Urban Pigeons (Columba livia) as a Source of Broad-Spectrum β-Lactamase-Producing Escherichia coli in Lisbon, Portugal
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Sample Collection and Bacterial Isolates
4.2. Antimicrobial Susceptibility Testing
4.3. Identification of Resistance Determinants
4.4. Molecular Typing
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Ma, Z.B.; Zeng, Z.L.; Yang, X.W.; Huang, Y.; Liu, J.H. The role of wildlife (wild birds) in the global transmission of antimicrobial resistance genes. Zool. Res. 2017, 38, 55–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veldman, K.; van Tulden, P.; Kant, A.; Testerink, J.; Mevius, D. Characteristics of cefotaxime-resistant Escherichia coli from wild birds in the Netherlands. Appl. Environ. Microbiol. 2013, 79, 7556–7561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, G.; Oka, C.; Asagi, M.; Ishiguro, N. Detection of conjugative R plasmids conferring chloramphenicol resistance in Escherichia coli isolated from domestic and feral pigeons and crows. Zentralbl. Bakteriol. Orig. A 1978, 241, 407–417. [Google Scholar] [PubMed]
- Bonnedahl, J.; Järhult, J.D. Antibiotic resistance in wild birds. Ups. J. Med. Sci. 2014, 119, 113–116. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Liang, B.; Jiang, B.W.; Zhu, L.W.; Wang, T.C.; Li, Y.G.; Liu, J.; Guo, X.J.; Ji, X.; Sun, Y. Migratory wild birds carrying multidrug-resistant Escherichia coli as potential transmitters of antimicrobial resistance in China. PLoS One 2021, 16, e0261444. [Google Scholar] [CrossRef]
- Peirano, G.; Pitout, J.D.D. Extended-spectrum β-lactamase-producing Enterobacteriaceae: Update on molecular epidemiology and treatment options. Drugs 2019, 79, 1529–1541. [Google Scholar] [CrossRef]
- Zeballos-Gross, D.; Rojas-Sereno, Z.; Salgado-Caxito, M.; Poeta, P.; Torres, C.; Benavides, J.A. The role of gulls as reservoirs of antibiotic resistance in aquatic environments: A scoping review. Front. Microbiol. 2021, 12, 703886. [Google Scholar] [CrossRef]
- Athanasakopoulou, Z.; Diezel, C.; Braun, S.D.; Sofia, M.; Giannakopoulos, A.; Monecke, S.; Gary, D.; Krähmer, D.; Chatzopoulos, D.C.; Touloudi, A.; et al. Occurrence and characteristics of ESBL- and carbapenemase-producing Escherichia coli from wild and feral birds in Greece. Microorganisms 2022, 10, 1217. [Google Scholar] [CrossRef]
- Ewbank, A.C.; Fuentes-Castillo, D.; Sacristán, C.; Cardoso, B.; Esposito, F.; Fuga, B.; de Macedo, E.C.; Lincopan, N.; Catão-Dias, J.L. Extended-spectrum β-lactamase (ESBL)-producing Escherichia coli survey in wild seabirds at a pristine atoll in the southern Atlantic Ocean, Brazil: First report of the O25b-ST131 clone harboring blaCTX-M-8. Sci. Total Environ. 2022, 806, 150539. [Google Scholar] [CrossRef]
- Skarżyńska, M.; Zając, M.; Bomba, A.; Bocian, Ł.; Kozdruń, W.; Polak, M.; Wiącek, J.; Wasyl, D. Antimicrobial resistance glides in the sky—free-living birds as a reservoir of resistant Escherichia coli with zoonotic potential. Front. Microbiol. 2021, 12, 656223. [Google Scholar] [CrossRef]
- Ben Yahia, H.; Chairat, S.; Gharsa, H.; Alonso, C.A.; Ben Sallem, R.; Porres-Osante, N.; Hamdi, N.; Torres, C.; Ben Slama, K. First report of KPC-2 and KPC-3-producing Enterobacteriaceae in wild birds in Africa. Microb. Ecol. 2020, 79, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Aires-de-Sousa, M.; Fournier, C.; Lopes, E.; de Lencastre, H.; Nordmann, P.; Poirel, L. High colonization rate and heterogeneity of ESBL- and carbapenemase-producing Enterobacteriaceae isolated from gull feces in Lisbon, Portugal. Microorganisms 2020, 8, 1487. [Google Scholar] [CrossRef]
- Ngaiganam, E.P.; Pagnier, I.; Chaalal, W.; Leangapichart, T.; Chabou, S.; Rolain, J.M.; Diene, S.M. Investigation of urban birds as source of β-lactamase-producing Gram-negative bacteria in Marseille city, France. Acta Vet. Scand. 2019, 61, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunha, M.P.V.; Oliveira, M.C.V.; Oliveira, M.G.X.; Menão, M.C.; Knöbl, T. CTX-M-producing Escherichia coli isolated from urban pigeons (Columba livia domestica) in Brazil. J. Infect. Dev. Ctries. 2019, 13, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Hasan, B.; Islam, K.; Ahsan, M.; Hossain, Z.; Rashid, M.; Talukder, B.; Ahmed, K.U.; Olsen, B.; Abul Kashem, M. Fecal carriage of multi-drug resistant and extended spectrum β-lactamases producing E. coli in household pigeons, Bangladesh. Vet. Microbiol. 2014, 168, 221–224. [Google Scholar] [CrossRef]
- Loucif, L.; Chelaghma, W.; Bendjama, E.; Cherak, Z.; Khellaf, M.; Khemri, A.; Rolain, J.M. Detection of blaOXA-48 and mcr-1 genes in Escherichia coli isolates from pigeon (Columba livia) in Algeria. Microorganisms 2022, 10, 975. [Google Scholar] [CrossRef]
- Vogt, N.A.; Stevens, C.P.G.; Pearl, D.L.; Taboada, E.N.; Jardine, C.M. Generalizability and comparability of prevalence estimates in the wild bird literature: Methodological and epidemiological considerations. Anim. Health Res. Rev. 2020, 21, 89–95. [Google Scholar] [CrossRef]
- Agência Portuguesa do Ambiente. Perfil de água balnear de Caxias. 2012. Available online: https://apambiente.pt/sites/default/files/_SNIAMB_A_APA/Comunicacao/Epoca_balnear/PerfisAB/ARH_TejoOeste/PerfisAguasBalneares/OEIRAS/Caxias_PTCQ9L.pdf (accessed on 25 August 2022).
- Carvalho, I.; Carvalho, J.A.; Martínez-Álvarez, S.; Sadi, M.; Capita, R.; Alonso-Calleja, C.; Rabbi, F.; Dapkevicius, M.L.N.E.; Igrejas, G.; Torres, C.; et al. Characterization of ESBL-producing Escherichia coli and Klebsiella pneumoniae isolated from clinical samples in a northern Portuguese hospital: Predominance of CTX-M-15 and high genetic diversity. Microorganisms 2021, 9, 1914. [Google Scholar] [CrossRef]
- Aires-de-Sousa, M.; Lopes, E.; Gonçalves, M.L.; Pereira, A.L.; Machado, E.; Costa, A.; de Lencastre, H.; Poirel, L. Intestinal carriage of extended-spectrum beta-lactamase-producing Enterobacteriaceae at admission in a Portuguese hospital. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 783–790. [Google Scholar] [CrossRef]
- Palmeira, J.D.; Cunha, M.V.; Carvalho, J.; Ferreira, H.; Fonseca, C.; Torres, R.T. Emergence and spread of cephalosporinases in wildlife: A review. Animals 2021, 11, 1765. [Google Scholar] [CrossRef]
- Marin, J.; Clermont, O.; Royer, G.; Mercier-Darty, M.; Decousser, J.W.; Tenaillon, O.; Denamur, E.; Blanquart, F. The population genomics of increased virulence and antibiotic resistance in human commensal Escherichia coli over 30 years in France. Appl. Environ. Microbiol. 2022, 88, e0066422. [Google Scholar] [CrossRef] [PubMed]
- Fournier, C.; Aires de Sousa, M.; Fuster Escriva, B.; Sales, L.; Nordmann, P.; Poirel, L. Epidemiology of extended-spectrum β-lactamase-producing Enterobacteriaceae among healthcare students, at the Portuguese Red Cross Health School of Lisbon, Portugal. J. Glob. Antimicrob. Resist. 2020, 22, 733–737. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, I.; Safia Chenouf, N.; Cunha, R.; Martins, C.; Pimenta, P.; Pereira, A.R.; Martínez-Álvarez, S.; Ramos, S.; Silva, V.; Igrejas, G.; et al. Antimicrobial resistance genes and diversity of clones among ESBL- and acquired AmpC-producing Escherichia coli isolated from fecal samples of healthy and sick cats in Portugal. Antibiotics 2021, 10, 262. [Google Scholar] [CrossRef] [PubMed]
- Mathers, A.J.; Peirano, G.; Pitout, J.D. The role of epidemic resistance plasmids and international high-risk clones in the spread of multidrug-resistant Enterobacteriaceae. Clin. Microbiol. Rev. 2015, 28, 565–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayeni, F.A.; Falgenhauer, J.; Schmiedel, J.; Schwengers, O.; Chakraborty, T.; Falgenhauer, L. Detection of blaCTX-M-27-encoding Escherichia coli ST206 in Nigerian poultry stocks. J. Antimicrob. Chemother. 2020, 75, 3070–3072. [Google Scholar] [CrossRef]
- Li, F.; Cheng, P.; Li, X.; Liu, R.; Liu, H.; Zhang, X. Molecular epidemiology and colistin-resistant mechanism of mcr-positive and mcr-negative Escherichia coli isolated from animal in Sichuan province, China. Front. Microbiol. 2022, 13, 818548. [Google Scholar] [CrossRef]
- Ho, P.L.; Chan, J.; Lo, W.U.; Lai, E.L.; Cheung, Y.Y.; Lau, T.C.K.; Chow, K.H. Prevalence and molecular epidemiology of plasmid-mediated fosfomycin resistance genes among blood and urinary Escherichia coli isolates. J. Med. Microbiol. 2013, 62, 1707–1713. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Y.; Guo, S.; Seow, K.L.G.; Ming, G.O.H.; Schlundt, J. Characterization of extended-spectrum beta-lactamase-producing Escherichia coli isolates from Jurong lake, Singapore with whole-genome-sequencing. Int. J. Environ. Res. Public Health 2021, 18, 937. [Google Scholar] [CrossRef]
- Hooban, B.; Fitzhenry, K.; O’Connor, L.; Miliotis, G.; Joyce, A.; Chueiri, A.; Farrell, M.L.; DeLappe, N.; Tuohy, A.; Cormican, M.; et al. A longitudinal survey of antibiotic-resistant Enterobacterales in the Irish environment, 2019-2020. Sci. Total Environ. 2022, 828, 154488. [Google Scholar] [CrossRef]
- Zhou, H.W.; Zhang, T.; Ma, J.H.; Fang, Y.; Wang, H.Y.; Huang, Z.X.; Wang, Y.; Wu, C.; Chen, G.X. Occurrence of plasmid- and chromosome-carried mcr-1 in waterborne Enterobacteriaceae in China. Antimicrob. Agents Chemother. 2017, 61, e00017-17. [Google Scholar] [CrossRef]
- Rangama, S.; Lidbury, I.D.E.A.; Holden, J.M.; Borsetto, C.; Murphy, A.R.J.; Hawkey, P.M.; Wellington, E.M.H. Mechanisms involved in the active secretion of CTX-M-15 β-lactamase by pathogenic Escherichia coli ST131. Antimicrob. Agents Chemother. 2021, 65, e0066321. [Google Scholar] [CrossRef] [PubMed]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 12.0. 2022. Available online: http://www.eucast.org (accessed on 25 August 2022).
- Lartigue, M.F.; Zinsius, C.; Wenger, A.; Bille, J.; Poirel, L.; Nordmann, P. Extended-spectrum beta-lactamases of the CTX-M type now in Switzerland. Antimicrob. Agents Chemother. 2007, 51, 2855–2860. [Google Scholar] [CrossRef] [Green Version]
- Kieffer, N.; Royer, G.; Decousser, J.W.; Bourrel, A.S.; Palmieri, M.; Ortiz De La Rosa, J.M.; Jacquier, H.; Denamur, E.; Nordmann, P.; Poirel, L. mcr-9, an inducible gene encoding an acquired phosphoethanolamine transferase in Escherichia coli, and its origin. Antimicrob. Agents Chemother. 2019, 63, e00965-19. [Google Scholar] [CrossRef]
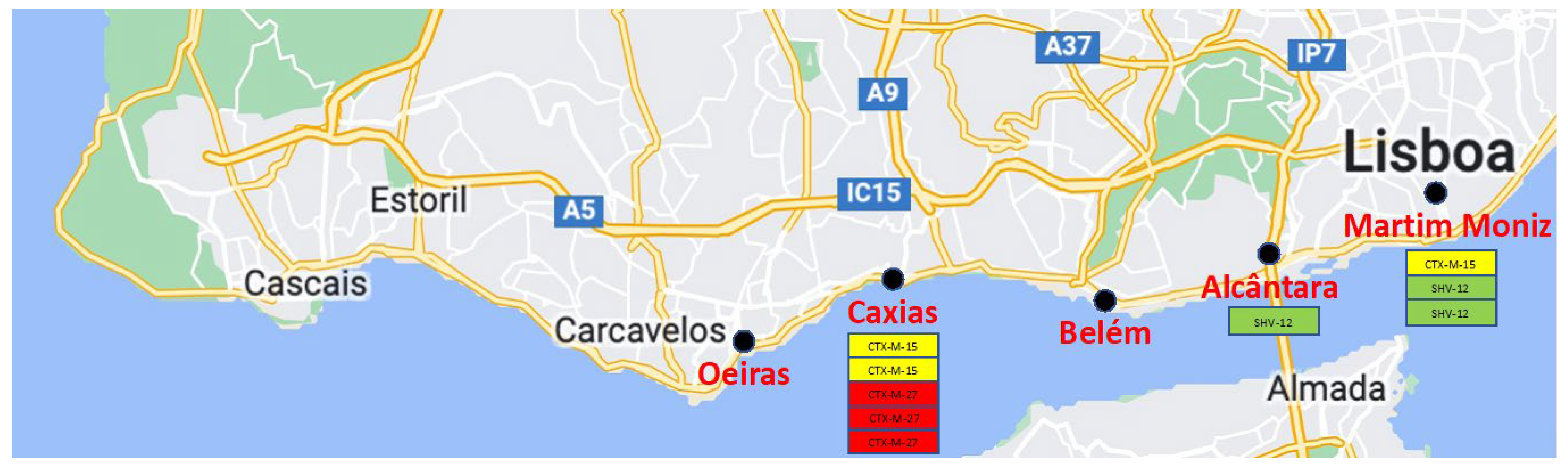
| Sampling Site | Sampling Date | Samples Recovered | Samples Colonized with ESBL-Producers No. (%) |
|---|---|---|---|
| Alcântara (near a waste disposal) | 21 September 2021 | 20 | 1 (5%) |
| Martim Moniz (near a hospital) | 30 October 2021 | 20 | 3 (15%) |
| Caxias (near an effluent) | 17 November 2021 | 20 | 5 (25%) |
| Oeiras (near an effluent) | 20 February 2022 | 20 | 0 |
| Belém (near Tejo River) | 15 May 2022 | 20 | 0 |
| Total | 100 | 9 (9%) |
| Fecal Sample | Sampling Site | Isolate | ESBL | MLST | TIC | AMC | CTX | CZD | TEM | FOX | ETP | IMP | CZA | ATM | CIP | SXT | TET | AMK | GEN | TOB | FOS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 14 | Alcântara | 14E R | SHV-12 | ST3576 | R | S | S | R | I | S | S | S | S | R | S | S | S | S | S | S | S |
| 35 | M. Moniz | 35E R | SHV-12 | ST10 | R | S | S | R | I | S | S | S | S | I | S | S | S | S | S | S | S |
| 36 | M. Moniz | 36E R | SHV-12 | ST154 | R | S | S | R | I | S | S | S | S | R | S | S | R | S | S | S | S |
| 39 | M. Moniz | 39E R | CTX-M-15 | ST206 | R | S | I | I | I | S | S | S | S | S | S | S | S | S | S | S | S |
| 44 | Caxias | 44E R | CTX-M-27 | ST131 | R | S | R | I | I | S | S | S | S | I | I | R | R | S | R | R | S |
| 47 | Caxias | 47E R | CTX-M-15 | ST2858 | R | S | I | I | I | S | S | S | S | I | S | S | S | S | S | S | S |
| 50 | Caxias | 50E R | CTX-M-27 | ST131 | R | S | I | I | I | S | S | S | S | I | S | R | R | S | R | R | S |
| 56 | Caxias | 56E R | CTX-M-15 | ST2858 | R | S | R | I | I | S | S | S | S | I | S | S | S | S | S | S | S |
| 60 | Caxias | 60E R | CTX-M-27 | ST1488 | R | S | R | R | I | S | S | S | S | I | I | R | R | S | S | S | S |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freire, S.; Grilo, T.; Poirel, L.; Aires-de-Sousa, M. Urban Pigeons (Columba livia) as a Source of Broad-Spectrum β-Lactamase-Producing Escherichia coli in Lisbon, Portugal. Antibiotics 2022, 11, 1368. https://doi.org/10.3390/antibiotics11101368
Freire S, Grilo T, Poirel L, Aires-de-Sousa M. Urban Pigeons (Columba livia) as a Source of Broad-Spectrum β-Lactamase-Producing Escherichia coli in Lisbon, Portugal. Antibiotics. 2022; 11(10):1368. https://doi.org/10.3390/antibiotics11101368
Chicago/Turabian StyleFreire, Samanta, Teresa Grilo, Laurent Poirel, and Marta Aires-de-Sousa. 2022. "Urban Pigeons (Columba livia) as a Source of Broad-Spectrum β-Lactamase-Producing Escherichia coli in Lisbon, Portugal" Antibiotics 11, no. 10: 1368. https://doi.org/10.3390/antibiotics11101368






