1. Introduction
Primary, secondary, and tertiary peritonitis can be distinguished among the large class of intra-abdominal infections (IAIs). Primary peritonitis is defined as peritoneal inflammation and infection caused by bacterial translocation, blood, or iatrogenic spread without macroscopically evident lesion of solid organs or viscera of the gastrointestinal or genitourinary tract. Spontaneous bacterial peritonitis is attributable to primary peritonitis. Tertiary peritonitis is the natural evolution of secondary peritonitis with treatment failure (persistence of peritonitis 48 h after treatment) [
1]. Secondary peritonitis, the main topic of this review, is among the major causes of community-acquired sepsis. It can be defined as the direct contamination of the peritoneum due to an injury (perforation) to a hollow viscus in the abdominal cavity (i.e., gastrointestinal tract or genitourinary tract). This lesion can be spontaneous (i.e., spontaneous ulceration/neoplastic expansion), or iatrogenic in the case of a postsurgical perforation (i.e., loss from an anastomosis). It is, therefore, to be noted that secondary peritonitis can frequently be due to complications of the surgical technique and related to exposure to polymicrobial contaminating sources. Contamination rarely comes from the injury of solid abdominal viscera or by suppurative abscesses. For the resolution of secondary peritonitis, it is necessary that surgical cleaning and washing of the infection site begin promptly and are associated with adequate and effective antibiotic treatment to prevent the development of tertiary peritonitis and sepsis [
2]. It has to be taken into account that in recent years surgical management procedures have evolved compared to the 1990s. In fact, it is expected that for a patient with signs and symptoms of disseminated peritonitis (i.e., tense abdomen, not treatable) or sepsis (delta of SOFA score>2) an early treatment should be provided once his or her vital functions have stabilized, with a radical surgical intervention associated with an aggressive empirical antibiotic therapy first and then modulated on the antibiogram obtained from the samples of peritoneal fluid or purulent material taken during the surgery [
3]. Most of the patients in this category are often already hospitalized in the ICU or will be managed in the ICU after surgery. In case of little signs and symptoms of peritonitis (i.e., localized and limited to a single abdominal quadrant or two at most) with laboratory and instrumental tests that confirm the circumscription of the inflammatory and of the infectious process, it is advisable to proceed with a less invasive intervention such as surgical cleaning and drainage associated with targeted antibiotic therapy after an antibiogram. Sometimes, only in highly selected (mild) cases, empirical antibiotic therapy can be evaluated as an early strategy alone before surgery [
4]. In addition to the extension of the pathology in the context of secondary peritonitis, in order to estimate the severity of the clinical case, it is also recommended to classify the patient on the basis of comorbidities and predisposing factors for therapeutic failure [
5].
Table 1 shows the predictive factors of failure in antimicrobial empiric treatment for secondary peritonitis.
Secondary peritonitis is among the most frequent causes of mortality (mortality rates 10–20%) in patients admitted to ICUs [
6]. In some studies, such as that conducted in 2014 by De Waele et al., the death rate exceeds that of respiratory infections, especially in the case of multidrug-resistant pathogens. In particular, postsurgical secondary peritonitis, being caused by nosocomial pathogens, is the most frequent cause of septic shock and acute or multi-organ renal failure in already compromised patients. These are, therefore, postsurgical complications with extremely poor prognosis and difficult therapeutic management if caused by multidrug-resistant pathogens [
7]. This comprehensive review of the literature aims to offer an overview of a very topical and complex management problem, secondary peritonitis, from multidrug-resistant pathogens with a focus on those caused by carbapenem-resistant
Enterobacterales.
2. Epidemiology of Secondary Peritonitis Caused by Carbapenemase-Producing Enterobacterales
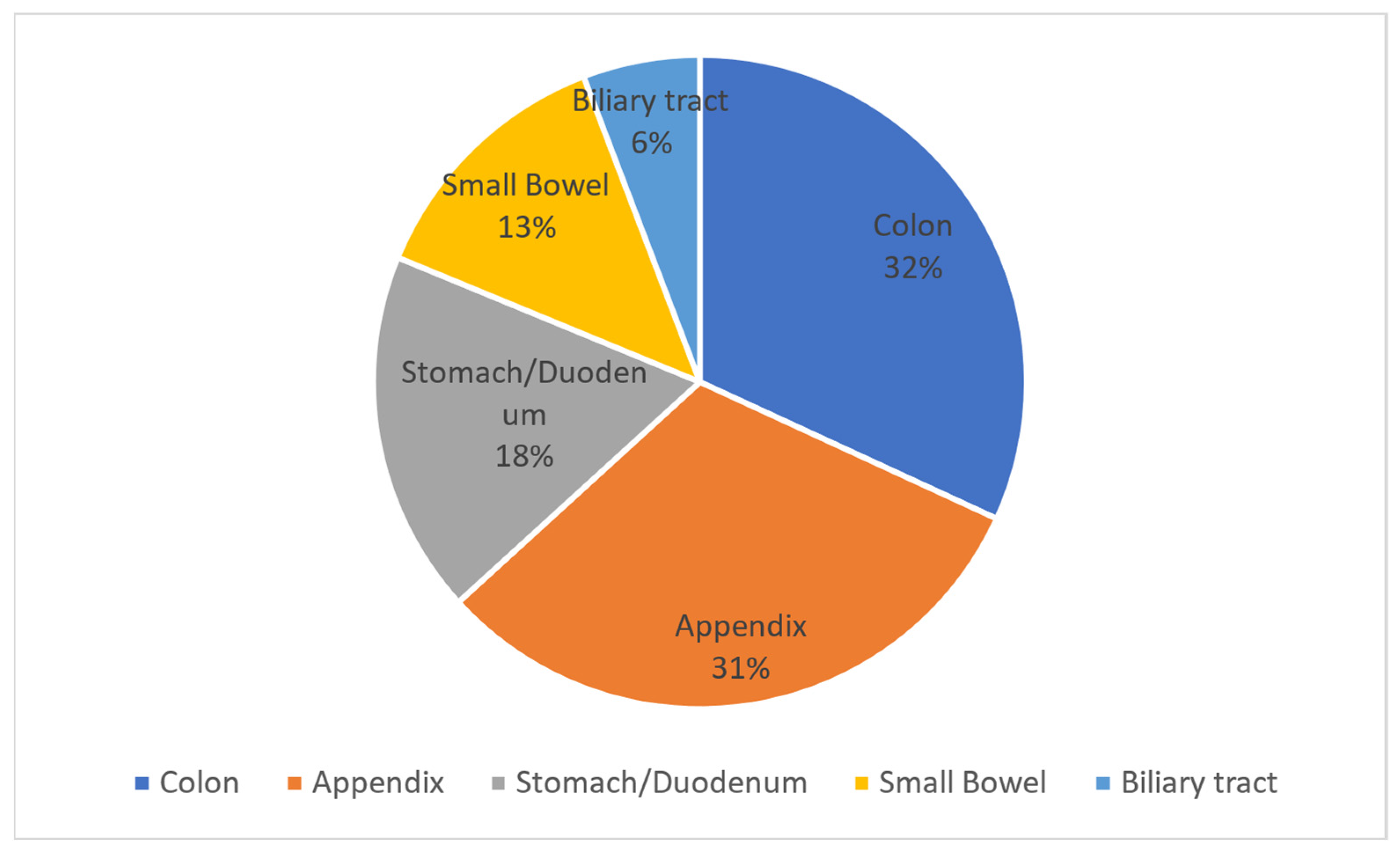
About 25% of cases of peritonitis are attributable to the secondary peritonitis group. Among the sites of origin of peritonitis in order of frequency, we find the colon, appendix, stomach/duodenum, small intestine, and biliary tract.
Two studies report data on the frequency of this pathology by stratifying patients according to the sites of origin of the infection. In 2009 Gauzit et al. reported the data in
Figure 1.
Figure 2, on the other hand, describes the percentages of the perforation site by anatomical site.
It has to be noted that in 39% of cases the perforation originates from the colorectal, in 33% of cases from the stomach/duodenum, in 25% of cases from the small intestine, and in 3% of cases there is multiple perforation [
6]. A second epidemiological study conducted in South Korea by Jang in 2015 reports, in agreement with what has already been reported, that secondary peritonitis derives mainly from the right colon (39.4% of cases). In 20% of cases it is associated with the positivity of the blood culture practiced on entry, and in more than 50% of cases it is associated with the positivity of the peritoneal fluid taken during surgical remediation. Secondary peritonitis is a clinical condition with high mortality (mortality rate 10–20%). This proportion increases up to 10% in the case of late or inadequate treatment [
8]. The peritonitis with the worst prognosis is identified by a SAPS II (Simplified Acute Physiology II score) > 38, and usually this is peritonitis with non-appendiceal origin (
Figure 3).
According to the findings of Gauzit et al. in 2009, the SAPS II had low values for peritonitis, starting with a better prognosis for peritonitis originating in the appendix and then increasing to high values for peritonitis originating in the colon [
6]. Obviously, it would be reductive to correlate the prognosis only with the score obtained with the SAPS II. It must be considered that secondary peritonitis is caused by heterogeneous groups of pathogens such as Gram-negative bacteria, Gram-positive bacteria, and fungi, and cases of peritonitis caused by more than one pathogen at the same time have been reported. From this heterogeneity derives the difficulty in establishing an early empirical antibiotic treatment that is effective and reduces the possibility of MDR or XDR pathogens developing once the results of the antibiogram have been obtained and the clinical response of the patient to treatment has been assessed (approximately 48 h after sampling upon entrance) [
8]. Focusing attention on carbapenemase-producing pathogens, as regards the year 2020, the European Center for Disease Prevention and Control reports the data on the incidence of infection by these pathogens.
Table 2 summarizes the cases of infection described by pathogen and geographic location.
Figure 4 shows the geographical distribution of resistance to carbapenems expressed for each type of pathogen. These are overall data and do not only concern intra-abdominal infections.
It is evident that in Europe there is a wide diffusion of carbapenemase producer micro-organisms. Northern European countries have lower percentages of detection, while Italy, Greece, and Spain have higher percentages.
From these data it is clear that the problem of carbapenemase-producing infections goes well beyond one part of the body and that sometimes several parts are involved at the same time, making treatment very complex. Prevention, consisting of identifying risk factors and treating them early with appropriate therapeutic schemes, is the strategy that will enable a reduction in the incidence of these difficult-to-manage infections. A Spanish study highlighted the possibility of predicting the presence of multi-resistant contaminating pathogens, justifying an increase in aggression in the empirical treatment of these selected cases.
The risk factors for the development of nosocomial infections from multidrug-resistant microorganisms are summarized in
Figure 5.
Patients with the risk factors shown in
Figure 5 or patients with sepsis are early candidates for empirical treatment based on an antimicrobial drug with a broad spectrum of action. In these cases, in the absence of early antibiotic aggression of the pathogen, there is a high probability that the patient has a bad prognosis and, for this reason, the empirical use of a combination of latest-generation broad-spectrum antibiotics is justified [
9].
As described in
Figure 5, there are many predisposing factors for colonization by carbapenemase-resistant
Enterobacterales: previous hospitalization in the ICU, previous long-term hospitalization, COPD, previous treatment with broad-spectrum antibiotics (beta-lactams + beta-lactamase inhibitors or carbapenems), previous MDR infection, and quinolone prophylaxis. Quinolone prophylaxis seems to play an important role in the emergence of GNB antimicrobial resistance. In fact, 70% of isolates with quinolone resistance show trimethoprim-sulfamethoxazole resistance.
Using these predisposing factors, we are able to identify the patients most predisposed to the onset of infections by resistant bacteria.
To effectively manage these infections, however, we must be aware of the characteristics expressed by each pathogen and the pathogen’s resistance profiles in order to adapt the treatment as much as possible, maximizing effectiveness and reducing doses and future resistance.
3. Resistance Patterns of Enterobacterales Causing Secondary Peritonitis
Antibiotic resistance is defined as the ability developed by some pathogens to inhibit the antimicrobial properties of the antibiotic classes used in the clinical setting and previously effective in limiting bacterial growth. It is a set of heterogeneous antimicrobial inactivation mechanisms which become increasingly complex to counteract. Currently, in fact, the development of new antimicrobial molecules effective against multidrug-resistant pathogens fails to keep pace with the rapid development of mechanisms of inactivation and evasion of pathogens that cause nosocomial infections. As regards secondary peritonitis, these are more frequently caused in the context of Gram-positive pathogens by methicillin-resistant Staphylococcus aureus (MRSA) or vancomycin-resistant Enterococcus faecium, and in the case of Gram-negative bacteria, non-fermenting pathogens such as
P. aeruginosa and
A. baumannii are highlighted.
A. baumannii,
B. fragilis, and
Enterobacterales such as
E. coli and
K. pneumoniae produce β-lactamases (ESBLs and/or AmpC and/or carbapenemases) [
10]. Therefore, given the increase in resistance both to vancomycin and to fluoroquinolones and aminoglycosides first, followed then by resistance to carbapenems, there is also difficulty in the use of these molecules which until now had been recognized as the last effective strategy of treatment. In summary, resistance to carbapenems is due to a combination of alterations in permeability and production of carbapenemases (non-metallo-carbapenemases, OXA-beta-lactamases, or IMP/VIM-beta-lactamases). Carbapenemases are β-lactamases capable of recognizing almost all hydrolysable beta-lactam antibiotics and of inactivating them. Carbapenemases can be classified according to the Amber Classification into four categories (A, B, C, and D). Class B carbapenemases are characterized by the presence of an active site containing zinc, while the other classes present serine at the active site [
11]. The carbapenemases most represented in
Enterobacterales are of categories A, B, and D. KPC and GES belong to class A, MBLs (IMP and VIM) belong to class B, and oxacillinases (OXA) belong to class D. KPC effectively hydrolyzes carbapenems, cephalosporins, penicillins, and aztreonam and is effectively inactivated by either clavulanic acid or tazobactam. IMP and VIM are similar in hydrolytic activity to class A carbapenemases, but unlike them do not hydrolyze aztreonam [
12].
Because zinc-chelating EDTA possesses activity linked to the activation of the galvanized domain of the active site, its use could be helpful in reducing the activity of these beta-lactamases [
13]. Type D carbapenemases are capable of hydrolyzing oxacillin and cloxacillin, and given the extreme variability of the active site (small and hydrophobic), they are poorly inhibited by clavulanic acid or EDTA [
14]. It should be noted that the active site of these oxacillinases is sensitive to CO
2 and is activated by high concentrations of CO
2 [
15]. In addition to the production of carbapenemases, multidrug-resistant pathogens are capable of inactivating carbapenems by associating the production of broad-spectrum beta-lactamases such as ESBLs and AmpCs with modified porins [
16]. The last but not negligible mechanism of resistance is represented by the expression of efflux pumps and alterations in the penicillin-binding target proteins.
4. The Emerging Problem of Carbapenem-Resistant Enterobacterales Causing Secondary Peritonitis
Among the Enterobacterales most frequently responsible for secondary peritonitis resistant to carbapenems, we find K. pneumoniae and E. coli, but other pathogens that do not belong to the class of Enterobacterales such as P. aeruginosa and A. baumannii cannot be neglected.
The worldwide spread, such as the European diffusion of carbapenem-resistant Enterobacterales, is mainly due to a selection of these pathogens over the years.
In fact, the increasingly frequent use, especially in monotherapy, of carbapenems to cope with infections caused by pathogens resistant to beta-lactams (penicillins and cephalosporins), fluoroquinolones, or aminoglycosides has made it possible for these pathogens to develop the ability to produce enzymes capable of degrading even carbapenems, making even one of our latest-generation molecules poorly effective. In Europe, from data reported in 2016, pathogens resistant also to carbapenems have already spread as endemic species [
17].
In any case, we can schematize the resistance profiles to carbapenems based on the production by the pathogen of different types of carbapenemases [
18].
Vancomycin-resistant enterococci deserve a separate treatment and will not be covered in detail in this review. For these pathogens, a study conducted by Wenstein has shown that in vitro resistance to penicillin, ampicillin, and vancomycin can predict resistance to imipenem in vivo. [
19] In this case, the association between doxycycline linezolid and daptomycin is useful.
Table 3,
Table 4,
Table 5 and
Table 6 summarize the sensitivity and resistance to different carbapenems commonly used in ICU for each of these carbapenemase-producing pathogens responsible for intra-abdominal infections. The data have been collected from the SENTRY database for the years 2019, 2020, and 2021. It was possible to extrapolate data on the resistance to carbapenems of each aforementioned pathogen.
5. Current Strategies of Antimicrobial Treatment of Secondary Peritonitis due to CRE
The increasingly frequent finding of multidrug-resistant pathogens is precisely the result of the abuse of broad-spectrum antibiotics. Thienamycin was discovered in 1978 from Streptomyces cattleya, and its carbapenems were synthesized in the laboratory in 1985. To date, these antibiotics have represented, for about thirty years, the most up-to-date treatment strategy for particularly aggressive pathogens [
20].
The development of resistance to these antimicrobials and the non-circumscription of these pathogens in the hospital setting marked a new era in the treatment of nosocomial and community-based infections.
The strategies currently used to treat secondary peritonitis and other diseases caused by multidrug-resistant pathogens include early and effective surgical therapy in association with antibiotic therapy [
21].
Preferably, an empirical combination antibiotic therapy should be evaluated in order to exploit the synergy between antibiotics, reduce their dosage, and at the same time decrease the mutagenic stress to which the bacteria causing the infection are subjected until data are available to undertake a targeted therapy [
22].
At the basis of correct and early treatment, there is, therefore, the need to use an empirical antibiotic therapy that avoids exacerbating the development of antibiotic resistance especially using an exaggerate and unnecessary dosage of drugs [
23]. However, at present there are no studies that define a clear superiority of combination therapy over monotherapy; it is therefore good to evaluate the epidemiology of the region of onset of the disease. Infections caused by CRE will usually be poorly responsive to treatment with penicillins, cephalosporins (with or without classical beta-lactamase inhibitors), and carbapenems; the sensitivity to aminoglycosides (gentamicin and amikacin), aztreonam, and trimethoprim/sulfamethoxazole is scarce or unpredictable. Once the possibility of a multidrug-resistant pathogen has been identified or resistance to carbapenems confirmed, our treatment options include the use of antibiotics alone or in combination. Although the therapeutic options seem multiple, it is difficult to use the correct antibiotic effectively while minimizing adverse events [
24]. Below is a brief description of the antibiotics effective on CRE, visually summarized in
Figure 6.
As shown already in 2011 by Falagas and colleagues, tigecycline with colistin (sinergistic action), colistin with a carbapenem (sinergistic action), fosfomycin with a carbapenem (additive or synergistic action), fosfomycin with an aminoglycoside (additive or synergistic action), and a carbapenem with an aminoglycoside (synergistic action) have been reported as antibiotic combinations effectively administered to series of patients infected with carbapenemase-producing
Enterobacterales [
25].
Colistin or Polymyxin E (PXE) is a molecule still used today in monotherapy or in combination therapy with other drugs. It is very active on non-fermenting pathogens; however, new findings are showing that it is becoming poorly effective against KPC producers [
26]. It has a narrow therapeutic window (an average concentration at steady state of 2 μg/mL is needed to achieve therapeutic targets, except where a concentration of approximately 2.5 μg/mL results in renal toxicity), which makes it unmanageable to use alone. A reduction in nephrotoxicity has been documented when associated with ascorbic acid [
27].
Polymyxin B (PXB) is less nephrotoxic than PXE and is also available on adsorption filter (Toraymyxin) for patients suffering from endotoxin-mediated septic shock unresponsive to conventional therapy. Already, in a randomized study conducted in 2015 by Payen et al., it was reported that Polymyxin B fails to give advantages if used early in patients with secondary peritonitis and septic shock in the initial phase. Mortality and organ failure could be assessed if used after hemodynamic stabilization of these septic patients [
28].
Fosfomycin (FOF) is a first-generation drug that is a valid aid in multi-antibiotic therapy (i.e., with aminoglycosides) in the management of both Gram-negative and Gram-positive infections. Its use as a single antimicrobial is not recommended given the proven speed of CRE in developing resistance [
29].
Plazomicin (PLZ) is a new-generation aminoglycoside with reduced side effects and MIC values lower than those of amikacin. PLZ received approval for the treatment of complicated urinary tract infections in patients who are not candidates for other treatments [
30]. It is active against Gram-negative producers of ESβL, KPC, and AmpC but not effective against MβL producers and poorly effective against non-fermenting pathogens [
31].
High-dose tigecycline (TG) is used as a last resort. Being a glycylcycline, it is a valuable aid in the treatment of both Gram-negative and Gram-positive infections including
Pseudomonas spp. and
Proteus spp. Resistance to TG in multitherapy (i.e., with aminoglycosides) is unlikely to develop, but should it occur for the production of efflux pumps or ribosomal protection mechanisms, an intervention using everacycline is still possible [
32].
Eravacycline (EV) is a fully synthetic fluorocyclin and an alternative to tigecycline in cases of resistance. It has good tolerability and a good safety profile. A further advantage is its excellent oral bioavailability, which facilitates administration even at home. It is approved for complicated intra-abdominal infections caused by pathogens producing ESβL, KPC, AmpC, MβL, and OXA, but it lacks activity against P.aeruginosa [
33].
Cefiderocol (CFD) is a novel cephalosporin with affinity mainly for the penicillin-binding protein 3 (PBP3) of
Enterobacterales and non-fermenting bacteria. It has shown a characteristic antibacterial spectrum with potent activity against a broad range of aerobic GNB, including carbapenem-resistant strains of
Enterobacterales and non-fermenting pathogens such as
A. baumannii and
P. aeruginosa; it should be a valid alternative to peritonitis caused by MDR pathogens. Due to its chemical structure, CFD possesses stability to hydrolysis by almost all β-lactamases, including serine- and metallo-β-lactamases. CFD is considered a valuable resource for the treatment of patients with infections due to aerobic Gram-negative carbapenemase-producing bacteria with limited therapeutic options [
34].
Ceftolozane/tazobactam (CTZ, with metronidazole M): The association ceftolozane/tazobactam can be considered as an efficient and safe carbapenem-sparing alternative for treating intra-abdominal infections [
35].
Ceftazidime/avibactam (CAZ) is a combination of third-generation cephalosporin ceftazidime and avibactam, a non-beta-lactam beta-lactamase inhibitor, which restores the activity of ceftazidime against many beta-lactamase–producing GNB. Ceftazidime-avibactam is safe and has high tolerability; it produces clinical cure rates comparable to meropenem and is suggested for indications of complicated intra-abdominal infections (in association with metronidazole) [
36].
Aztreonam/avibactam (AZAV): The AZAV combination has been shown to have in vitro activity against metallo-β-lactamase–producing bacteria, such as NDM/VIM/IMP. AZAV is the only β-lactam insensitive to the hydrolysis of metallo-β-lactamases. Unfortunately, metallo-β-lactamase–producing strains are often coproducers of β-lactamases capable of degrading Aztreonam (i.e., AmpC). Avibactam is able to inhibit β-lactamases which degrade Aztreonam, making this combination an effective tool in the treatment of infections caused by class B carbapenemase-producing strains. The association has been promoted to offer an excellent therapeutic opportunity against metallo-β-lactamase–producing strains [
37,
38].
Meropenem/vaborbactam (MV) in monotherapy was associated with increased clinical cure, decreased mortality, and reduced nephrotoxicity. Vaborbactam is a non-beta-lactam beta-lactamase inhibitor with a structure unlike any other currently marketed beta-lactamase inhibitor. Meropenem acquires more effectiveness by adding vaborbactam against most species of
Enterobacterales. In vitro and in vivo pharmacodynamic studies have reported bactericidal activity against various Gram-negative strains, including carbapenem-resistant strains, with the exception of bacteria-producing MBL [
39].
The relebactam + imipenem-cilastatin (RIC) association uses relebactam, a parenteral, small-molecule beta-lactamase inhibitor that is active against beta-lactamases; in vitro susceptibility studies have demonstrated that relebactam restores imipenem susceptibility to many imipenem-resistant bacteria. In vivo infection models show that relebactam given with imipenem-cilastatin could be used to treat severe Gram-negative infections [
40,
41].
Having briefly described the antibiotics currently used to treat carbapenem-resistant
Enterobacterales infections, we outline possible therapeutic strategies in
Figure 7.
6. Future Perspectives
Future perspectives on AMR management are currently focused on the advice of precision medicine and artificial intelligence. According to WHO data collected in 2020, more patients are expected to die from infections with MDR pathogens by 2050 than from cancer today, only 50% of antibiotics are used correctly globally, and drug-resistant infections cause at least 700,000 deaths worldwide per year (projected to rise to 10 million deaths per year by 2050). Therefore, corrective measures have to be taken using any means [
42].
Compelling evidence has demonstrated the effectiveness of antibiotic stewardship programs in reducing inappropriate antimicrobial use, AMR rates, hospital-acquired AMR, hospitalization time, and costs. Despite these successes, ongoing challenges for antibiotic stewardship programs include rapid differentiation between bacterial and viral infections, early pathogen identification and characterization (i.e., antibiotic susceptibility), and reduction in antimicrobial abuse overwhelmed during the COVID-19 pandemic. Approximately 75% of the patients diagnosed with COVID-19 received antibiotics, increasing the risk for acquiring AMR infections. Hence the necessity to improve antimicrobial management, supplementing antibiotic stewardship with precision medicine.
Precision medicine (PM) is an innovative multicomponent medical approach designed to optimize efficiency and advantages for particular groups of patients using genetic or molecular profiling. More practically, precision medicine involves rapidly identifying altered biology within a patient and using the findings to guide therapy.
Precision medicine was first developed in the field of oncology. Its use in infectious diseases is at an early stage, but it is showing great potential, especially in employing “omics”-based biomarkers such as proteomics, metabolomics, and lipidomics to estimate disease prognosis, predict treatment response, and improve clinical outcomes [
43].
In the case of abdominal infections and sepsis, some of these approaches are still in the early stages of research, while others, even if they are not specific markers (i.e., biomarkers), are already in routine use in clinical practice [
44].
Biomarkers such as procalcitonin (PCT) and the mid-region fragment of pro-adrenomedullin (MR-proADM) may be less specific but useful drivers to properly detect the efficacy of the ongoing therapeutic approach. Dosing these molecules several times during hospitalization is expensive, so it is fundamental to identify subpopulations who could benefit from this marker’s detection to improve a poor prognosis.
The use of PCT-guided algorithms for antimicrobial stewardship in sepsis has shown a reduction in mortality. A value of PCT > 2 ng/mL seems to identify patients that may benefit from receiving adjuvant therapy with hydrocortisone, vitamin C, and thiamine to reduce the progression of organ dysfunction in case of septic shock, while in patients with high initial levels of PCT a more aggressive treatment can be justified because levels of PCT > 6 ng/mL predict progressive organ dysfunction and increased risk of mortality [
45].
Interestingly, PCT repetitive dosage can be used as a predictor of adverse outcomes and treatment failure because the non-clearance of PCT by more than 80% is a significant independent predictor of mortality [
46].
The other biomarker, produced by vascular endothelial cells, is the MR-proADM, which directly reflects plasma levels of adrenomedullin (a vasodilator agent with metabolic and immune-modulating properties). MR-proADM if detected early in plasma reflects a worse prognosis, predicting mortality better than lactate rise and SOFA score, ICU admission, and the need for urgent treatment. High-plasma MR-proADM clearance at day 5 is related to better outcomes [
44,
47].
Furthermore, artificial intelligence and machine learning algorithms hold great promise for integrating precision medicine approaches with antibiotic stewardship. There are three main areas in which investigators are currently focused: (a) antimicrobial resistance prediction in interpreting genomic data; (b) deepening the cellular functions disrupted by antibiotics and developing novel antimicrobial agents; and (c) taking antimicrobial stewardship decisions using data extracted from electronic medical recorders [
48].
A huge amount of healthcare information (e.g., signs and symptoms, genetics, risk factors, immune response, molecular enzymes, virulence factors, environment, and epidemiology) can be managed by artificial intelligence and used to deliver the most efficient treatment or preventive care in a timely manner, thus minimizing risks of mistakes and adverse events.
In the near future, both precision medicine and artificial intelligence will a play a leading role in antibiotic stewardship, especially in ICU-complicated infections.
7. Search Strategy
Several internet databases were consulted (Ovid MEDLINE, EMBASE, and Google Scholar), using a combination of terms such as “carbapenem resistance,” “secondary peritonitis,” “severe infections,” “intensive care unit,” “multi-resistant bacteria,” and “Enterobacterales” to identify studies (without date limitations) reporting secondary peritonitis due to carbapenem-resistant Enterobacterales. The resulting studies were listed using a reference manager software (Endnote 20. Clarivate Analytics, Philadelphia, PA, USA), and duplicates were removed. Results and primary evidence of the studies thus obtained were summarized in narrative form and through figures and tables.
The SENTRY Public Dataset by JMI laboratories and the European Centre for Disease Prevention and Control was consulted to find the resistance patterns of all infections and intra-abdominal infections. The data obtained are summarized in tables and discussed in the epidemiology section.