Res. 2014, 472, 3305–3310. [Google Scholar] [CrossRef] [Green Version]
- Zampieri, M.; Enke, T.; Chubukov, V.; Ricci, V.; Piddock, L.; Sauer, U. Metabolic constraints on the evolution of antibiotic resistance. Mol. Syst. Biol. 2017, 13, 917. [Google Scholar] [CrossRef] [PubMed]
- Mack, D.; Fischer, W.; Krokotsch, A.; Leopold, K.; Hartmann, R.; Egge, H.; Laufs, R. The Intercellular Adhesin Involved in Biofilm Accumulation of Staphylococcus epidermidis Is a Line-ar-1,6-Linked Glucosaminoglycan: Purification and Structural Analysis. J. Bacteriol. 1996, 178, 175–183. [Google Scholar] [CrossRef] [Green Version]
- Heilmann, C.; Schweitzer, O.; Gerke, C.; Vanittanakom, N.; Mack, D.; Götz, F. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol. Microbiol. 1996, 20, 1083–1091. [Google Scholar] [CrossRef]
- Earciola, C.R.; Campoccia, D.; Ravaioli, S.; Montanaro, L. Polysaccharide intercellular adhesin in biofilm: Structural and regulatory aspects. Front. Cell. Infect. Microbiol. 2015, 5, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, H.T.; Nguyen, T.H.; Otto, M. The staphylococcal exopolysaccharide PIA–Biosynthesis and role in biofilm formation, colonization, and infection. Comput. Struct. Biotechnol. J. 2020, 18, 3324–3334. [Google Scholar] [CrossRef] [PubMed]
- Conlon, K.M.; Humphreys, H.; O’Gara, J. icaR Encodes a Transcriptional Repressor Involved in Environmental Regulation of ica Operon Expression and Biofilm Formation in Staphylococcus epidermidis. J. Bacteriol. 2002, 184, 4400–4408. [Google Scholar] [CrossRef] [Green Version]
- Jeng, W.-Y.; Ko, T.-P.; Liu, C.-I.; Guo, R.-T.; Liu, C.-L.; Shr, H.-L.; Wang, A.H.-J. Crystal structure of IcaR, a repressor of the TetR family implicated in biofilm formation in Staphylococcus epidermidis. Nucleic Acids Res. 2008, 36, 1567–1577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Gara, J.P. icaand beyond: Biofilm mechanisms and regulation inStaphylococcus epidermidisandStaphylococcus aureus. FEMS Microbiol. Lett. 2007, 270, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Marques, M.R.C.; Loebenberg, R.; Almukainzi, M. Simulated Biological Fluids with Possible Application in Dissolution Testing. Dissolut. Technol. 2011, 18, 15–28. [Google Scholar] [CrossRef]
- Johnson, V.L.; Hunter, D.J. The epidemiology of osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2014, 28, 5–15. [Google Scholar] [CrossRef]
- Ravi, S.; Zhu, M.; Luey, C.; Young, S.W. Antibiotic resistance in early periprosthetic joint infection. ANZ J. Surg. 2016, 86, 1014–1018. [Google Scholar] [CrossRef]
- Koban, I.; Matthes, R.; Hübner, N.-O.; Welk, A.; Sietmann, R.; Lademann, J.; Kramer, A.; Kocher, T. XTT assay of ex vivo saliva biofilms to test antimicrobial influences. GMS Krankenhhyg Interdiszip 2012, 7. [Google Scholar] [CrossRef]
- Cerca, F.; França, Â.; Pérez-Cabezas, B.; Carvalhais, V.; Ribeiro, A.; Azeredo, J.; Pier, G.; Cerca, N.; Vilanova, M. Dormant bacteria within Staphylococcus epidermidis biofilms have low inflammatory properties and maintain tolerance to vancomycin and penicillin after entering planktonic growth. J. Med. Microbiol. 2014, 63, 1274–1283. [Google Scholar] [CrossRef] [Green Version]




| Genes | Strains | Primer Sequences | Amplicon Length (bp) | References |
|---|---|---|---|---|
| 16S rRNA | All strains | F: 5′-GAAAGCCACGGCTAACTACG-3′ R: 5′-CATTTCACCGCTACACATGG-3′ | 368 | [23] |
| icaA | S. epidermidis | F: 5′-TCTCTTGCAGGAGCAATCAA-3′ R: 5′-TCAGGCACTAACATCCAGCA-3′ | 188 | [24] |
| S. simulans | F: 5′-GGAGGTCTTTGGAAGCAACG-3′ R: 5′-AACCTTTTCGTTTTCATTGTGCT-3′ | 92 | This study | |
| S. lugdunensis | F: 5′- ACCTTGTGCCCATCGAAGAC-3′ R: 5′-TGCGGTAACTGGCAATCCTC-3′ | 337 | This study | |
| S. haemolyticus | F: 5′-TTTGCTGGATGTTGGTGCCT-3′ R: 5′-ACTTCATGCCCACCTTGAGC -3′ | 79 | This study | |
| icaD | S. epidermidis | F: 5′-ATGGTCAAGCCCAGACAGAG-3′ R: 5′-CGTGTTTTCAACATTTAATGCAA-3′ | 198 | [24] |
| S. simulans | F: 5′- ACTCATGTGGTACAGCTCTCTT-3′ R: 5′-GACAAGTCCAGACAGAGGGA-3′ | 307 | This study | |
| S. lugdunensis | F: 5′- TGTCACTCATCGTAACTGCTTC-3′ R: 5′- ATCCATGCTTTTTGCCATGC-3′ | 178 | This study | |
| S. haemolyticus | F: 5′- AAGCCCAGACAGAGGCAATA -3′ R: 5′-CAAACAAACTCATCCATCCGAA -3′ | 229 | This study | |
| icaB | S. epidermidis | F: 5′-AATGACGAATGCAACACCAA-3′ R: 5′-CGTGTGCTTTAAGCCATTGA-3′ | 227 | [25] |
| S. simulans | F: 5′-GGTCCCCTACATGATCCGTT-3′ R: 5′-ACTTGGTGGCGAGAAAAATGG-3′ | 350 | This study | |
| S. lugdunensis | F: 5′- TCCAGACACTTTTAGGTGGGA-3′ R: 5′- CTTAAAGCACATGGGGCACA-3′ | 88 | This study | |
| S. haemolyticus | F: 5′- AGCGCACTCGCGTTAAACTA -3′ R: 5′-AACTTTGCGTCGTGTGCTTT -3′ | 158 | This study | |
| icaC | S. epidermidis | F: 5′-CGCTGTTTCCGGTAGTGATT-3′ R: 5′-TTGGGTGCAACAAATAAATGA-3′ | 176 | [26] |
| S. simulans | F: 5′- TCCGCATATCACAGAGTTCCA-3′ R: 5′- AAGCACGGTGTCGCACTAAA-3′ | 75 | This study | |
| S. lugdunensis | F: 5′- TAAAGCAAGGCGTGCCAAAG-3′ R: 5′- ATGCGAGGTATTGATGGCGA-3′ | 88 | This study | |
| S. haemolyticus | F: 5′- TCGCTGTTTCCGGTAGTGATT -3′ R: 5′-TCCAGTTAGGCTGGTATTGGTC -3′ | 372 | This study |
| Strains | Genes | Accession Numbers |
|---|---|---|
| S. simulans | icaA | AF500263.1 |
| icaD | NZ_KQ957518.1 | |
| icaB | NZ_KQ957518.1 | |
| icaC | LR134264.1 | |
| S. lugdunensis | icaA | FR870271.1 |
| icaD | FR870271.1 | |
| icaB | FR870271.1 | |
| icaC | FR870271.1 | |
| S. haemolyticus | icaA | FJ472951 |
| icaD | FJ472951 | |
| icaB | FJ472951 | |
| icaC | FJ472951 |
| MIC (µg/mL) | |||||
| Antibiotics | S. epidermids R | S. epidermids | S. simulans | S. lugdunensis | S. haemolyticus |
| Gentamicin | 3 | 0.094 | 0.016 | 0.016 | 4 |
| Clindamycin | >256 | 0.064 | 0.016 | 0.023 | 2 |
| Vancomycin | 1 | 1.5 | 0.5 | 1.5 | 1.5 |
| Rifampicin | >32 | 0.012 | 0.003 | 0.008 | 0.008 |
| BIC (µg/mL) | |||||
| Antibiotics | S. epidermids R | S. epidermids | S. simulans | S. lugdunensis | S. haemolyticus |
| Gentamicin | >1024 | 256 | 512 | >1024 | >1024 |
| Clindamycin | >1024 | >1024 | >1024 | >1024 | >1024 |
| Vancomycin | >1024 | >1024 | >1024 | >1024 | >1024 |
| Rifampicin | >1024 | >1024 | >1024 | >1024 | 1 |
| Antibiotic Concentrations (µg/mL) | |||||
|---|---|---|---|---|---|
| Antibiotics | S. epidermids R | S. epidermids | S. simulans | S. lugdunensis | S. haemolyticus |
| Gentamicin | 1024 | 16 | 16 | 64 | 256 |
| Clindamycin | 1024 | 16 | 16 | 1024 | 256 |
| Vancomycin | 32 | 16 | 64 | 1024 | 1 |
| Rifampicin | 8 | 4 | 0.25 | 0.25 | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steixner, S.J.M.; Spiegel, C.; Dammerer, D.; Wurm, A.; Nogler, M.; Coraça-Huber, D.C. Influence of Nutrient Media Compared to Human Synovial Fluid on the Antibiotic Susceptibility and Biofilm Gene Expression of Coagulase-Negative Staphylococci In Vitro. Antibiotics 2021, 10, 790. https://doi.org/10.3390/antibiotics10070790
Steixner SJM, Spiegel C, Dammerer D, Wurm A, Nogler M, Coraça-Huber DC. Influence of Nutrient Media Compared to Human Synovial Fluid on the Antibiotic Susceptibility and Biofilm Gene Expression of Coagulase-Negative Staphylococci In Vitro. Antibiotics. 2021; 10(7):790. https://doi.org/10.3390/antibiotics10070790
Chicago/Turabian StyleSteixner, Stephan Josef Maria, Christopher Spiegel, Dietmar Dammerer, Alexander Wurm, Michael Nogler, and Débora Cristina Coraça-Huber. 2021. "Influence of Nutrient Media Compared to Human Synovial Fluid on the Antibiotic Susceptibility and Biofilm Gene Expression of Coagulase-Negative Staphylococci In Vitro" Antibiotics 10, no. 7: 790. https://doi.org/10.3390/antibiotics10070790
APA StyleSteixner, S. J. M., Spiegel, C., Dammerer, D., Wurm, A., Nogler, M., & Coraça-Huber, D. C. (2021). Influence of Nutrient Media Compared to Human Synovial Fluid on the Antibiotic Susceptibility and Biofilm Gene Expression of Coagulase-Negative Staphylococci In Vitro. Antibiotics, 10(7), 790. https://doi.org/10.3390/antibiotics10070790








