Factors Affecting Antibiotic Prescription among Hospital Physicians in a Low-Antimicrobial-Resistance Country: A Qualitative Study
Abstract
:1. Introduction
2. Results
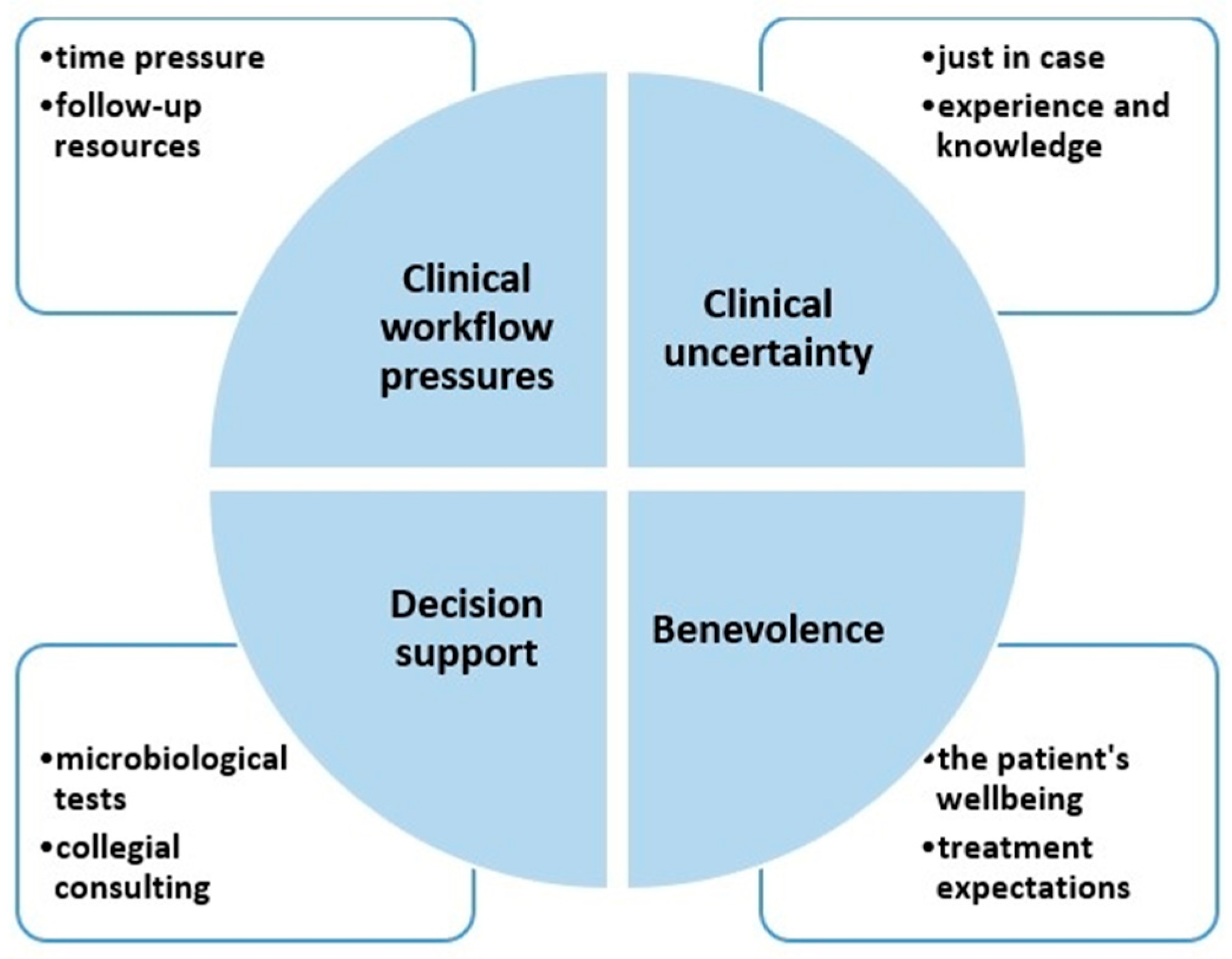
2.1. Theme (i) Clinical Workflow Pressures
“I think a lot is due to the pressure in the hospital; we hear all the time, ‘Discharge, discharge, discharge’ (…) We get whipped up like that, (…) and then I think the threshold (…) to give broader spectrum antibiotics is lower.”(Respondent 11, 22 years of experience).
“It is important that the patient is properly followed up; that is what’s scary in an understaffed hospital full of patients: a lack of nurses. Then, it might be more rational to use broader spectrum antibiotics than in a more orderly situation (…) A typical example is that we actually shift from penicillin to cefotaxime (i.e., a more broad-spectrum antibiotic) on Fridays due to lesser capacity during weekends”(R7, 13 years).
2.2. Theme (ii) Clinical Uncertainty
“We know that, theoretically, they often don’t need antibiotics, but it is hard to select the patients who actually don’t need antibiotics and have the courage to send them home without any (…) you are scared the patient will come back in worse shape, and you will be asked why you didn’t continue antibiotics”(R12, 10 years).
“It is partly the experience level that allows you to have the guts to be restrictive”(R7, 13 years).
2.3. Theme (iii) Decision Support
“Often the material is inadequate. It is an extreme pressure in the emergency ward sometimes, and you choose the easiest solution, which gives non-representative results”(R6, 4 years).
“What works well is to seek collegial support when you are in doubt, call an infectious disease specialist”(R8, 23 years).
“(…) If we had time for consultation, it would be fine, but when it (the consultation phone) buzzes on top of everything else you have to do, then you don’t have time (…), and the advice might be suboptimal”(R7, 13 years).
2.4. Theme (iv) Benevolence
“(…) Sometimes I think, ‘Why am I doing this?’ when I, e.g., give broad-spectrum antibiotics to an old patient who has dementia and has not improved on other antibiotics—it feels wrong, but at the same time, I feel I don’t have a choice when I stand in front of the patient”(R7, 13 years).
“It is obvious that every patient, who can and wishes to get well, should receive all available treatment (…). For some patients, this would mean complicated antibiotic regimens to keep them alive during the treatment (e.g., transplant or cancer treatment). (…) This is worrisome due to the development of resistant bacteria that may kill other individuals”(R1, 12 years).
“You often feel pressured; you know (when a relative asks), “Can you guarantee that my father doesn’t have this and this condition?”—in situations like that you might push the boundaries more than you intended to”(R4, 25 years).
3. Discussion
4. Materials and Methods
4.1. Design and Setting
4.2. Recruitment
4.3. Interview Guide and Interviews
| 1 | What are your thoughts about rational antibiotic prescription? |
| 2 | What are your thoughts about antimicrobial resistance? |
| 3 | How would you describe the antibiotic prescription in this hospital, from your perspective? |
| 4 | What influences you when you prescribe antibiotics? |
| 5 | Can you please tell me about a situation where you had to decide when to start, not start, or stop antibiotics that you remember in particular? |
| 6 | Do you have any final comments on rational antibiotic prescription? |
4.4. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chandler, C.I.R. Current accounts of antimicrobial resistance: Stabilisation, individualisation and antibiotics as infrastructure. Palgrave Commun. 2019, 5, 53. [Google Scholar] [CrossRef] [Green Version]
- Jee, Y.; Carlson, J.; Rafai, E.; Musonda, K.; Huong, T.T.G.; Daza, P.; Sattayawuthipong, W.; Yoon, T. Antimicrobial resistance: A threat to global health. Lancet Infect. Dis. 2018, 18, 939–940. [Google Scholar] [CrossRef]
- Podolsky, S.H. The evolving response to antibiotic resistance (1945–2018). Palgrave Commun. 2018, 4, 124. [Google Scholar] [CrossRef]
- Antibiotic Resistance: Global Report on Surveillance. World Health Organisation, 2014. Available online: https://apps.who.int/iris/bitstream/handle/10665/112642/9789241564748_eng.pdf (accessed on 10 December 2021).
- Dyar, O.J.; Huttner, B.; Schouten, J.; Pulcini, C. What is antimicrobial stewardship? Clin. Microbiol. Infect. 2017, 23, 793–798. [Google Scholar] [CrossRef] [Green Version]
- Fridkin, S.; Baggs, J.; Fagan, R.; Magill, S.; Pollack, L.A.; Malpiedi, P.; Slayton, R.; Khader, K.; Rubin, M.A.; Jones, M.; et al. Vital signs: Improving antibiotic use among hospitalised patients. MMWR Morb. Mortal. Wkly. Rep. 2014, 63, 194–200. [Google Scholar] [PubMed]
- Hulscher, M.; Prins, J.M. Antibiotic stewardship: Does it work in hospital practice? A review of the evidence base. Clin. Microbiol. Infect. 2017, 23, 799–805. [Google Scholar] [CrossRef] [Green Version]
- Edwards, R.; Charani, E.; Sevdalis, N.; Alexandrou, B.; Sibley, E.; Mullett, D.; Loveday, H.P.; Drumright, L.N.; Holmes, A. Optimisation of infection prevention and control in acute health care by use of behaviour change: A systematic review. Lancet Infect. Dis. 2012, 12, 318–329. [Google Scholar] [CrossRef]
- Krockow, E.; Colman, A.M.; Chattoe-Brown, E.; Jenkins, D.; Perera, N.; Mehtar, S.; Tarrant, C. Balancing the risks to individual and society: A systematic review and synthesis of qualitative research on antibiotic prescribing behaviour in hospitals. J. Hosp. Infect. 2019, 101, 428–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, A.T.; Roque, F.; Falcão, A.; Figueiras, A.; Herdeiro, M.T. Understanding physician antibiotic prescribing behaviour: A systematic review of qualitative studies. Int. J. Antimicrob. Agents 2013, 41, 203–212. [Google Scholar] [CrossRef]
- McCullough, A.; Rathbone, J.; Parekh, S.; Hoffmann, T.; Del Mar, C. Not in my backyard: A systematic review of clinicians’ knowledge and beliefs about antibiotic resistance. J. Antimicrob. Chemother. 2015, 70, 2465–2473. [Google Scholar] [CrossRef] [Green Version]
- Charani, E.; Edwards, R.; Sevdalis, N.; Alexandrou, B.; Sibley, E.; Mullett, D.; Franklin, B.; Holmes, A. Behaviour change strategies to influence antimicrobial prescribing in acute care: A systematic review. Clin. Infect. Dis. 2011, 53, 651–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davey, P.; Marwick, C.A.; Scott, C.L.; Charani, E.; McNeil, K.; Brown, E.; Gould, I.M.; Ramsay, C.R.; Michie, S. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst. Rev. 2017, 2, CD003543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Centre for Disease Prevention and Control. Antimicrobial Resistance in the EU/EEA (EARS-Net)—Annual Epidemiological Report 2019; ECDC: Stockholm, Sweden, 2020. [Google Scholar]
- Hobæk, B.; Kveim Lie, A. Less Is More—Norwegian Drug Regulation, Antibiotic Policy and the Need Clause. Milbank Q. 2019, 97, 762–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NORM/NORM-VET 2020. Usage of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Norway; NORM/NORM-VET: Tromsø/Oslo, The Netherlands, 2021; ISSN 1502-2307. [Google Scholar]
- Sikkens, J.J.; Gerritse, S.L.; Peters, E.; Kramer, M.H.H.; Van Agtmael, A.M. The ‘morning dip’ in antimicrobial appropriateness: Circumstances determining appropriateness of antimicrobial prescribing. J. Antimicrob. Chemother. 2018, 73, 1714–1720. [Google Scholar] [CrossRef]
- Coombes, I.; Stowasser, A.D.; Coombes, J.; Mitchell, C. Why do interns make prescribing errors? A qualitative study. Med. J. Aust. 2008, 188, 89–94. [Google Scholar] [CrossRef]
- Warreman, E.; Lambregtsa, M.; Wouters, R.; Visser, L.; Staats, H.; van Dijk, E.; de Boer, M. Determinants of in-hospital antibiotic prescription behaviour: A systematic review and formation of a comprehensive framework. Clin. Microbiol. Infect. 2019, 25, 538–545. [Google Scholar] [CrossRef]
- Broom, A.; Kenny, K.; Prainsack, B.; Broom, J. Antimicrobial resistance as a problem of values? Views from three continents. Crit. Public Health 2020, 31, 451–463. [Google Scholar] [CrossRef]
- Skodvin, B.; Aase, K.; Brekken, A.L.; Charani, E.; Lindemann, P.C.; Smith, I. Addressing the key communication barriers between microbiology laboratories and clinical units: A qualitative study. J. Antimicrob. Chemother. 2017, 72, 2666–2672. [Google Scholar] [CrossRef] [PubMed]
- Hardin, G. The tragedy of the commons. The population problem has no technical solution; it requires a fundamental extension in morality. Science 1968, 162, 1243–1248. [Google Scholar] [CrossRef] [Green Version]
- Leibovici, L.; Paul, M.; Ezra, O. Ethical dilemmas in antibiotic treatment. J. Antimicrob. Chemother. 2012, 67, 12–16. [Google Scholar] [CrossRef]
- Baker, E.F.; Geiderman, J.M.; Kraus, C.K.; Goett, R. The role of hospital ethics committees in emergency medicine practice. J. Am. Coll. Emerg. Physicians Open 2020, 1, 403–407. [Google Scholar] [CrossRef]
- Report on the Modified Delphi Process for Common Structure and Process Indicators for Hospital Antimicrobial Stewardship Programs. Available online: https://www.cdc.gov/drugresistance/pdf/tatfar_rec1-finalreport_2015.pdf (accessed on 17 December 2021).
- Fusch, P.; Fusch, G.E.; Ness, L.R. Denzin’s Paradigm Shift: Revisiting Triangulation in Qualitative Research. J. Soc. Chang. 2018, 10, 19–32. [Google Scholar] [CrossRef]
- Barrett, A.; Kajamaa, A.; Johnston, J. How to… be reflexive when conducting qualitative research. Clin. Teach. 2020, 17, 9–12. [Google Scholar] [CrossRef] [PubMed]
- The Norwegian Health Directorate. National Guidelines for Use of Antimicrobial Agents in Hospital Care. 2020. Available online: https://www.helsedirektoratet.no/retningslinjer/antibiotika-i-sykehus (accessed on 5 March 2021). (In Norwegian).
- Point Prevalence Survey of Healthcare-Associated Infections and Antimicrobial Use in Acute Care Hospitals. Protocol Version 5.3—Full Scale Survey; European Centre for Disease Control and Prevention: Stockholm, Sweden, 2016; Available online: https://op.europa.eu/en/publication-detail/-/publication/39a84b73-dee0-11e6-ad7c-01aa75ed71a1 (accessed on 29 August 2019).
- Kallio, H.; Pietilä, A.-M.; Johnson, M.; Kangasniemi, M. Systematic methodological review: Developing a framework for a qualitative semi-structured interview guide. J. Adv. Nurs. 2016, 72, 2954–2965. [Google Scholar] [CrossRef] [PubMed]
- Skodvin, B.; Aase, K.; Charani, E.; Holmes, A.; Smith, I. An antimicrobial stewardship program initiative: A qualitative study on prescribing practices among hospital doctors. Antimicrob. Resist. Infect. Control 2015, 4, 24. [Google Scholar] [CrossRef]
- Bate, P.; Mendel, P.; Robert, G. Organizing for Quality: The Improvement Journey of Leading Hospitals in Europe and the United States. Nuffield Trust; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Braun, V.; Clarke, V. Using thematic analysis in psychology. Qual. Res. Psychol. 2006, 3, 77–101. [Google Scholar] [CrossRef] [Green Version]
| Age | (Years) |
| Median (IQR) | 36.5 (14.5) |
| Range | 29–66 |
| Working Experience as a Physician (Years) | (n) |
| <5 years | 4 |
| 5–10 years | 3 |
| 10–20 years | 4 |
| >20 years | 3 |
| Gender | (n) |
| Female | 9 |
| Male | 5 |
| Theme | Subtheme | Quotes (Respondent Number, Years of Experience) |
|---|---|---|
| Clinical workflow pressures | Time pressure | There is too much pressure, no time to just observe the patient (…) so most doctors will give what they know works, which means broad-spectrum antibiotics, and the problem is that it works! (…) (R4, 25 years) For (patients with) chronic obstructive pulmonary disease patients and patients like that, it is difficult to know if they have an infection or not, and I feel it is more efficient just to put them on antibiotics (R14, 1 years) |
| Follow-up resources | If you know the patient gets followed up, and clinical deterioration would easily get picked up, you would probably be more comfortable starting penicillin (instead of more broad-spectrum antibiotics), as opposed to a patient who gets left alone in a corridor, which is often the case (R9, 8 years) It is not the nurses’ fault, but it often happens that they say no if I ask for close follow-up (of an ill patient) during night shifts. They tell me they don’t have the capacity and no one to step in (R13, 4 years) | |
| Clinical uncertainty | Just in case | 9/10 doctors would, in cases of insecurity, rather ensure themselves (and give broad-spectrum antibiotics) (R9, 8 years) (Physicians continue antibiotics because) (…) it is the fear of doing wrong, of being appealed, both legally but also from colleagues. (R8, 23 years) |
| Knowledge and experience | It is an intuition you get with clinical experience that allowed us to trust the clinical picture and withhold antibiotics (R6, 4 years) I got this idea that I wish to use narrower antibiotics, but then I don’t know enough to do so (R5, 4 years) | |
| Decision support | Microbiological tests | The most important thing is to have a resistance pattern, then I can feel safe that I use the right antibiotic (and don’t need to give more broad-spectrum antibiotics) (R5, 4 years) We need to become better at sputum tests (…), often the patient has had a lot of productive cough and no collected sputum (…), which is a shame, because if you find the right microbe to target, then we will actually succeed (with narrow-spectrum antibiotics) (R11, 22 years) |
| Collegial consulting | We often call up infectious medicine and ask for help (…) if we are in doubt. (R14, 1 years) I almost never make difficult decisions alone; I just talk to a colleague (R8, 23 years) Unfortunately, the reality is that you usually have several tasks in addition to giving consultations, and that certainly affects the quality of the advice we give (ID specialist, R4, 25 years) If you don’t have time, then the advice (on antibiotics) you give may be bad and sometimes even dangerous (ID specialist, R2, 18 years) | |
| Benevolence | The patient’s wellbeing | If you’ve got an, e.g., immunosuppressed patient who is ill, then we treat with what is available in antibiotics now without worrying about the problem of resistance, sure we do (R10, 16 years) It is an analogy to peeing in one’s pants; it is solving the problem now (by prescribing antibiotics), everyone know it will be uncomfortable later (…), but that is a problem for tomorrow (…) (R2, 18 years) |
| Treatment expectations | In cancer and transplant patients, we often choose more broad-spectrum antibiotics, as they are immunocompromised, though you know broader spectrum means more resistance (…), and that is problematic, because patients who could have been cured from cancer could later die due the complications of the treatment (multi-resistant bacteria) (R10, 8 years) When, e.g., you are 90 years old and sick, it is probably okay to die of pneumonia. We try to tell relatives that, but they have a high level of expectations and will say, “But pneumonia is treatable”, and then we start treatment anyways, and then it doesn’t work, and we often try a more broad-spectrum antibiotic, and it doesn’t work either, and that’s a vicious circle (R4, 25 years). The family was literally praying to me, ‘She must live, what can be done?’ and then we continued to treat (…), but it won’t be any better (R5, 5 years) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christensen, I.; Haug, J.B.; Berild, D.; Bjørnholt, J.V.; Skodvin, B.; Jelsness-Jørgensen, L.-P. Factors Affecting Antibiotic Prescription among Hospital Physicians in a Low-Antimicrobial-Resistance Country: A Qualitative Study. Antibiotics 2022, 11, 98. https://doi.org/10.3390/antibiotics11010098
Christensen I, Haug JB, Berild D, Bjørnholt JV, Skodvin B, Jelsness-Jørgensen L-P. Factors Affecting Antibiotic Prescription among Hospital Physicians in a Low-Antimicrobial-Resistance Country: A Qualitative Study. Antibiotics. 2022; 11(1):98. https://doi.org/10.3390/antibiotics11010098
Chicago/Turabian StyleChristensen, Ingrid, Jon Birger Haug, Dag Berild, Jørgen Vildershøj Bjørnholt, Brita Skodvin, and Lars-Petter Jelsness-Jørgensen. 2022. "Factors Affecting Antibiotic Prescription among Hospital Physicians in a Low-Antimicrobial-Resistance Country: A Qualitative Study" Antibiotics 11, no. 1: 98. https://doi.org/10.3390/antibiotics11010098
APA StyleChristensen, I., Haug, J. B., Berild, D., Bjørnholt, J. V., Skodvin, B., & Jelsness-Jørgensen, L. -P. (2022). Factors Affecting Antibiotic Prescription among Hospital Physicians in a Low-Antimicrobial-Resistance Country: A Qualitative Study. Antibiotics, 11(1), 98. https://doi.org/10.3390/antibiotics11010098






