Potential for Phages in the Treatment of Bacterial Sexually Transmitted Infections
Abstract
:1. Introduction
2. Threat of Bacterial Sexually Transmitted Infections
2.1. Epidemiology
2.2. Antibiotic Resistance and Current Treatment Methods
2.3. Symptoms of Infection
3. Challenges Associated with Application of Phage Therapy in Bacterial Sexually Transmitted Infections
3.1. Phage Availability
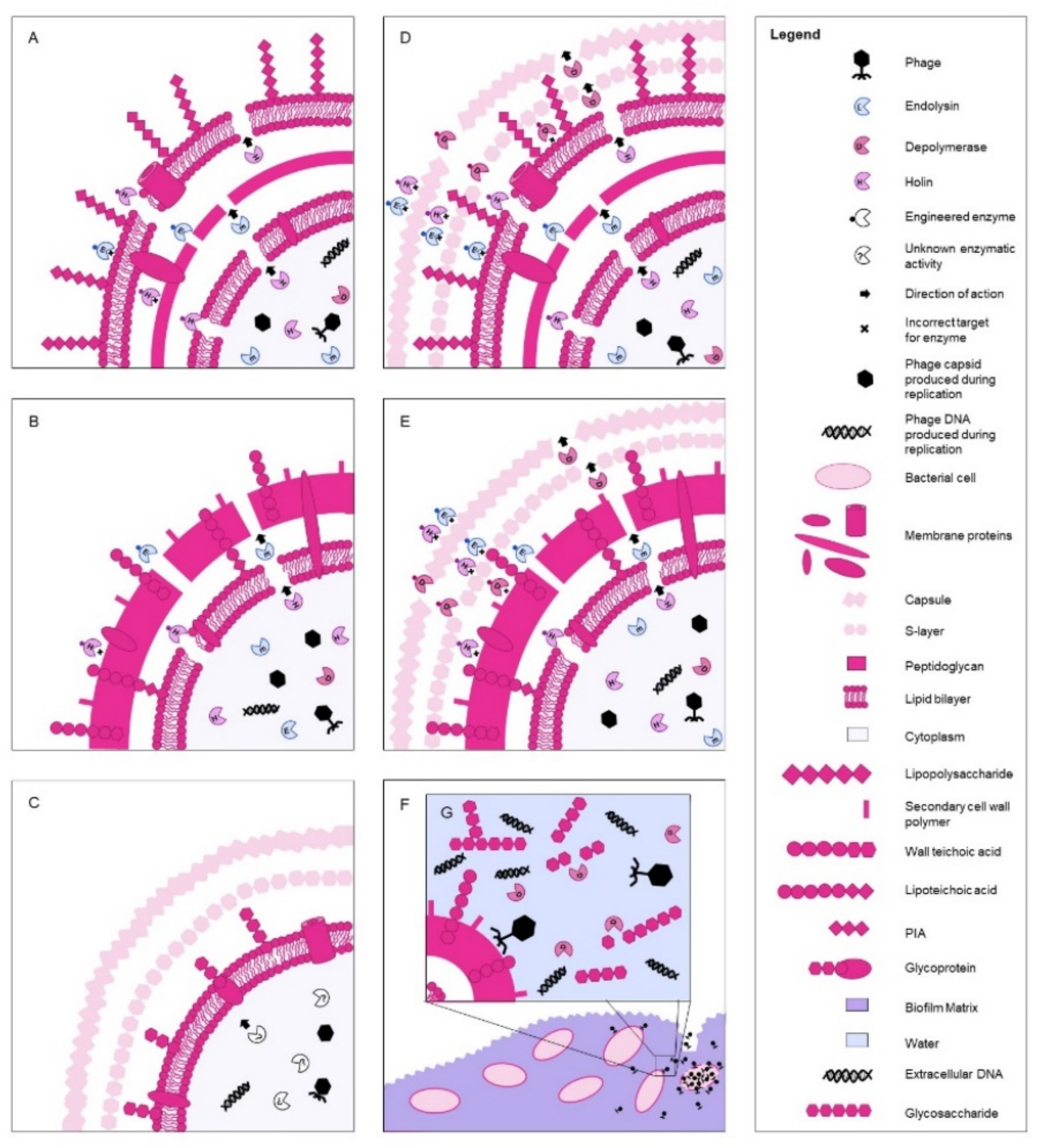
3.2. Potential for Enzymatic Phage Therapy
3.3. Current Clinical Possibilities and Challenges
3.4. Challenges Posed by Bacterial Properties and Conditions
4. Conclusions
5. Expert Opinion
6. Article Highlights
- S. flexneri and S. sonnei phages have been utilized historically in phage therapy, and their transition to treatment in BSTIs may become possible.
- M. genitalium, H. ducreyi, U. parvum and U. urealyticum are rarer BSTIs that pose antibiotic-resistant threats and present challenges associated with culturing. Despite their rarity, phage therapy should eventually be investigated as an alternative treatment method.
- Due to their antibiotic susceptibility, C. granulomatis and T. pallidum do not necessarily require future phage therapy, although this kind of treatment poses potential.
- S. agalactiae and C. trachomatis pose more potential, as in vitro and in vivo studies have already shown success with phage enzyme treatment.
- N. gonorrhoeae with prophages and lysogenic bacteriophages identified holds potential for therapy using phage-derived enzymes, and due to its already high and still increasing antibiotic resistance rates, this should be investigated as an alternative treatment method.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fair, R.J.; Tor, Y. Antibiotics and bacterial resistance in the 21st century. Perspect. Med. Chem. 2014, 6, 25–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paterson, D. The challenge of treating superbugs. Semin. Respir. Crit. Care Med. 2015, 36, 1–2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations the Review on Antimicrobial Resistance. 2016. Available online: https://amr-review.org/sites/default/files/160518_Finalpaper_withcover.pdf (accessed on 4 July 2021).
- Centers for Disease Control and Prevention. New CDC Analysis Shows Steep and Sustained Increases in Stds in Recent Years. 2018. Available online: https://www.cdc.gov/nchhstp/newsroom/2018/press-release-2018-std-prevention-conference.html (accessed on 10 June 2019).
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States. 2013. Available online: https://www.cdc.gov/drugresistance/threat-report-2013/pdf/ar-threats-2013-508.pdf (accessed on 10 June 2019).
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Malhotra, M.; Sood, S.; Mukherjee, A.; Muralidhar, S.; Bala, M. Genital Chlamydia trachomatis: An update. Indian J. Med. Res. 2013, 138, 303–316. [Google Scholar] [PubMed]
- Peeling, R.W.; Mabey, D.; Kamb, M.L.; Chen, X.S.; Radolf, J.D.; Benzaken., A.S. Syphilis. Nat. Rev. Dis. Prim. 2017, 3, 17073. [Google Scholar] [CrossRef] [PubMed]
- Daley, G.M.; Russell, D.B.; Tabrizi, S.N.; McBride, J. Mycoplasma genitalium: A review. Int. J. STD AIDS 2014, 25, 457–487. [Google Scholar] [CrossRef]
- Sethi, S.; Zaman, K.; Jain, N. Mycoplasma genitalium infections: Current treatment options and resistance issues. Infect. Drug Resist. 2017, 10, 283–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, J.S.; Cusini, M.; Gomberg, M.; Moi, H. 2016 European guideline on Mycoplasma genitalium infections. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1650–1656. [Google Scholar] [CrossRef] [Green Version]
- Hunjak, B.; Sabol, I.; Vojnović, G.; Fistonić, I.; Erceg, A.B.; Peršić, Z.; Grce, M. Ureaplasma urealyticum and Ureaplasma parvum in women of reproductive age. Arch. Gynecol. Obstet. 2014, 289, 407–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morse, S.A. Chancroid and Haemophilus ducreyi. Clin. Microbiol. Rev. 1989, 2, 137–157. [Google Scholar] [CrossRef]
- Lautenschlager, S.; Kemp, M.; Christensen, J.J.; Mayans, M.V.; Moi, H. 2017 European guideline for the management of chancroid. Int. J. STD AIDS 2017, 28, 324–329. [Google Scholar] [CrossRef]
- Alfa, M. The laboratory diagnosis of Haemophilus ducreyi. Can. J. Infect. Dis. Med. Microbiol. 2005, 16, 31–34. [Google Scholar] [CrossRef] [Green Version]
- O’Farrell, N.; Moi, H. 2016 European guideline on donovanosis. Int. J. STD AIDS 2016, 27, 605–607. [Google Scholar] [CrossRef] [PubMed]
- O’Farrell, N. Donovanosis. Sex. Transm. Infect. 2002, 78, 452–457. [Google Scholar] [CrossRef]
- Kharsany, A.B.M.; Hoosen, A.A.; Kiepiela, P.; Naicker, T.; Sturm, A.W. Growth and cultural characteristics of Calymmatobacterium granulomatis—The aetiological agent of granuloma inguinale (Donovanosis). J. Med. Microbiol. 1997, 46, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.; Sahly, H.; Jessen, A.; Ingiliz, P.; Stellbrink, H.J.; Neifer, S.; Schewe, K.; Dupke, S.; Baumgarten, A.; Kuschel, A.; et al. High rates of quinolone-resistant strains of Shigella sonnei in HIV-infected MSM. Infection 2013, 41, 999–1003. [Google Scholar] [CrossRef] [PubMed]
- Mook, P.; Gardiner, D.; Kanagarajah, S.; Kerac, M.; Hughes, G.; Field, N.; McCarthy, N.; Rawlings, C.; Simms, I.; Lane, C.; et al. Use of gender distribution in routine surveillance data to detect potential transmission of gastrointestinal infections among men who have sex with men in England. Epidemiol. Infect. 2018, 146, 1468–1477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mook, P.; McCormick, J.; Bains, M.; Cowley, L.A.; Chattaway, M.A.; Jenkins, C.; Mikhail, A.; Hughes, G.; Elson, R.; Day, M.; et al. ESBL-Producing and Macrolide-Resistant Shigella sonnei Infections among Men Who Have Sex with Men, England, 2015. Emerg. Infect. Dis. 2016, 22, 1948–1952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simms, I.; Field, N.; Jenkins, C.; Childs, T.; Gilbart, V.L.; Dallman, T.J.; Mook, P.; Crook, P.; Hughes, G. Intensified shigellosis epidemic associated with sexual transmission in men who have sex with men—Shigella flexneri and S. sonnei in England, 2004 to end of February 2015. Eurosurveillance 2015, 20, 21097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinic, V.; Seth-Smith, H.; Stöckle, M.; Goldenberger, D.; Egli, A. First report of sexually transmitted multi-drug resistant Shigella sonnei infections in Switzerland, investigated by whole genome sequencing. Swiss Med. Wkly. 2018, 148, w14645. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.S.; Dallman, T.J.; Ashton, P.M.; Day, M.; Hughes, G.; Crook, P.D.; Gilbart, V.L.; Zittermann, S.; Allen, V.G.; Howden, B.P.; et al. Intercontinental dissemination of azithromycin-resistant shigellosis through sexual transmission: A cross-sectional study. Lancet Infect. Dis. 2015, 15, 913–921. [Google Scholar] [CrossRef] [Green Version]
- Międzybrodzki, R.; Hoyle, N.; Zhvaniya, F.; Łusiak-Szelachowska, M.; Weber-Dąbrowska, B.; Łobocka, M.; Borysowski, J.; Alavidze, Z.; Kutter, E.; Górski, A. Current updates from the long-standing phage research centers in Georgia, Poland, and Russia. In Bacteriophages; Harper, D., Abedon, S., Burrowes, B., McConville, M., Eds.; Springer: Cham, Switzerland, 2018; pp. 1–31. [Google Scholar]
- Sulakvelidze, A.; Alavidze, Z.; Morris, J.G. Bacteriophage therapy. Antimicrob. Agents Chemother. 2001, 45, 649–659. [Google Scholar] [CrossRef] [Green Version]
- LaVergne, S.; Hamilton, T.; Biswas, B.; Kumaraswamy, M.; Schooley, R.T.; Wooten, D. Phage therapy for a multidrug-resistant Acinetobacter baumannii craniectomy site infection. Open Forum Infect. Dis. 2018, 5, ofy064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Workowski, K.A.; Bolan, G.A. Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm. Rep. 2015, 64, 1–137. [Google Scholar]
- Wi, T.; Lahra, M.M.; Ndowa, F.; Bala, M.; Dillon, J.R.; Ramon-Pardo, P.; Eremin, S.R.; Bolan, G.; Unemo, M. Antimicrobial resistance in Neisseria gonorrhoeae: Global surveillance and a call for international collaborative action. PLoS Med. 2017, 14, e1002344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unemo, M.; Shafer, W.M. Antibiotic resistance in Neisseria gonorrhoeae: Origin, evolution, and lessons learned for the future. Ann. N. Y. Acad. Sci. 2011, 1230, E19–E28. [Google Scholar] [CrossRef] [PubMed]
- Unemo, M.; Seifert, H.S.; Hook, E.W., 3rd; Hawkes, S.; Ndowa, F.; Dillon, J.R. Gonorrhoea. Nat. Rev. Dis. Primers 2019, 5, 79. [Google Scholar] [CrossRef] [PubMed]
- Richardson, W.P.; Sadoff, J.C. Production of a capsule of Neisseria gonorrhoeae. Infect. Immun. 1977, 15, 663–664. [Google Scholar] [CrossRef] [Green Version]
- Griffin, P.J.; Rieder, S.V. A study on the growth requirements of Neisseria gonorrhoeae and its clinical application. Yale J. Biol. Med. 1957, 29, 613–621. [Google Scholar]
- Piekarowicz, A.; Majchrzak, M.; Kłyz, A.; Adamczyk-Popławska, M. Analysis of the filamentous bacteriophage genomes integrated into Neisseria gonorrhoeae FA1090 chromosome. Polish J. Microbiol. 2006, 55, 251–260. [Google Scholar]
- Piekarowicz, A.; Kłyz, A.; Majchrzak, M.; Adamczyk-Popławska, M.; Maugel, T.K.; Stein, D.C. Characterization of the dsDNA prophage sequences in the genome of Neisseria gonorrhoeae and visualization of productive bacteriophage. BMC Microbiol. 2007, 7, 66. [Google Scholar] [CrossRef] [Green Version]
- Piekarowicz, A.; Kłyż, A.; Majchrzak, M.; Szczęsna, E.; Piechucki, M.; Kwiatek, A.; Maugel, T.K.; Stein, D.C. Neisseria gonorrhoeae filamentous phage NgoΦ6 is capable of infecting a variety of Gram-negative bacteria. J. Virol. 2014, 88, 1002–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, I.N. Evolution of Chlamydia trachomatis. Ann. N. Y. Acad. Sci. 2011, 1230, E11–E18. [Google Scholar] [CrossRef]
- Ceovic, R.; Gulin, S.J. Lymphogranuloma venereum: Diagnostic and treatment challenges. Infect. Drug Resist. 2015, 8, 39–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawlikowska-Warych, M.; Śliwa-Dominiak, J.; Deptuła, W. Chlamydial plasmids and bacteriophages. J. Polish Biochem. Soc. Comm. Biochem. Biophys. 2015, 62, 1–6. [Google Scholar] [CrossRef]
- International Committee on Taxonomy of Viruses. ICTV Master Species List 2020.v1. 2021. Available online: https://talk.ictvonline.org/files/master-species-lists/m/msl/12314 (accessed on 21 August 2021).
- Wei, S.; Liu, Q.; Lian, T.; Shao, L. The ΦCPG1 chlamydiaphage can infect Chlamydia trachomatis and significantly reduce its infectivity. Virus Res. 2019, 267, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Guo, R.; Zhou, Q.; Sun, C.; Zhang, X.; Liu, Y.; Liu, Q. Chlamydiaphage φCPG1 capsid protein Vp1 inhibits Chlamydia trachomatis growth via the mitogen-activated protein kinase pathway. Viruses 2016, 8, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.; Liu, Y.; Liu, Y.; Li, L.; Hou, S.; Gao, X.; Qi, M.; Liu, Q. The presence of Chlamydia phage φCPG1 capsid protein VP1 genes and antibodies in patients infected with Chlamydia trachomatis. J. Polish Biochem. Soc. Comm. Biochem. Biophys. 2016, 63, 501–504. [Google Scholar]
- Ma, J.; Sun, Y.; Sun, C.; Zhou, Q.; Qi, M.; Kong, J.; Wang, J.; Liu, Y.; Liu, Q. Identification of proteins differentially expressed by Chlamydia trachomatis treated with chlamydiaphage capsid protein VP1 during intracellular growth. Arch. Microbiol. 2017, 199, 1121–1131. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Guo, R.; Guo, Y.; Shao, L.L.; Liu, Y.; Wei, S.J.; Liu, Y.J.; Liu, Q.Z. Biological effects of chlamydiaphage φCPG1 capsid protein Vp1 on chlamydia trachomatis in vitro and in vivo. J. Huazhong Univ. Sci. Technol. Med. Sci. 2017, 37, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Rank, R.G.; Bowlin, A.K.; Cané, S.; Shou, H.; Liu, Z.; Nagarajan, U.M.; Bavoil, P.M. Effect of chlamydiaphage φCPG1 on the course of conjunctival infection with “Chlamydia caviae” in guinea pigs. Infect. Immun. 2009, 77, 1216–1221. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Howell, J.K.; Bradley, S.D.; Zheng, Y.; Zhou, Z.H.; Norris, S.J. Cellular architecture of Treponema pallidum: Novel flagellum, periplasmic cone, and cell envelope as revealed by cryo electron tomography. J. Mol. Biol. 2010, 403, 546–561. [Google Scholar] [CrossRef] [Green Version]
- French, P. Syphilis. BMJ 2007, 334, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Radolf, J.D.; Deka, R.K.; Anand, A.; Šmajs, D.; Norgard, M.V.; Yang, X.F. Treponema pallidum, the syphilis spirochete: Making a living as a stealth pathogen. Nat. Rev. Microbiol. 2016, 14, 744–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagier, J.C.; Edouard, S.; Pagnier, I.; Mediannikov, O.; Drancourt, M.; Raoult, D. Current and past strategies for bacterial culture in clinical microbiology. Clin. Microbiol. Rev. 2015, 28, 208–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demirkan, I.; Williams, H.F.; Dhawi, A.; Carter, S.D.; Winstanley, C.; Bruce, K.D.; Hart, C.A. Characterization of a spirochaete isolated from a case of bovine digital dermatitis. J. Appl. Microbiol. 2006, 101, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, A.E.; Robinson, I.M.; Joens, L.A.; Kinyon, J.M. A bacteriophage for Treponema hyodysenteriae. Vet. Rec. 1978, 103, 34–35. [Google Scholar] [CrossRef]
- Mitchell, H.L.; Dashper, S.G.; Catmull, D.V.; Paolini, R.A.; Cleal, S.M.; Slakeski, N.; Tan, K.H.; Reynolds, E.C. Treponema denticola biofilm-induced expression of a bacteriophage, toxin-antitoxin systems and transposases. Microbiology 2010, 156, 774–788. [Google Scholar] [CrossRef] [Green Version]
- Eggers, C.H.; Casjens, S.; Hayes, S.F.; Garon, C.F.; Damman, C.J.; Oliver, D.B.; Samuels, D.S. Bacteriophages of spirochetes. J. Mol. Microbiol. Biotechnol. 2000, 2, 365–373. [Google Scholar]
- Le Doare, K.; Heath, P.T. An overview of global GBS epidemiology. Vaccine 2013, 31 (Suppl. 4), D7–D12. [Google Scholar] [CrossRef]
- Morgan, J.A.; Zafar, N.; Cooper, D.B. Group B Streptococcus and Pregnancy. [Updated 31 July 2021]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482443/ (accessed on 21 August 2021).
- Armistead, B.; Oler, E.; Adams Waldorf, K.; Rajagopal, L. The double life of group B streptococcus: Asymptomatic colonizer and potent pathogen. J. Mol. Biol. 2019, 431, 2914–2931. [Google Scholar] [CrossRef]
- Kapatai, G.; Patel, D.; Efstratiou, A.; Chalker, V. Comparison of molecular serotyping approaches of Streptococcus agalactiae from genomic sequences. BMC Genom. 2017, 18, 429. [Google Scholar] [CrossRef]
- Domelier, A.S.; van der Mee-Marquet, N.; Sizaret, P.Y.; Héry-Arnaud, G.; Lartigue, M.F.; Mereghetti, L.; Quentin, R. Molecular characterization and lytic activities of Streptococcus agalactiae bacteriophages and determination of lysogenic-strain features. J. Bacteriol. 2009, 191, 4776–4785. [Google Scholar] [CrossRef] [Green Version]
- Oechslin, F.; Daraspe, J.; Giddey, M.; Moreillon, P.; Resch, G. In vitro characterization of PlySK1249, a novel phage lysin, and assessment of its antibacterial activity in a mouse model of Streptococcus agalactiae bacteremia. Antimicrob. Agents Chemother. 2013, 57, 6276–6283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, H.; Norcross, N.L.; Kahn, D.E. Isolation and Characterization of Streptococcus agalactiae Bacteriophage. Microbiology Society. J. Gen. Virol. 1969, 5, 315–317. [Google Scholar] [CrossRef]
- Furfaro, L.L.; Payne, M.S.; Chang, B.J. Host range, morphological and genomic characterisation of bacteriophages with activity against clinical Streptococcus agalactiae isolates. PLoS ONE 2020, 15, e0235002. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, D.G.; Dong, S.; Baker, J.R.; Engler, J.A. The bifunctional peptidoglycan lysin of Streptococcus agalactiae bacteriophage B30. Microbiology 2004, 150, 2079–2087. [Google Scholar] [CrossRef]
- Donovan, D.M.; Foster-Frey, J.; Dong, S.; Rousseau, G.M.; Moineau, S.; Pritchard, D.G. The cell lysis activity of the Streptococcus agalactiae bacteriophage B30 endolysin relies on the cysteine, histidine-dependent amidohydrolase/peptidase domain. Appl. Environ. Microbiol. 2006, 72, 5108–5112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmelcher, M.; Powell, A.M.; Camp, M.J.; Pohl, C.S.; Donovan, D.M. Synergistic streptococcal phage λSA2 and B30 endolysins kill streptococci in cow milk and in a mouse model of mastitis. Appl. Microbiol. Biotechnol. 2015, 99, 8475–8486. [Google Scholar] [CrossRef]
- Pritchard, D.G.; Dong, S.; Kirk, M.C.; Cartee, R.T.; Baker, J.R. LambdaSa1 and LambdaSa2 prophage lysins of Streptococcus agalactiae. Appl. Environ. Microbiol. 2007, 73, 7150–7154. [Google Scholar] [CrossRef] [Green Version]
- Shan, Y.; Yang, N.; Teng, D.; Wang, X.; Mao, R.; Hao, Y.; Ma, X.; Fan, H.; Wang, J. Recombinant of the staphylococcal bacteriophage lysin CHAPk and its elimination against Streptococcus agalactiae biofilms. Microorganisms 2020, 8, 216. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Q.; Nelson, D.; Zhu, S.; Fischetti, V. Removal of group B streptococci colonizing the vagina and oropharynx of mice with a bacteriophage lytic enzyme. Antimicrob. Agents Chemother. 2005, 49, 111–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; Luo, D.; Gondil, V.S.; Gong, Y.; Jia, M.; Yan, D.; He, J.; Hu, S.; Yang, H.; Wei, H. Construction and characterization of a chimeric lysin ClyV with improved bactericidal activity against Streptococcus agalactiae in vitro and in vivo. Appl. Microbiol. Biotechnol. 2020, 104, 1609–1619. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Li, N.; Yan, Y.; Wang, H.; Li, Y.; Lu, C.; Sun, J. Combined Antibacterial Activity of Phage Lytic Proteins Holin and Lysin from Streptococcus suis Bacteriophage SMP. Curr. Microbiol. 2012, 65, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Köller, T.; Kreikemeyer, B.; Nelson, D. Rapid degradation of Streptococcus pyogenes biofilms by PlyC, a bacteriophage-encoded endolysin. J. Antimicrob. Chemother. 2013, 68, 1818–1824. [Google Scholar] [CrossRef] [PubMed]
- Hoopes, J.T.; Stark, C.J.; Kim, H.A.; Sussman, D.J.; Donovan, D.M.; Nelson, D.C. Use of a bacteriophage lysin, PlyC, as an enzyme disinfectant against Streptococcus equi. Appl. Environ. Microbiol. 2009, 75, 1388–1394. [Google Scholar] [CrossRef] [Green Version]
- Nelson, D.; Loomis, L.; Fischetti, V.A. Prevention and elimination of upper respiratory colonization of mice by group A streptococci by using a bacteriophage lytic enzyme. Proc. Natl. Acad. Sci. USA 2001, 98, 4107–4112. [Google Scholar] [CrossRef] [Green Version]
- Loeffler, J.M.; Nelson, D.; Fischetti, V.A. Rapid killing of Streptococcus pneumoniae with a bacteriophage cell wall hydrolase. Science 2001, 294, 2170–2172. [Google Scholar] [CrossRef]
- Manhart, L.E.; Broad, J.M.; Golden, M.R. Mycoplasma genitalium: Should we treat and how? Clin. Infect. Dis. 2011, 53 (Suppl. 3), S129–S142. [Google Scholar] [CrossRef] [Green Version]
- Sethi, S.; Sing, H.G.; Samanta, P.; Sharma, M. Mycoplasma genitalium: An emerging sexually transmitted pathogen. Indian J. Med. Res. 2012, 136, 942–955. [Google Scholar]
- Tu, A.H.; Voelker, L.L.; Shen, X.; Dybvig, K. Complete nucleotide sequence of the mycoplasma virus P1 genome. Plasmid 2001, 45, 122–126. [Google Scholar] [CrossRef]
- Voelker, L.L.; Dybvig, K. Sequence analysis of the Mycoplasma arthritidis bacteriophage MAV1 genome identifies the putative virulence factor. Gene 1999, 233, 101–107. [Google Scholar] [CrossRef]
- Gourlay, R.N.; Wyld, S.G.; Garwes, D.J. Some properties of mycoplasma virus Br 1. Arch. Virol. 1983, 75, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Röske, K.; Calcutt, M.J.; Wise, K.S. The Mycoplasma fermentans prophage φMFV1: Genome organization, mobility and variable expression of an encoded surface protein. Mol. Microbiol. 2004, 52, 1703–1720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dybvig, K.; Nowak, J.A.; Sladek, T.L.; Maniloff, J. Identification of an enveloped phage, mycoplasma virus L172, that contains a 14-kilobase single-stranded DNA genome. J. Virol. 1985, 53, 384–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gourlay, R.N.; Wyld, S.G.; Garwes, D.J.; Pocock, D.H. Comparison of mycoplasmatales virus MV-Lg-pS2-L172 with plasmavirus MV-L2 and the other mycoplasma viruses. Arch. Virol. 1979, 61, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Kokkayil, P.; Dhawan, B. Ureaplasma: Current perspectives. Indian J. Med. Microbiol. 2015, 33, 205–214. [Google Scholar] [CrossRef]
- Marques, L.M.; Rezende, I.S.; Barbosa, M.S.; Guimarães, A.M.; Martins, H.B.; Campos, G.B.; do Nascimento, N.C.; Dos Santos, A.P.; Amorim, A.T.; Santos, V.M.; et al. Ureaplasma diversum genome provides new insights about the interaction of the surface molecules of this bacterium with the host. PLoS ONE 2016, 11, e0161926. [Google Scholar] [CrossRef] [PubMed]
- Rutanarugsa, A.; Vorachit, M.; Polnikorn, N.; Jayanetra, P. Drug resistance of Haemophilus ducreyi. Southeast Asian J. Trop. Med. Public Health 1990, 21, 185–193. [Google Scholar]
- González-Beiras, C.; Marks, M.; Chen, C.Y.; Roberts, S.; Mitjà, O. Epidemiology of Haemophilus ducreyi Infections. Emerg. Infect. Dis. 2016, 22, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Spinola, S.M. Haemophilus ducreyi as a cause of skin ulcers. Lancet Glob. Health 2014, 2, e387. [Google Scholar] [CrossRef] [Green Version]
- Roberts, S.A.; Taylor, S.L. Haemophilus ducreyi: A newly recognised cause of chronic skin ulceration. Lancet Glob. Health 2014, 2, e187–e188. [Google Scholar] [CrossRef] [Green Version]
- Albritton, W.L. Biology of Haemophilus ducreyi. Microbiol. Rev. 1989, 53, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Spinola, S.M.; Bauer, M.E.; Munson, R.S. Immunopathogenesis of Haemophilus ducreyi infection (chancroid). Infect. Immun. 2002, 70, 1667–1676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pillay, A.; Hoosen, A.A.; Loykissoonlal, D.; Glock, C.; Odhav, B.; Sturm, A.W. Comparison of culture media for the laboratory diagnosis of chancroid. J. Med. Microbiol. 1998, 47, 1023–1026. [Google Scholar] [CrossRef] [PubMed]
- Szafrański, S.P.; Kilian, M.; Yang, I.; Bei der Wieden, G.; Winkel, A.; Hegermann, J.; Stiesch, M. Diversity patterns of bacteriophages infecting Aggregatibacter and Haemophilus species across clades and niches. ISME J. 2019, 13, 2500–2522. [Google Scholar] [CrossRef]
- Skowronek, K.; Piekarowicz, A.; Kauc, L. Comparison of HP1c1 and S2 phages of Haemophilus influenzae. Acta Microbiol. Pol. 1986, 35, 227–232. [Google Scholar]
- Williams, B.J.; Golomb, M.; Phillips, T.; Brownlee, J.; Olson, M.V.; Smith, A.L. Bacteriophage HP2 of Haemophilus influenzae. J. Bacteriol. 2002, 184, 6893–6905. [Google Scholar] [CrossRef] [Green Version]
- Zehr, E.S.; Tabatabai, L.B.; Bayles, D.O. Genomic and proteomic characterization of SuMu, a Mu-like bacteriophage infecting Haemophilus parasuis. BMC Genom. 2012, 13, 331. [Google Scholar] [CrossRef] [Green Version]
- Richens, J. Donovanosis (granuloma inguinale). Sex. Transm. Infect. 2006, 82 (Suppl. 4), iv21–iv22. [Google Scholar] [CrossRef] [Green Version]
- Santiago-Wickey, J.N.; Crosby, B. Granuloma inguinale [Updated 11 August 2021]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK513306/ (accessed on 21 August 2021).
- Międzybrodzki, R.; Borysowski, J.; Weber-Dąbrowska, B.; Fortuna, W.; Letkiewicz, S.; Szufnarowski, K.; Pawełczyk, Z.; Rogóż, P.; Kłak, M.; Wojtasik, E.; et al. Clinical aspects of phage therapy. Adv. Virus Res. 2012, 83, 73–121. [Google Scholar]
- Pu, X.Y.; Zhang, Q.; Pan, J.C.; Shen, Z.; Zhang, W. Spontaneous mutation frequency and molecular mechanisms of Shigella flexneri fluoroquinolone resistance under antibiotic selective stress. World J. Microbiol. Biotechnol. 2013, 29, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Gilbart, V.L.; Simms, I.; Jenkins, C.; Furegato, M.; Gobin, M.; Oliver, I.; Hart, G.; Gill, O.N.; Hughes, G. Sex, drugs and smart phone applications: Findings from semistructured interviews with men who have sex with men diagnosed with Shigella flexneri 3a in England and Wales. Sex. Transm. Infect. 2015, 91, 598–602. [Google Scholar] [CrossRef] [Green Version]
- Caboni, M.; Pédron, T.; Rossi, O.; Goulding, D.; Pickard, D.; Citiulo, F.; MacLennan, C.A.; Dougan, G.; Thomson, N.R.; Saul, A.; et al. An O Antigen Capsule Modulates Bacterial Pathogenesis in Shigella sonnei. PLoS Pathog. 2015, 11, e1004749. [Google Scholar] [CrossRef]
- Jun, J.W.; Kim, J.H.; Shin, S.P.; Han, J.E.; Chai, J.Y.; Park, S.C. Characterization and complete genome sequence of the Shigella bacteriophage pSf-1. Res. Microbiol. 2013, 164, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, S.; Rousseau, G.M.; Labrie, S.J.; Tremblay, D.M.; Kourda, R.S.; Ben Slama, K.; Moineau, S. Characterization of two polyvalent phages infecting Enterobacteriaceae. Sci. Rep. 2016, 7, 40349. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, H.; Ma, H.; Bao, R.; Liu, H.; Yang, L.; Liang, B.; Jia, L.; Xie, J.; Xiang, Y.; et al. Characterization and genomic analysis of SFPH2, a novel T7virus infecting Shigella. Front. Microbiol. 2018, 9, 3027. [Google Scholar] [CrossRef] [PubMed]
- Shahin, K.; Bouzari, M.; Wang, R. Isolation, characterization and genomic analysis of a novel lytic bacteriophage vB_SsoS-ISF002 infecting Shigella sonnei and Shigella flexneri. J. Med. Microbiol. 2018, 67, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, S.T.; Roy, B.; Basu, U.; Dutta, S.; Ghosh, A.N.; Bandyopadhyay, B.; Giri, N. Genomic and proteomic characterizations of Sfin-1, a novel lytic phage infecting multidrug-resistant Shigella spp. and Escherichia coli C. Front. Microbiol. 2019, 10, 1876. [Google Scholar] [CrossRef] [Green Version]
- Chanishvili, N. Literature Review of the Practical Application of Bacteriophage Research; Sharp, R., Ed.; Eliava Institute of Bacteriophage, Microbiology and Virology: Tbilisi, Georgia, 2009. [Google Scholar]
- Furfaro, L.L.; Chang, B.J.; Payne, M.S. Perinatal Streptococcus agalactiae Epidemiology and Surveillance Targets. Clin. Microbiol. Rev. 2018, 31, e00049-18. [Google Scholar] [CrossRef] [Green Version]
- Hughes, G.; Silalang, P.; Were, J.; Patel, H.; Childs, T.; Alexander, S.; Duffell, S.; Saxon, C.; Ison, C.; Mitchell, H.; et al. Prevalence and characteristics of gastrointestinal infections in men who have sex with men diagnosed with rectal chlamydia infection in the UK: An “unlinked anonymous” cross-sectional study. Sex. Transm. Infect. 2017, 94, 518–521. [Google Scholar] [CrossRef]
- Ng, L.-K.; Martin, I.E. The laboratory diagnosis of Neisseria gonorrhoeae. Can. J. Infect. Dis. Med. Microbiol. 2005, 16, 15–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comunián-Carrasco, G.; Peña-Martí, G.E.; Martí-Carvajal, A.J. Antibiotics for treating gonorrhoea in pregnancy. Cochrane Database Syst. Rev. 2018, 2, CD011167. [Google Scholar] [CrossRef]
- Gourlay, R.N.; Wyld, S.G.; Poulton, M.E. Some characteristics of mycoplasma virus Hr 1, isolated from and infecting Mycoplasma hyorhinis. Brief report. Arch. Virol. 1983, 77, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Furfaro, L.L.; Chang, B.J.; Payne, M.S. Applications for bacteriophage therapy during pregnancy and the perinatal period. Front. Microbiol. 2018, 8, 2660. [Google Scholar] [CrossRef] [Green Version]
- Łobocka, M.; Dąbrowska, K.; Górski, A. Engineered Bacteriophage Therapeutics: Rationale, Challenges and Future. BioDrugs 2021, 35, 255–280. [Google Scholar] [CrossRef]
- Bhattarai, S.R.; Yoo, S.Y.; Lee, S.W.; Dean, D. Engineered phage-based therapeutic materials inhibit Chlamydia trachomatis intracellular infection. Biomaterials 2012, 33, 5166–5174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, T.; Baba, T.; Hiramatsu, K.; Schneewind, O. Prophages of Staphylococcus aureus Newman and their contribution to virulence. Mol. Microbiol. 2006, 62, 1035–1047. [Google Scholar] [CrossRef] [PubMed]
- Young, R. Phage lysis: Do we have the hole story yet? Curr. Opin. Microbiol. 2013, 16, 790–797. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Mao, J.; Xie, J. Bacteriophage polysaccharide depolymerases and biomedical applications. BioDrugs 2014, 28, 265–274. [Google Scholar] [CrossRef]
- Young, R. Phage lysis: Three steps, three choices, one outcome. J. Microbiol. 2014, 52, 243–258. [Google Scholar] [CrossRef]
- Fischetti, V.A. Bacteriophage lysins as effective antibacterials. Curr. Opin. Microbiol. 2008, 11, 393–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez, D.; Rodríguez-Rubio, L.; Martínez, B.; Rodríguez, A.; García, P. Bacteriophages as weapons against bacterial biofilms in the food industry. Front. Microbiol. 2016, 7, 825. [Google Scholar] [CrossRef] [PubMed]
- Schmelcher, M.; Loessner, M.J. Bacteriophage endolysins-extending their application to tissues and the bloodstream. Curr. Opin. Biotechnol. 2021, 68, 51–59. [Google Scholar] [CrossRef]
- Briers, Y.; Walmagh, M.; Van Puyenbroeck, V.; Cornelissen, A.; Cenens, W.; Aertsen, A.; Oliveira, H.; Azeredo, J.; Verween, G.; Pirnay, J.P.; et al. Engineered endolysin-based “Artilysins” to combat multidrug-resistant gram-negative pathogens. MBio 2014, 5, e01379-14. [Google Scholar] [CrossRef] [Green Version]
- Zampara, A.; Sørensen, M.C.H.; Grimon, D.; Antenucci, F.; Vitt, A.R.; Bortolaia, V.; Briers, Y.; Brøndsted, L. Exploiting phage receptor binding proteins to enable endolysins to kill Gram-negative bacteria. Sci. Rep. 2020, 10, 12087. [Google Scholar] [CrossRef]
- Donovan, D.M.; Dong, S.; Garrett, W.; Rousseau, G.M.; Moineau, S.; Pritchard, D.G. Peptidoglycan hydrolase fusions maintain their parental specificities. Appl. Environ. Microbiol. 2006, 72, 2988–2996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Y.; Barros, M.; Vennemann, T.; Gallagher, D.T.; Yin, Y.; Linden, S.B.; Heselpoth, R.D.; Spencer, D.J.; Donovan, D.M.; Moult, J. A bacteriophage endolysin that eliminates intracellular streptococci. eLife 2016, 5, e13152. [Google Scholar] [CrossRef] [PubMed]
- São-José, C. Engineering of Phage-Derived Lytic Enzymes: Improving Their Potential as Antimicrobials. Antibiotics 2018, 7, 29. [Google Scholar] [CrossRef] [Green Version]
- Abdelrahman, F.; Easwaran, M.; Daramola, O.I.; Ragab, S.; Lynch, S.; Oduselu, T.J.; Khan, F.M.; Ayobami, A.; Adnan, F.; Torrents, E.; et al. Phage-EncodedEndolysins. Antibiotics 2021, 10, 124. [Google Scholar] [CrossRef]
- Mueller, N.T.; Bakacs, E.; Combellick, J.; Grigoryan, Z.; Dominguez-Bello, M.G. The infant microbiome development: Mom matters. Trends Mol. Med. 2015, 21, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Ruiz, I.; Coutinho, F.H.; Rodriguez-Valera, F. Thousands of Novel Endolysins Discovered in Uncultured Phage Genomes. Front. Microbiol. 2018, 9, 1033. [Google Scholar] [CrossRef] [Green Version]
- Landlinger, C.; Tisakova, L.; Oberbauer, V.; Schwebs, T.; Muhammad, A.; Latka, A.; Van Simaey, L.; Vaneechoutte, M.; Guschin, A.; Resch, G.; et al. Engineered Phage Endolysin Eliminates Gardnerella Biofilm without Damaging Beneficial Bacteria in Bacterial Vaginosis Ex Vivo. Pathogens 2021, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Belopolskaya, K.A.; Sidorova, I.S.; Shakhgireeva, L.S.; Belopolskiy, A.A. Perspectives of phages therapy for gynecological infections. Трудный Пациент [Difficult Patient] 2014, 12, 6–9. (In Russian) [Google Scholar]
- Jault, P.; Leclerc, T.; Jennes, S.; Pirnay, J.P.; Que, Y.A.; Resch, G.; Rousseau, A.F.; Ravat, F.; Carsin, H.; Le Floch, R.; et al. Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): A randomised, controlled, double-blind phase 1/2 trial. Lancet Infect. Dis. 2019, 19, 35–45. [Google Scholar] [CrossRef]
- Jończyk-Matysiak, E.; Łodej, N.; Kula, D.; Owczarek, B.; Orwat, F.; Międzybrodzki, R.; Neuberg, J.; Bagińska, N.; Weber-Dąbrowska, B.; Górski, A. Factors determining phage stability/activity: Challenges in practical phage application. Expert Rev. Anti Infect. Ther. 2019, 17, 583–606. [Google Scholar] [CrossRef] [PubMed]
- Malik, D.J.; Sokolov, I.J.; Vinner, G.K.; Mancuso, F.; Cinquerrui, S.; Vladisavljevic, G.T.; Clokie, M.R.J.; Garton, N.J.; Stapley, A.G.F.; Kirpichnikova, A. Formulation, stabilisation and encapsulation of bacteriophage for phage therapy. Adv. Colloid Interface Sci. 2017, 249, 100–133. [Google Scholar] [CrossRef] [Green Version]
- Rosner, D.; Clark, J. Formulations for Bacteriophage Therapy and the Potential Uses of Immobilization. Pharmaceuticals 2021, 14, 359. [Google Scholar] [CrossRef]
- Duyvejonck, H.; Merabishvili, M.; Vaneechoutte, M.; de Soir, S.; Wright, R.; Friman, V.P.; Verbeken, G.; De Vos, D.; Pirnay, J.P.; Van Mechelen, E.; et al. Evaluation of the Stability of Bacteriophages in Different Solutions Suitable for the Production of Magistral Preparations in Belgium. Viruses 2021, 13, 865. [Google Scholar] [CrossRef]
- Leitner, L.; Ujmajuridze, A.; Chanishvili, N.; Goderdzishvili, M.; Chkonia, I.; Rigvava, S.; Chkhotua, A.; Changashvili, G.; McCallin, S.; Schneider, M.P.; et al. Intravesical bacteriophages for treating urinary tract infections in patients undergoing transurethral resection of the prostate: A randomised, placebo-controlled, double-blind clinical trial. Lancet Infect. Dis. 2021, 21, 427–436. [Google Scholar] [CrossRef]
- Plaut, R.D.; Stibitz, S. Regulatory Considerations for Bacteriophage Therapy Products. In Phage Therapy: A Practical Approach; Górski, A., Międzybrodzki, R., Borysowski, J., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2019; pp. 337–350. [Google Scholar]
- Pelfrene, E.; Sebris, Z.; Cavaleri, M. Developing Phages into Medicines for Europe. In Phage Therapy: A Practical Approach; Górski, A., Międzybrodzki, R., Borysowski, J., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2019; pp. 351–362. [Google Scholar]
- Górski, A.; Borysowski, J.; Międzybrodzki, R. Phage Therapy: Towards a Successful Clinical Trial. Antibiotics 2020, 9, 827. [Google Scholar] [CrossRef]
- Letkiewicz, S.; Łusiak-Szelachowska, M.; Międzybrodzki, R.; Żaczek, M.; Weber-Dąbrowska, B.; Górski, A. Low Immunogenicity of Intravesical Phage Therapy for Urogenitary Tract Infections. Antibiotics 2021, 10, 627. [Google Scholar] [CrossRef]
- Dedrick, R.M.; Freeman, K.G.; Nguyen, J.A.; Bahadirli-Talbott, A.; Smith, B.E.; Wu, A.E.; Ong, A.S.; Lin, C.T.; Ruppel, L.C.; Parrish, N.M.; et al. Potent antibody-mediated neutralization limits bacteriophage treatment of a pulmonary Mycobacterium abscessus infection. Nat. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Łusiak-Szelachowska, M.; Żaczek, M.; Weber-Dąbrowska, B.; Międzybrodzki, R.; Letkiewicz, S.; Fortuna, W.; Rogóż, P.; Szufnarowski, K.; Jończyk-Matysiak, E.; Olchawa, E.; et al. Antiphage activity of sera during phage therapy in relation to its outcome. Future Microbiol. 2017, 12, 109–117. [Google Scholar] [CrossRef]
- Łusiak-Szelachowska, M.; Międzybrodzki, R.; Fortuna, W.; Borysowski, J.; Górski, A. Anti-phage serum antibody responses and the outcome of phage therapy. Folia Microbiol. 2021, 66, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Elwell, C.; Mirrashidi, K.; Engel, J. Chlamydia cell biology and pathogenesis. Nat. Rev. Microbiol. 2016, 14, 385–400. [Google Scholar] [CrossRef]
- Sagar, A.; Klemm, C.; Hartjes, L.; Mauerer, S.; van Zandbergen, G.; Spellerberg, B. The β-hemolysin and intracellular survival of Streptococcus agalactiae in human macrophages. PLoS ONE 2013, 8, e60160. [Google Scholar]
- Yan, W.; Banerjee, P.; Xu, M.; Mukhopadhyay, S.; Ip, M.; Carrigy, N.B.; Lechuga-Ballesteros, D.; To, K.K.W.; Leung, S.S.Y. Formulation strategies for bacteriophages to target intracellular bacterial pathogens. Adv. Drug Deliv. Rev. 2021, 113864. [Google Scholar] [CrossRef] [PubMed]
- Weber-Dąbrowska, B.; Jończyk-Matysiak, E.; Żaczek, M.; Łobocka, M.; Łusiak-Szelachowska, M.; Górski, A. Bacteriophage Procurement for Therapeutic Purposes. Front. Microbiol. 2016, 7, 1177. [Google Scholar] [CrossRef]
- Hyman, P. Phages for Phage Therapy: Isolation, Characterization, and Host Range Breadth. Pharmaceuticals 2019, 12, 35. [Google Scholar] [CrossRef] [Green Version]
- Loś, J.M.; Golec, P.; Wegrzyn, G.; Wegrzyn, A.; Loś, M. Simple method for plating Escherichia coli bacteriophages forming very small plaques or no plaques under standard conditions. Appl. Environ. Microbiol. 2008, 74, 5113–5120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serwer, P. Gels for the Propagation of Bacteriophages and the Characterization of Bacteriophage Assembly Intermediates. In Bacteriophages; Kurtböke, İ., Ed.; IntechOpen Limited: London, UK, 2012. [Google Scholar]
- Garner, S.A.; Everson, J.S.; Lambden, P.R.; Fane, B.A.; Clarke, I.N. Isolation, molecular characterisation and genome sequence of a bacteriophage (Chp3) from Chlamydophila pecorum. Virus Genes 2004, 28, 207–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, B.K.; Abedon, S.T.; Loc-Carrillo, C. Phage cocktails and the future of phage therapy. Future Microbiol. 2013, 8, 769–783. [Google Scholar] [CrossRef] [PubMed]
- Pirnay, J.P.; Verbeken, G.; Ceyssens, P.J.; Huys, I.; De Vos, D.; Ameloot, C.; Fauconnier, A. The Magistral Phage. Viruses 2018, 10, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Haddad, L.; Harb, C.P.; Gebara, M.A.; Stibich, M.A.; Chemaly, R.F. A systematic and critical review of bacteriophage therapy against multi-drug resistant ESKAPE organisms in humans. Clin. Infect. Dis. 2019, 69, 167–178. [Google Scholar] [CrossRef] [PubMed]
| Bacteria | Antibiotic Resistance | Frequency | Symptoms | Treatment | Cell Surface | Culture Conditions | Phages Available |
|---|---|---|---|---|---|---|---|
| Neisseria gonorrhoeae | Considered an urgent threat by the CDC [5], cephalosporins are the last class of antibiotics antibiotic-resistant N. gonorrhoeae remain susceptible to for treatment [28]; high resistance (23%) to tetracycline reported as well as cases of reduced susceptibility to azithromycin and/or cephalosporins [5] | Estimated 78 million per year worldwide [29], second most common bacterial STI [30] | Usually asymptomatic, may include: urethritis, proctitis, pharyngitis, cervicitis, chronic pelvic pain, pelvic inflammatory disease (PID), infertility, ectopic pregnancy, epididymitis, prostatitis, urethral stricture and disseminated gonococcal infection [5,29]. During pregnancy can result in premature delivery, gonococcal ophthalmia neonatorum and systemic neonatal infections in the newborn and pelvic sepsis in the mother [31] | Combination ceftriaxone plus azithromycin or doxycycline [5,28] | Gram-negative with capsule [32] | Requires 5% CO2 [33] | Nine identified prophages present in N. gonorrhoeae genome [34,35], including lysogenic filamentous phage Ngoφ6 with demonstrated activity against a variety of Gram-negative bacteria [36] |
| Chlamydia trachomatis | Some reports of macrolide and tetracycline resistance [37], treatment of lymphogranuloma venereum or LGV, with antibiotics is still considered successful [38] | Estimated 101 million per year worldwide, most common bacterial STI [7]; LGV is rare in the developed world, but outbreaks occur sporadically [38] | Asymptomatic in 75% of women and 50% of males. Symptoms include urethritis, epididymitis, proctitis, cervicitis, salpingitis, endometritis, pelvic inflammatory disease in 20% of women, infertility and ectopic pregnancy [7]. Infection can increase the risk of HIV transmission and cervical cancer [7]. Can cause preterm delivery, chorioamnionitis, neonatal conjunctivitis and neonatal pneumonia [7]. LGV causes inguinal or femoral lymphadenopathy; untreated LGV can result in secondary infections as well as genital and rectal proctocolitis, ulcers and fistulas [28,38] | Azithromycin, Doxycycline or Erythromycin [28,37] | Gram-negative intracellular parasite without capsule [28] | Propagated within cell cultures [7] | To date, five Chlamydiaphages described (Chp1, Chp2, Chp3, Chp4, ΦCPG1, ΦCPAR39) [39]; these bacteriophages have circular single-stranded DNA genomes, an estimated 6 kbp in length. Chlamydiamicrovirus have icosahedral, non-enveloped capsids with diameters of approximately 30 nm. They are group II bacteriophages from the family Microviridae and the subfamily Gokushovirinae [40]. Reports indicate that ΦCPG1 capsid protein VP1, as well as ΦCPG1 phage itself, has disruptive effects on the growth of C. trachomatis in vitro [41,42,43,44,45]. In vivo φCPG1 delays appearance of Chlamydia caviae and decreases pathological response in a Guinea pig animal model [46]. |
| Treponema pallidum subspecies pallidum | Reported macrolide resistance in the US, Europe, China and Australia [8] | Estimated 12 million per year worldwide [47], from 2013 to 2017 in the US, number of cases increased by 76 percent [4] | Primary syphilis typically presents with a painless localized lesion healing on its own. Secondary syphilis often presents with a papular rash. Latent syphilis can occur for any amount of time in which a person is infected with syphilis but is asymptomatic. Tertiary syphilis occurs in 35% of people with latent syphilis, resulting in life-threatening conditions including cardiovascular syphilis, gummatous syphilis and neurosyphilis. If a baby is born when the mother is infected with T. pallidum, or up to 4 years after, it can cause infection in the fetus (1/3) or stillbirth (1/3); 1/3 the babies are unaffected [48] | Long-acting penicillin [8] | Weakly Gram-negative without capsule [49] | Propagated within rabbits through intratesticular, intradermal, intravenous or intracisternal inoculation; slow doubling time (30–33 h) [50] | Phages observed for the Treponema genus (T. phagedenis, T. hyodysenteriae [51,52]) but none characterized for T. pallidum, T. denticola phage described (lysogenic φtd1) [53]; phages from the spirochete phylum have been isolated and described with the majority being Myoviridae [54] |
| Streptococcus agalactiae (GBS) | Considered a concerning threat by CDC, clindamycin resistance prevalent, some erythromycin, azithromycin and vancomycin resistance reported [5] | Considered a part of normal flora for 10–30% of women [55], the CDC estimates 27,000 severe cases of GBS infections in the US, with 49% (~13,230) being erythromycin-resistant and with 28% (~7560) being clindamycin-resistant [5]; a majority of infants colonized with GBS do not develop a GBS infection; about 60% of cases of early-onset GBS infection occur in neonates born to patients with negative GBS culture at 35–37 weeks [56] | Leading cause of neonatal sepsis and meningitis [55]; asymptomatic in colonized women [57] | Intravenous penicillin G (during labor); ampicillin or vancomycin may be substituted [56] | Gram-positive, with ten different capsular types (Ia, Ib, II-XI) [58] | Growth observed with normal laboratory conditions (37 °C and enriched media) [59,60] | Temperate phages have been isolated and characterized for S. agalactiae [59,61,62]; phage lysins have successfully shown activity in vitro (lysins from S. agalactiae phages B30 [63,64], λSA2 [65] and λSA1 [66], as well as CHAPk lysin derived from S. aureus [67]) and in vivo (PlyGBS from phage NCTC11261 [68] and chimeric ClyV [69]). There can be a wide host range with streptococcal phages and phage enzymes [70,71] and streptococcal lysins from other species have demonstrated successful activity in vitro (S. dysgalactiae subsp. equisimilis SK1249 prophage lysin PlySK1249 [60], S. equisimilis subsp. equi lysin PlyC [72]) and in vivo (C1 phage lysin [73]), while whole phages such as S. pneumoniae lytic phage PaI have had successful in vivo activity [74] |
| Mycoplasma genitalium | Reported resistance to tetracyclines, quinolones (moxifloxacin) and macrolides (azithromycin) [9], with resistance increasing at a rapid rate [10]; later-generation antibiotics are last line of defense [10] | Highly variable rates of prevalence geographically (ranging from 0% to 47.5%); estimated rates of 2.0% in low-risk groups and 7.3% in high-risk groups [9]; cause of 10–35% of non-chlamydial non-gonococcal urethritis in men [11] | Frequently presents asymptomatically; however, can cause vaginal discharge, dysuria, urethritis, cervicitis, pelvic inflammatory disease (PID), abdominal pain and dyspareunia [11]; linked to female [75] and male infertility (decreased sperm count) [9] | First line: azithromycin or josamycin; second line: moxifloxacin; third line: doxycycline or pristinamycin [10] | No cell wall (Mycoplasma) [76], no capsule demonstrated on M. genitalium [10,76] | Requires supplemental media (recommended SP4 media); fastidious and slow-growing (may take several weeks or months to grow in culture) [10] | No bacteriophages reported for M. genitalium. Two sequences reported for other mycoplasma viruses (M. pulmonis virus P1 [77] and M. arthritidis virus MAV1 [78]); additional mycoplasma viruses have been reported without sequence information (M. hyorhinis virus Hr 1 [78], M. bovirhinis virus Br1 [79], M. fermentans prophage φMFV1 [80] and mycoplasma viruses L1, L2, L3, BN1 and L172 [81,82]) |
| Ureaplasma parvum and Ureaplasma urealyticum | Reported resistance to macrolides, tetracyclines and fluoroquinolones [83] | High prevalence of ureaplasma colonization in the healthy population (70–80%); however, infection can be dangerous. More often found in symptomatic women than asymptomatic women, U. parvum more frequently isolated than U. urealyticum [12] | Can cause renal infections as well as adverse outcomes in pregnancy such as premature labor, miscarriage or stillbirth. Additionally, may cause infertility if left untreated, may present asymptomatically or with severe symptoms in urogenital infections in women, while in men, typical presents with urethritis. Ureaplasma parvum and Ureaplasma urealyticum considered pathogenic isolates [12] | Azithromycin, doxycycline or erythromycin [83] | No cell wall (Mycoplasma) [83]; capsule experimentally shown to exist in U. urealyticum and hypothesized to exist in U. parvum [84] | Requires serum, growth factors and metabolic substrate (recommended SP4 media); grows without turbidity (pH indicator required for growth detection) [83] | No characterized bacteriophages reported for U. parvum or U. urealyticum [40] |
| Haemophilus ducreyi | Reported resistance to ampicillin, tetracyclines, sulfamethoxazoles, trimethoprim, [85] sulfonamides, chloramphenicol, streptomycin, kanamycin, penicillin and gentamicin [13] | As a causative agent of chancroid endemic to Africa, Asia and Latin America [15], rates appear to be decreasing (before 2000, rates ranged from 0.0 to 69.0% geographically; after 2000, rates range from 0.0 to 15.0%) [86] except in India and Malawi [14]. Was recently identified as a causative agent of skin ulcers in children in tropical areas [87], with rates ranging from 9.0% to 60.0% [86] | Chancroid manifests as genital ulcers, in 50% of patients with genital ulcers, painful and tender inguinal lymphadenopathy may be present [13]; recently recognized to caused chronic skin ulcerations [88] | First line: ceftriaxone or azithromycin; second line: ciprofloxacin or erythromycin [14] | Gram-negative [13], despite a loose capsular structure being observed with electron microscopy [89]; H. ducreyi does not possess capsule-like genes, so the capsular structure produced is likely not a classical capsule [90] | Shown to require hemin and albumin [89]; studies also show media requirements differ between strains of H. ducreyi [91], recommended hydrolyzed protein base supplemented with complex media [89] (Mueller-Hinton, chocolatized blood agar, IsovitaleX [89,91]) at 33 °C in micro-aerophilic (increased CO2 levels) conditions for 48 h [13,91] | Genome screening of clinical isolates of H. ducreyi enabled to identification of some phage clusters containing predicted DNA prophages [92]. No H. ducreyi bacteriophages were isolated; however, other Haemophilus phages have been reported (H. influenzae phages HP1c1 [93], S2A, HP2, B, C, N3 and φflu [94] and H. parasuis phage SuMu [95], of which only the HP1/S2 family have been characterized in detail [94]) |
| Calymmatobacterium granulomatis/ Klebsiella granulomatis | C. granulomatis has not been reported as an antibiotic resistance threat [17] | Endemic to specific areas of the world (India, Papua New Guinea, Brazil, South Africa [96], central Africa, northwestern Australia and the Caribbean [18]), data support a trending decrease in donovanosis over time [17] | Causes donovanosis, also known as granuloma inguinale. Infection begins with ulceration of site of inoculation, followed by lymphadenopathy. Classically there are four types of infections: ulcerogranulomatous (most common with beefy red, non-tender ulcers that bleed readily), hypertrophic or verrucous (growths with irregular edges, occasionally dry) necrotic (smelly ulcers causing deep tissue destruction) and dry, sclerotic or cicatricial lesions. Disseminated infection may occur and is usually associated with pregnancy and cervical infection [17] | Azithromycin (for a minimum of 3 weeks or until symptoms resolve) [17,97]; surgery may be required for extensive tissue damage [97] | Gram-negative intracellular encapsulated parasite of monocytes [18,97], C. granulomatis cells within monocyte are colloquially known as Donovan bodies [17] | Propagated within monocyte co-cultures incubated for 48 h at 37 °C in 5% CO2 [17,18]; bacteria observed intra- and extra-cellularly in monocyte co-cultures after rapid Giemsa stain [17,18] | No C. granulomatis bacteriophages isolated; observation of bacteriophage particles attached to and within the bacteria cell via electron microscopy has been reported, although it has also been strongly refuted [18]. A proposal exists to reclassify C. granulomatis as Klebsiella granulomatis, but there is debate based on the genetics observed [16,17]. Although no evidence supports that they may be effective against C. granulomatis, there are many isolated and characterized Klebsiella phages [40,98], with some even being used in clinical phage therapy [25,98] |
| Shigella flexneri and Shigella sonnei | Considered a serious threat by CDC [5], resistance to ampicillin and trimethoprim-sulfamethoxazole is nearly ubiquitous, with increasing resistance to ciprofloxacin [19], azithromycin [5] and fluoroquinolones reported [19,99] | Accounting for 5–10% of diarrheal illnesses worldwide with more than 165 million cases and 1 million deaths yearly, and despite being a gastrointestinal bacteria, Shigella is emerging as an STI [99], particularly among men who have sex with men (MSM). Considered an STI since 1970s [24]. Emerging epidemics in the UK of S. flexneri (subtype 3a—2009, 2a—2011) and S. sonnei (2011) among men, while rates in women have remained low [22]; epidemics suspected to target gay and bisexual men (MSM) [19]. Transmission across Europe has been observed [23] | May cause shigellosis, an acute, severe bacterial colitis [24]. Infection usually results in diarrhea (sometimes bloody), fever and abdominal pain. May cause more serious complications such as reactive arthritis [5] | Cephalosporins [21] | Gram-negative S. sonnei has an immunogenic O antigen group 4 capsule [100,101] | Growth observed with normal laboratory conditions (37 °C and enriched media) [102] | Many Shigella flexneri and Shigella sonnei bacteriophages have been isolated [98], characterized and sequenced (including S. flexneri virulent Siphophages S6 [103], pSf-2 [102] and Podophage SFPH2 [104], S. flexneri/S. sonnei virulent Siphophages vB SsoS-ISF002 [105] and pSf-1 [102], virulent S. flexneri Myophage S7 [103] and S. flexineri, S. dysenteriae, S. sonnei and E. coli C lytic Sfin-1 Siphophage [106]). Additionally, Shigella flexneri and Shigella sonnei phages have been utilized in clinical phage therapy [25,107] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cater, K.; Międzybrodzki, R.; Morozova, V.; Letkiewicz, S.; Łusiak-Szelachowska, M.; Rękas, J.; Weber-Dąbrowska, B.; Górski, A. Potential for Phages in the Treatment of Bacterial Sexually Transmitted Infections. Antibiotics 2021, 10, 1030. https://doi.org/10.3390/antibiotics10091030
Cater K, Międzybrodzki R, Morozova V, Letkiewicz S, Łusiak-Szelachowska M, Rękas J, Weber-Dąbrowska B, Górski A. Potential for Phages in the Treatment of Bacterial Sexually Transmitted Infections. Antibiotics. 2021; 10(9):1030. https://doi.org/10.3390/antibiotics10091030
Chicago/Turabian StyleCater, Kathryn, Ryszard Międzybrodzki, Vera Morozova, Sławomir Letkiewicz, Marzanna Łusiak-Szelachowska, Justyna Rękas, Beata Weber-Dąbrowska, and Andrzej Górski. 2021. "Potential for Phages in the Treatment of Bacterial Sexually Transmitted Infections" Antibiotics 10, no. 9: 1030. https://doi.org/10.3390/antibiotics10091030
APA StyleCater, K., Międzybrodzki, R., Morozova, V., Letkiewicz, S., Łusiak-Szelachowska, M., Rękas, J., Weber-Dąbrowska, B., & Górski, A. (2021). Potential for Phages in the Treatment of Bacterial Sexually Transmitted Infections. Antibiotics, 10(9), 1030. https://doi.org/10.3390/antibiotics10091030






