The Effects of Different UVA Photoperiods on the Growth Performance, Immune Responses, Antioxidant Status and Apoptosis-Related Gene Expression of the Pacific White Shrimp (Penaeus vannamei)
Abstract
:1. Introduction
2. Results
2.1. Growth Performance
2.2. Immune Enzyme Activity and Relative Expression of Immune-Related Genes
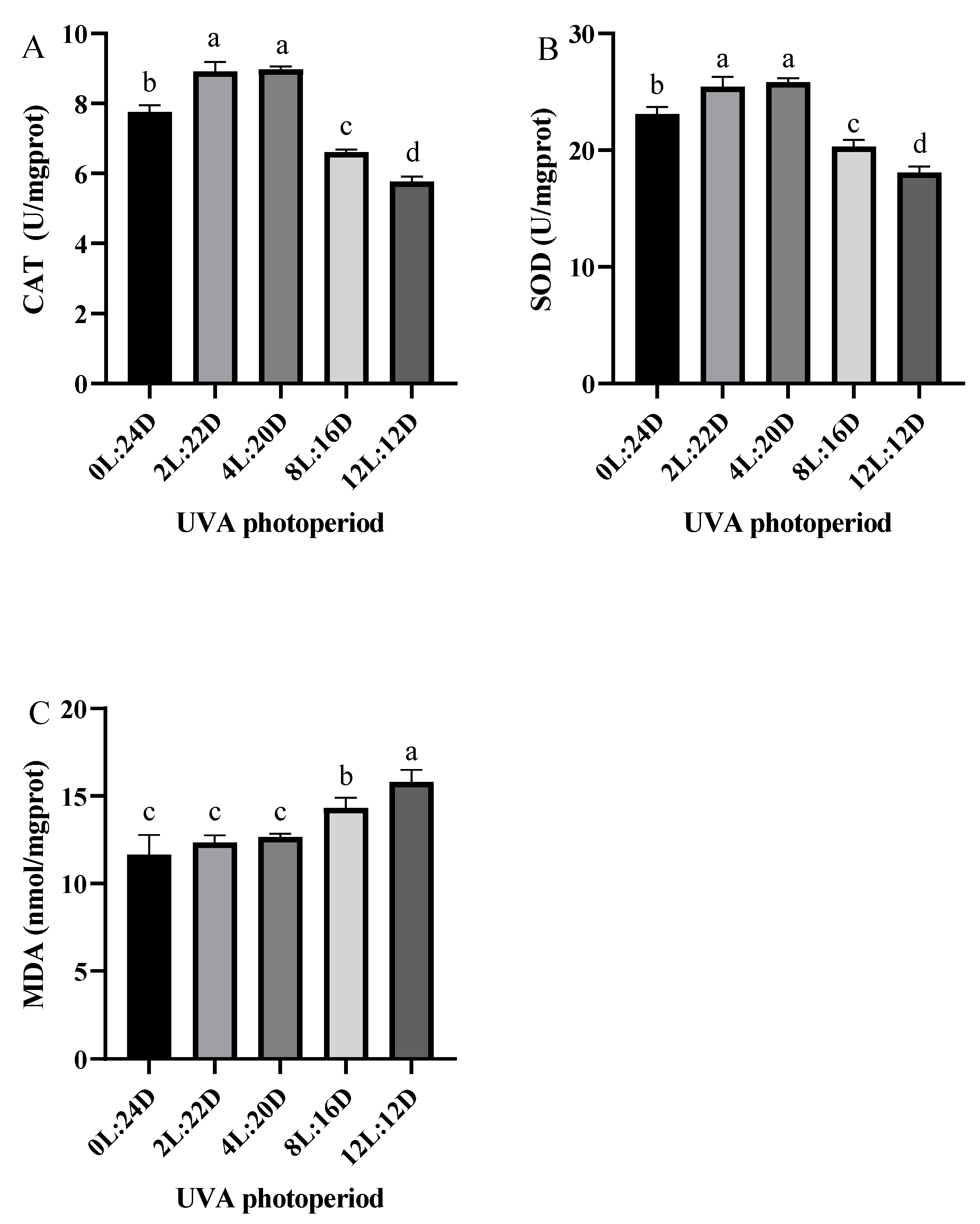
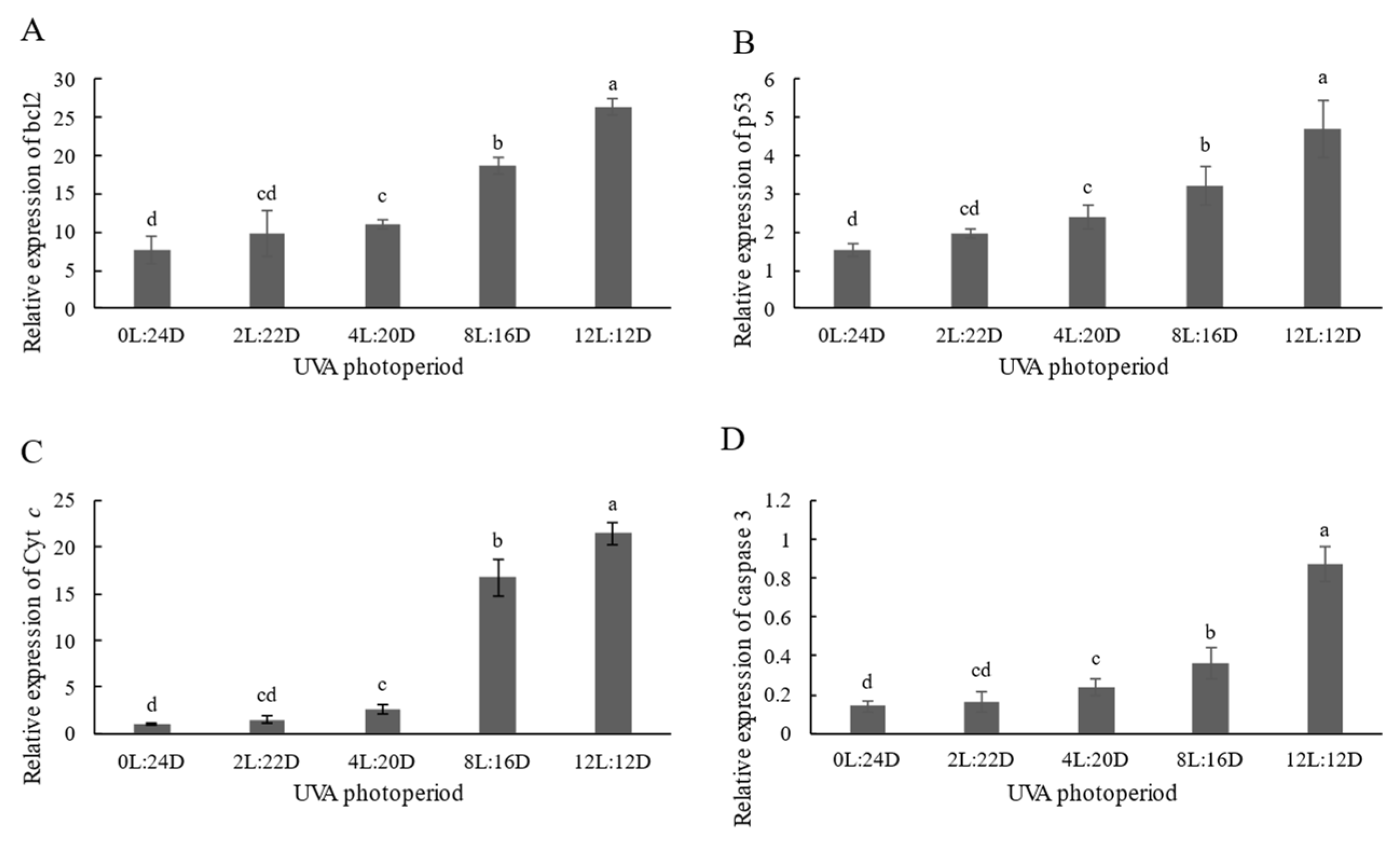
2.3. Antioxidant Capacity and Relative Expression of Apoptosis-Related Genes
3. Discussion
3.1. Growth Performance
3.2. Effects of Five UVA Photoperiod on Immune Responses
3.3. Effects of Five UVA Photoperiods on Antioxidant Enzyme Activity
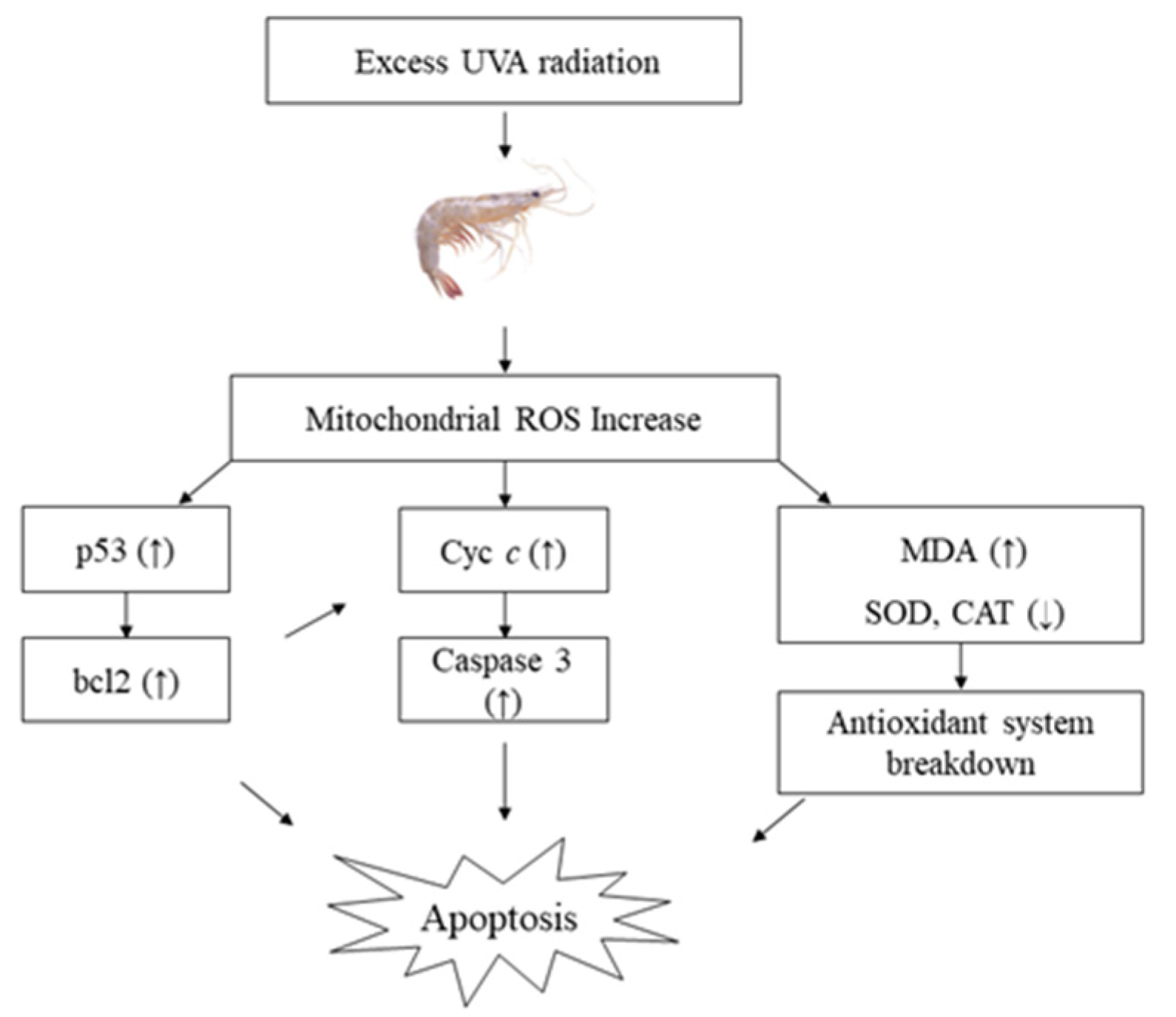
3.4. Effects of Five UVA Photoperiods on Apoptosis-Related Gene Expression
4. Materials and Methods
4.1. Experimental Shrimp
4.2. Experimental Design
4.3. Sample Collection
4.4. Analysis of Enzyme Activity
4.5. Real-Time PCR Analysis
4.6. Data Processing and Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Madronich, S.; McKenzie, R.L.; Caldwell, M.; Björn, L.O. Changes in ultraviolet-radiation reaching the earths surface. Ambio 1995, 24, 143–152. [Google Scholar]
- Williamson, C.E.; Zepp, R.G.; Lucas, R.M.; Madronich, S.; Austin, A.T.; Ballaré, C.L.; Norval, M.; Sulzberger, B.; Bais, A.F.; McKenzie, R.L. Solar ultraviolet radiation in a changing climate. Nat. Clim. Chang. 2014, 4, 434–441. [Google Scholar] [CrossRef]
- Pulgar, J.; Waldisperg, M.; Galbán-Malagón, C.; Maturana, D.; Pulgar, V.M.; Aldana, M. UV radiation impacts body weight, oxygen consumption, and shelter selection in the intertidal vertebrate Girella laevifrons. Sci. Total. Environ. 2017, 578, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Browman, H.I.; Skiftesvik, A.B.; Kuhn, P. The relationship between ultraviolet and polarized light and growth rate in the early larval stages of turbot (Scophtalmus maximus), Atlantic cod (Gadus morhua) and Atlantic herring (Clupea harengus) reared in intensive culture conditions. Aquaculture 2006, 256, 296–301. [Google Scholar] [CrossRef]
- Alves, R.N.; Agusti, S. Effect of ultraviolet radiation (UVR) on the life stages of fish. Rev. Fish Biol. Fish. 2020, 30, 335–372. [Google Scholar] [CrossRef]
- Groff, A.A.; da Silva, J.; Nunes, E.A.; Ianistcki, M.; Guecheva, T.N.; de Oliveira, A.M.; de Oliveira, C.P.; Val, A.L.; Henriques, J.A. UVA/UVB-induced genotoxicity and lesion repair in Colossoma macropomum and Arapaima gigas Amazonian fish. J. Photochem. Photobiol. B 2010, 99, 93–99. [Google Scholar] [CrossRef]
- Bok, M.J.; Roberts, N.W.; Cronin, T.W. Behavioural evidence for polychromatic ultraviolet sensitivity in mantis shrimp. Proc. Biol. Sci. 2018, 285, 20181384. [Google Scholar] [CrossRef] [Green Version]
- Salo, H.M.; Jokinen, E.I.; Markkula, S.E.; Aaltonen, T.M.; Penttilä, H.T. Comparative effects of UVA and UVB irradiation on the immune system of fish. J. Photochem. Photobiol. B 2000, 56, 154–162. [Google Scholar] [CrossRef]
- Rakete, S.; Nagaraj, R.H. UVA Light-mediated Ascorbate Oxidation in Human Lenses. Photochem. Photobiol. 2017, 93, 1091–1095. [Google Scholar] [CrossRef]
- Fei, F.; Liu, B.; Gao, X.; Wang, X.; Liu, Y.; Bin, H. Effects of supplemental ultraviolet light on growth, oxidative stress responses, and apoptosis-related gene expression of the shrimp Litopenaeus vannamei. Aquaculture 2020, 520, 735013. [Google Scholar] [CrossRef]
- Li, E.; Wang, X.; Chen, K.; Xu, C.; Qin, J.G.; Chen, L. Physiological change and nutritional requirement of Pacific white shrimp Litopenaeus vannamei at low salinity. Rev. Aquac. 2017, 9, 57–75. [Google Scholar] [CrossRef]
- Coyle, S.D.; Bright, L.A.; Wood, D.R.; Neal, R.S.; Tidwell, J.H. Performance of Pacific white shrimp, Litopenaeus vannamei, reared in zero-exchange tank systems exposed to different light Sources and intensities. J. World Aquac. Aquac. Soc. 2011, 42, 687–695. [Google Scholar] [CrossRef]
- You, K.; Yang, H.; Liu, Y.; Liu, S.; Zhou, Y.; Zhang, T. Effects of different light sources and illumination methods on growth and body color of shrimp Litopenaeus vannamei. Aquaculture 2006, 252, 557–565. [Google Scholar] [CrossRef]
- Sucré, E.; Vidussi, F.; Mostajir, B.; Charmantier, G.; Lorin-Nebel, C. Impact of ultraviolet-B radiation on planktonic fish larvae: Alteration of the osmoregulatory function. Aquat. Toxicol. 2012, 109, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Key, P.B.; Chung, K.W.; West, J.B.; Pennington, P.L.; DeLorenzo, M.E. Developmental and reproductive effects in grass shrimp (Palaemon pugio) following acute larval exposure to a thin oil sheen and ultraviolet light. Aquat. Toxicol. 2020, 228, 105651. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.N.; Justo, M.S.S.; Laranja, J.L.Q.; Alarcon, J.F.; Al Suwailem, A.; Agusti, S. Exposure to natural ultraviolet B radiation levels has adverse effects on growth, behavior, physiology, and innate immune response in juvenile European seabass (Dicentrarchus labrax). Aquaculture 2021, 533, 736215. [Google Scholar] [CrossRef]
- Jordan, R.; Howe, D.; Juanes, F.; Stauffer, J., Jr.; Loew, E. Ultraviolet radiation enhances zooplanktivory rate in ultraviolet sensitive cichlids. Afr. J. Ecol. 2004, 42, 228–231. [Google Scholar] [CrossRef]
- Sinha, R.P.; Häder, D.P. UV-induced DNA damage and repair: A review. Photochem. Photobiol. Sci. 2002, 1, 225–236. [Google Scholar] [CrossRef]
- Vitt, S.; Rahn, A.K.; Drolshagen, L.; Bakker, T.C.M.; Scharsack, J.P.; Rick, I.P. Enhanced ambient UVB light affects growth, body condition and the investment in innate and adaptive immunity in three-spined sticklebacks (Gasterosteus aculeatus). Aquat. Ecol. 2017, 51, 499–509. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Esteban, M.Á.; Cuesta, A.; Sun, Y.-Z. Prebiotics and Fish Immune Response: A Review of Current Knowledge and Future Perspectives. Rev. Fish. Sci. Aquac. 2015, 23, 315–328. [Google Scholar] [CrossRef]
- Wu, B.; Wang, Q.; Cao, J.; Mei, J.; Xie, J. Effects of Ascorbic Acid and β-1,3-Glucan on Survival, Physiological Response and Flesh Quality of Cultured Tiger Grouper (Epinephelus fuscoguttatus) during Simulated Transport in Water. Biology (Basel) 2020, 9, 37. [Google Scholar] [CrossRef] [Green Version]
- Middlemiss, K.L.; Daniels, C.L.; Urbina, M.A.; Wilson, R.W. Combined effects of UV irradiation, ozonation, and the probiotic Bacillus spp. on growth, survival, and general fitness in European lobster (Homarus gammarus). Aquaculture 2015, 444, 99–107. [Google Scholar] [CrossRef]
- Xu, W.N.; Chen, D.H.; Chen, Q.Q.; Liu, W.B. Growth performance, innate immune responses and disease resistance of fingerling blunt snout bream, Megalobrama amblycephala adapted to different berberine-dietary feeding modes. Fish. Shellfish Immunol. 2017, 68, 458–465. [Google Scholar] [CrossRef]
- Dorts, J.; Silvestre, F.; Tu, H.T.; Tyberghein, A.E.; Phuong, N.T.; Kestemont, P. Oxidative stress, protein carbonylation and heat shock proteins in the black tiger shrimp, Penaeus monodon, following exposure to endosulfan and deltamethrin. Environ. Toxicol. Pharmacol. 2009, 28, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.N.; Zhou, J.; Wang, P.; Tian, T.T.; Zheng, Y.; Liu, Y.; Mai, W.J.; Wang, A.L. Oxidative stress, DNA damage and antioxidant enzyme gene expression in the Pacific white shrimp, Litopenaeus vannamei when exposed to acute pH stress. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2009, 150, 428–435. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, L.; Xin, Y.; Wang, W.N.; He, W.Y.; Wang, A.L.; Liu, Y. Effect of temperature on antioxidant enzyme gene expression and stress protein response in white shrimp, Litopenaeus vannamei. J. Therm. Biol. 2010, 35, 284–289. [Google Scholar] [CrossRef]
- Zagarese, H.E.; Williamson, C.E. The implications of solar UV radiation exposure for fish and fisheries. Fish. Fish. 2010, 2, 250–260. [Google Scholar] [CrossRef]
- Lorenz, B.T. Food for Thought: A Review of the Carotenoid, Astaxanthin, as a Pigment Source and Vitamin for Cultured Penaeus Prawn. Available online: http://www.brineshrimpdirect.com/Food-for-Thought-c85.html (accessed on 17 November 2021).
- Alonso-Alvarez, C.; Bertrand, S.; Devevey, G.; Gaillard, M.; Prost, J.; Faivre, B.; Sorci, G. An experimental test of the dose-dependent effect of carotenoids and immune activation on sexual signals and antioxidant activity. Am. Nat. 2004, 164, 651–659. [Google Scholar] [CrossRef]
- Pike, T.W.; Blount, J.D.; Bjerkeng, B.; Lindström, J.; Metcalfe, N.B. Carotenoids, oxidative stress and female mating preference for longer lived males. Proc. Biol. Sci. 2007, 274, 1591–1596. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T. Phytochemicals as protectors against ultraviolet radiation: Versatility of effects and mechanisms. Planta Med. 2008, 74, 1548–1559. [Google Scholar] [CrossRef] [Green Version]
- Ermak, G.; Davies, K.J. Calcium and oxidative stress: From cell signaling to cell death. Mol. Immunol. 2002, 38, 713–721. [Google Scholar] [CrossRef]
- Orrenius, S. Reactive oxygen species in mitochondria-mediated cell death. Drug Metab. Rev. 2007, 39, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Yeh, M.S.; Cheng, W. Cold shock-induced norepinephrine triggers apoptosis of haemocytes via caspase-3 in the white shrimp, Litopenaeus vannamei. Fish. Shellfish Immunol. 2009, 27, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Ju, C.; Zhang, X. The miR-1000-p53 pathway regulates apoptosis and virus infection in shrimp. Fish. Shellfish Immunol. 2015, 46, 516–522. [Google Scholar] [CrossRef]
- Chang, C.C.; Yeh, M.S.; Lin, H.K.; Cheng, W. The effect of Vibrio alginolyticus infection on caspase-3 expression and activity in white shrimp Litopenaeus vannamei. Fish. Shellfish Immunol. 2008, 25, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.C.; Young, L.C.; Maeda, T.; Tron, V.A.; Andrew, S.E. Mammalian DNA mismatch repair protects cells from UVB-induced DNA damage by facilitating apoptosis and p53 activation. DNA Repair (Amst.) 2003, 2, 427–435. [Google Scholar] [CrossRef]
- Hollmann, G.; Linden, R.; Giangrande, A.; Allodi, S. Increased p53 and decreased p21 accompany apoptosis induced by ultraviolet radiation in the nervous system of a crustacean. Aquat. Toxicol. 2016, 173, 1–8. [Google Scholar] [CrossRef]
- Qian, Z.; Liu, T.; Liu, Q.; He, S.; Liu, Y.; Hou, F.; Wang, X.; Mi, X.; Cai, C.; Liu, X. p53 is involved in shrimp survival via its regulation roles on MnSOD and GPx in response to acute environmental stresses. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2014, 159, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shao, D.; Wu, Y.; Cai, C.; Hu, C.; Shou, X.; Dai, B.; Ye, B.; Wang, M.; Jia, X. Apoptotic responses of Carassius auratus lymphocytes to nodularin exposure in vitro. Fish. Shellfish Immunol. 2012, 33, 1229–1237. [Google Scholar] [CrossRef]
- Porter, A.G.; Jänicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Zhang, G. Gene discovery, comparative analysis and expression profile reveal the complexity of the Crassostrea gigas apoptosis system. Dev. Comp. Immunol. 2011, 35, 603–610. [Google Scholar] [CrossRef]
- Jin, G.H.; Liu, Y.; Jin, S.Z.; Liu, X.D.; Liu, S.Z. UVB induced oxidative stress in human keratinocytes and protective effect of antioxidant agents. Radiat. Environ. Biophys. 2007, 46, 61–68. [Google Scholar] [CrossRef]
- Yabu, T.; Ishibashi, Y.; Yamashita, M. Stress-induced apoptosis in larval embryos of Japanese flounder. Fish. Sci. 2003, 69, 1218–1223. [Google Scholar] [CrossRef]
- Ricart-Jané, D.; Llobera, M.; López-Tejero, M.D. Anticoagulants and other preanalytical factors interfere in plasma nitrate/nitrite quantification by the Griess method. Nitric Oxide 2002, 6, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Cho, J.H.; Kim, S.R.; Hur, Y.B. Toxic effects of waterborne ammonia exposure on hematological parameters, oxidative stress and stress indicators of juvenile hybrid grouper, Epinephelus lanceolatus ♂ × Epinephelus fuscoguttatus ♀. Environ. Toxicol. Pharmacol. 2020, 80, 103453. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]


| UVA Photoperiod | Initial Weight (g) | Final Weight (g) | Growth Rate (%) | FCR (%) | SGR (%) | Mortality (%) |
|---|---|---|---|---|---|---|
| 0L:24D | 9.60 ± 0.04 | 17.68 ± 0.46 b | 84.13 ± 5.59 b | 1.17 ± 0.14 c | 2.18 ± 0.11 b | 26.67 ± 3.33 c |
| 2L:22D | 9.55 ± 0.09 | 19.35 ± 0.64 a | 102.69 ± 6.28 a | 0.86 ± 0.04 d | 2.52 ± 0.11 a | 18.89 ± 1.92 d |
| 4L:20D | 9.62 ± 0.17 | 19.68 ± 0.40 a | 104.78 ± 6.89 a | 0.74 ± 0.02 d | 2.56 ± 0.12 a | 17.78 ± 1.92 d |
| 8L:16D | 9.53 ± 0.05 | 16.14 ± 0.17 c | 69.44 ± 1.88 c | 1.59 ± 0.08 b | 1.88 ± 0.04 c | 35.56 ± 3.85 b |
| 12L:12D | 9.51 ± 0.12 | 14.73 ± 0.67 d | 54.79 ± 5.16 d | 2.34 ± 0.22 a | 1.56 ± 0.12 d | 43.33 ± 5.77 a |
| Ingredients | Content (%) |
|---|---|
| Crude protein | 43.25% |
| Crude fat | 7.41% |
| Crude fiber | 3.76% |
| Crude ash | 13.24% |
| Moisture | 11.88% |
| Total phosphorus | 1.05% |
| Lysine | 2.43% |
| Gene | Accession No. | Primer |
|---|---|---|
| bcl2 | MH559339.1 | F: ATGTTGCTGTGCACCAAGTG |
| R: AAGGCAGCACATGAACACGA | ||
| p53 | KX179650.1 | F: GTGGAAGTGTTGCCAAGCAG |
| R: CGAATTTGTGACGACCTGCC | ||
| cytochrome C (Cyt c) | KX096890.1 | F: CGTACACGTCCAGCAAAAGC |
| R: GGTGTACACGTAGCCTGGTG | ||
| caspase-3 | EU421939.1 | F: GGTGGACAAAGGCGTGAGTA |
| R: CTCGGCCAAGAAGTGGATGA | ||
| crustin | AY486426.1 | F: ACCTGTTCCAACGGCTACAA |
| R: AACCTGCGATCCGAGGAATG | ||
| penaeidin 3a | AF390139.1 | F: GCCGGGGAATTTCCTTCTCA |
| R: ACAGGTTGTCAAGCGAGGTT | ||
| C-type lectin (Lc1) | KY937940.1 | F: AGCTGGCACGAAAGACATCA |
| R: GAGACACCGCTCGTCGTTAT | ||
| LGBP | EU102286.1 | F: CGTCTCCGAACCATGTCCAA |
| R: CAAAGTTGTCGTTGCCCCTG | ||
| β-Actin | AF300705.2 | F: CTCGCAGTCCAACCCGAG |
| R: TCTACAACCAGGGCGGCTA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Liu, B.; Gao, X.; Wang, X.; Li, H.; Xu, L.; Wang, G.; Zhao, K.; Huang, B. The Effects of Different UVA Photoperiods on the Growth Performance, Immune Responses, Antioxidant Status and Apoptosis-Related Gene Expression of the Pacific White Shrimp (Penaeus vannamei). Antibiotics 2022, 11, 37. https://doi.org/10.3390/antibiotics11010037
Wang X, Liu B, Gao X, Wang X, Li H, Xu L, Wang G, Zhao K, Huang B. The Effects of Different UVA Photoperiods on the Growth Performance, Immune Responses, Antioxidant Status and Apoptosis-Related Gene Expression of the Pacific White Shrimp (Penaeus vannamei). Antibiotics. 2022; 11(1):37. https://doi.org/10.3390/antibiotics11010037
Chicago/Turabian StyleWang, Xinyi, Baoliang Liu, Xiaoqiang Gao, Xi Wang, Hongxu Li, Liang Xu, Guiming Wang, Kuifeng Zhao, and Bin Huang. 2022. "The Effects of Different UVA Photoperiods on the Growth Performance, Immune Responses, Antioxidant Status and Apoptosis-Related Gene Expression of the Pacific White Shrimp (Penaeus vannamei)" Antibiotics 11, no. 1: 37. https://doi.org/10.3390/antibiotics11010037
APA StyleWang, X., Liu, B., Gao, X., Wang, X., Li, H., Xu, L., Wang, G., Zhao, K., & Huang, B. (2022). The Effects of Different UVA Photoperiods on the Growth Performance, Immune Responses, Antioxidant Status and Apoptosis-Related Gene Expression of the Pacific White Shrimp (Penaeus vannamei). Antibiotics, 11(1), 37. https://doi.org/10.3390/antibiotics11010037





