Prior Antibiotic Use Increases Risk of Urinary Tract Infections Caused by Resistant Escherichia coli among Elderly in Primary Care: A Case-Control Study
Abstract
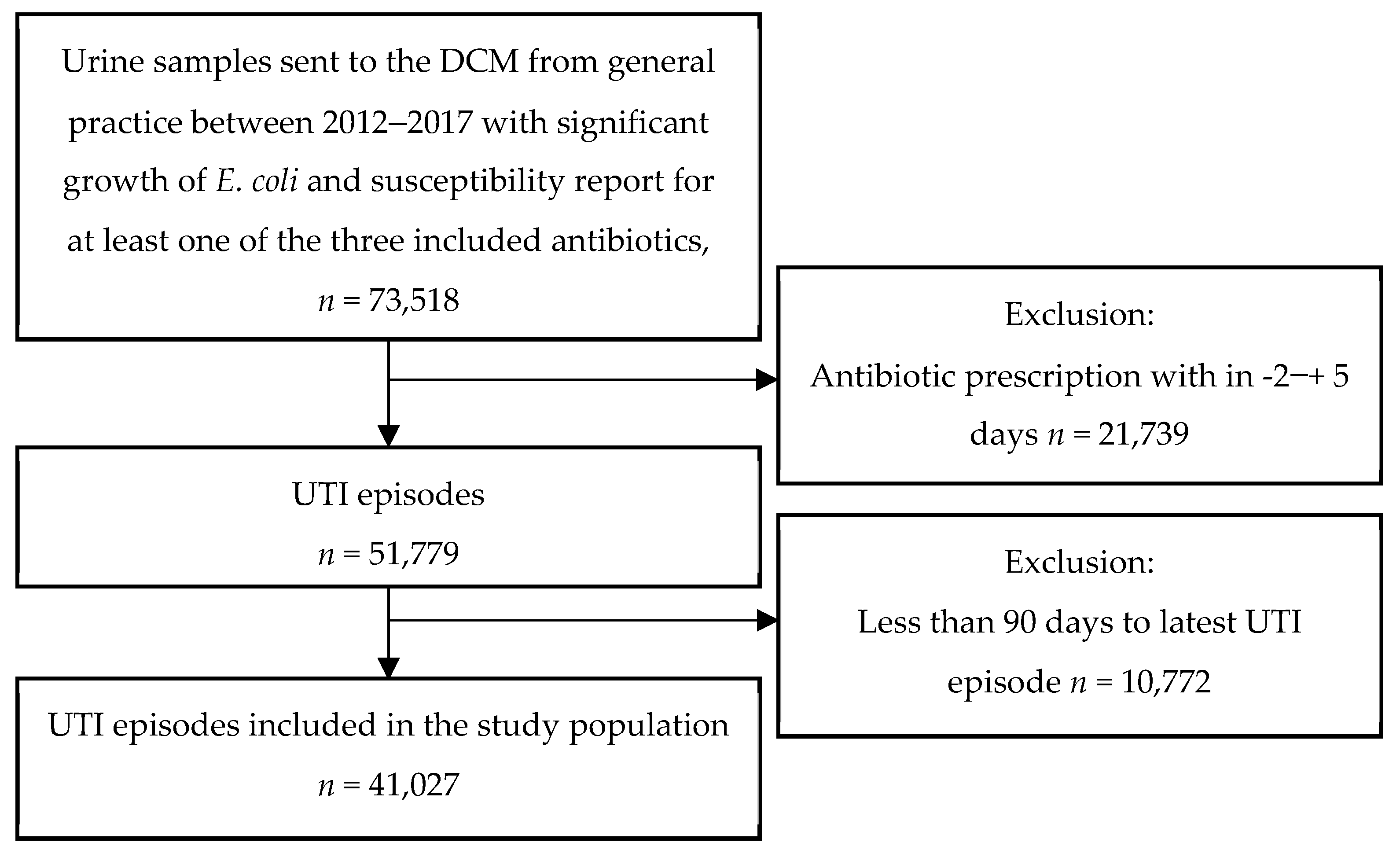
:1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Laboratory Methods
2.3. Outcome Measure and Classification of Cases and Controls
2.4. Antibiotic Exposure
2.5. Covariates
2.6. Statistical Analysis
2.7. Sensitivity Analysis
3. Results
3.1. General Characteristics
3.2. Total Exposure
3.3. Time since Last Antibiotic Exposure
3.4. Specific Antibiotic Exposure
3.5. Sensitivity Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC); European Food Safety Authority (EFSA); European Medicines Agency (EMA). Third joint inter-agency report on integrated analysis of consumption of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from humans and food-producing animals in the EU/EEA. EFSA J. 2021, 19, e06712. [Google Scholar] [CrossRef]
- Statens Serum Institut; National Food Institute; Technical University of Denmark. DANMAP 2019—Use of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria from Food Animals, Food and Humans in Denmark. Available online: https://backend.orbit.dtu.dk/ws/portalfiles/portal/235092204/DANMAP_2019.pdf (accessed on 30 March 2022).
- Søraas, A.; Sundsfjord, A.; Jørgensen, S.; Liestøl, K.; Jenum, P.A. High Rate of Per Oral Mecillinam Treatment Failure in Community-Acquired Urinary Tract Infections Caused by ESBL-Producing Escherichia coli. PLoS ONE 2014, 9, e85889. [Google Scholar] [CrossRef] [PubMed]
- Bell, B.G.; Schellevis, F.; Stobberingh, E.; Goossens, H.; Pringle, M. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect. Dis. 2014, 14, 13. [Google Scholar] [CrossRef]
- Costelloe, C.; Metcalfe, C.; Lovering, A.; Mant, D.; Hay, A.D. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: Systematic review and meta-analysis. BMJ 2010, 340, c2096. [Google Scholar] [CrossRef]
- Bakhit, M.; Hoffmann, T.; Scott, A.M.; Beller, E.; Rathbone, J.; Del Mar, C. Resistance decay in individuals after antibiotic exposure in primary care: A systematic review and meta-analysis. BMC Med. 2018, 16, 126. [Google Scholar] [CrossRef]
- Opatowski, M.; Brun-Buisson, C.; Touat, M.; Salomon, J.; Guillemot, D.; Tuppin, P.; Watier, L. Additional file 1 of Antibiotic prescriptions and risk factors for antimicrobial resistance in patients hospitalized with urinary tract infection: A matched case-control study using the French health insurance database (SNDS). BMC Infect. Dis. 2021, 21, 571. [Google Scholar] [CrossRef]
- Vellinga, A.; Tansey, S.; Hanahoe, B.; Bennett, K.; Murphy, A.W.; Cormican, M. Trimethoprim and ciprofloxacin resistance and prescribing in urinary tract infection associated with Escherichia coli: A multilevel model. J. Antimicrob. Chemother. 2012, 67, 2523–2530. [Google Scholar] [CrossRef]
- Hillier, S.; Roberts, Z.; Dunstan, F.; Butler, C.; Howard, A.; Palmer, S. Prior antibiotics and risk of antibiotic-resistant community-acquired urinary tract infection: A case-control study. J. Antimicrob. Chemother. 2007, 60, 92–99. [Google Scholar] [CrossRef]
- Rossignol, L.; Maugat, S.; Blake, A.; Vaux, S.; Heym, B.; Le Strat, Y.; Kerneis, S.; Blanchon, T.; Coignard, B.; Hanslik, T. Risk factors for resistance in urinary tract infec-tions in women in general practice: A cross-sectional survey. J. Infect. 2015, 71, 302–311. [Google Scholar] [CrossRef]
- Statens Serum Institut; National Food Institute; Technical University of Denmark. DANMAP 2020—Use of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria from Food Animals, Food and Humans in Denmark. Available online: https://www.ssi.dk/-/media/arkiv/subsites/antibiotikaresistens/danmap_2020_07102021_version-2_low.pdf?la=da (accessed on 1 July 2022).
- Aabenhus, R.; Hansen, M.P.; Siersma, V.; Bjerrum, L. Clinical indications for antibiotic use in Danish general practice: Results from a nationwide electronic prescription database. Scand. J. Prim. Health Care 2017, 35, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Blix, H.S.; Engeland, A.; Litleskare, I.; Rønning, M. Age- and gender-specific antibacterial prescribing in Norway. J. Antimicrob. Chemother. 2007, 59, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Brabazon, E.; Carton, M.; Dornikova, G.; Bedford, D. Epidemiology and resistance patterns in urinary pathogens from long-term care facilities and GP populations. Ir. Med. J. 2012, 105, 177–180. [Google Scholar] [PubMed]
- Colodner, R.; Kometiani, I.; Chazan, B.; Raz, R. Risk Factors for Community-Acquired Urinary Tract Infection Due to Quinolone-Resistant E. coli. Infection 2008, 36, 41–45. [Google Scholar] [CrossRef]
- Su, Y.-C.; Kung, L.-C.; Lee, C.-H.; Chang, W.; Hung, C.-L.; Tsao, C.-C.; Huang, M.-Y. Antimicrobial-Resistant Bacteremia in the Elderly: Risk of Previous Hospitalization. Int. J. Gerontol. 2017, 11, 27–30. [Google Scholar] [CrossRef]
- Thornley, T.; Ashiru-Oredope, D.; Normington, A.; Beech, E.; Howard, P. Antibiotic prescribing for residents in long-term-care facilities across the UK. J. Antimicrob. Chemother. 2019, 74, 1447–1451. [Google Scholar] [CrossRef]
- Azaizi, H.; Veimer Jensen, M.L.; Scheel Rasmussen, I.; Jarloev, J.O.; Nygaard Jensen, J. Antibiotic prescribing among elderly living in long-term care facilities versus elderly living at home: A Danish registry-based study. Infect. Dis. 2022, 19, 651–655. [Google Scholar] [CrossRef]
- Kildemoes, H.W.; Sørensen, H.T.; Hallas, J. The Danish National Prescription Registry. Scand. J. Public Health 2011, 39, 38–41. [Google Scholar] [CrossRef]
- Aspevall, O.; Hallander, H.; Gant, V.; Kouri, T. European guidelines for urinalysis: A collaborative document produced by European clinical microbiologists and clinical chemists under ECLM in collaboration with ESCMID. Clin. Microbiol. Infect. 2001, 7, 173–178. [Google Scholar] [CrossRef]
- EUCAST. EUCATS Breakpoints. Available online: https://www.eucast.org/clinical_breakpoints/ (accessed on 4 April 2022).
- Hardin, J.W.; Hilbe, J.M. Generalized Estimating Equations, 2nd ed.; Chapman and Hall/CRC: Boca Raton, FL, USA, 2012; Available online: https://www.taylorfrancis.com/books/9781439881149 (accessed on 4 April 2022).
- Veimer Jensen, M.L.; Aabenhus, R.M.; Holzknecht, B.J.; Bjerrum, L.; Jensen, J.N.; Siersma, V.; Cordoba, G. Antibiotic prescribing in Danish general practice in the elderly population from 2010 to 2017. Scand. J. Prim. Health Care 2021, 39, 498–505. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Antimicrobial Resistance in the EU/EEA (EARS-Net)—Annual Epidemiological Report for 2019. 2020. Available online: https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-resistance-europe-2019 (accessed on 2 November 2021).
- Søraas, A.; Sundsfjord, A.; Sandven, I.; Brunborg, C.; Jenum, P.A. Risk Factors for Community-Acquired Urinary Tract Infections Caused by ESBL-Producing Enterobacteriaceae—A Case–Control Study in a Low Prevalence Country. PLoS ONE 2013, 8, e69581. [Google Scholar] [CrossRef] [PubMed]
- Hertz, F.B.; Schønning, K.; Rasmussen, S.C.; Littauer, P.; Knudsen, J.D.; Løbner-Olesen, A.; Frimodt-Møller, N. Epidemiological factors associated with ESBL- and non ESBL-producing E. coli causing urinary tract infection in general practice. Infect. Dis. 2016, 48, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Kresken, M.; Pfeifer, Y.; Wagenlehner, F.; Werner, G.; Wohlfarth, E.; on behalf of Study Group ‘Antimicrobial Resistance‘ of the Paul Ehrlich Society for Infection Therapy. Resistance to Mecillinam and Nine Other Antibiotics for Oral Use in Escherichia coli Isolated from Urine Specimens of Primary Care Patients in Germany, 2019/20. Antibiotics 2022, 11, 751. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, H.O.; Johansson, A.F.; Granholm, S.; Kahlmeter, G.; Sundqvist, M. High genetic diversity of nitrofurantoin- or mecillinam-resistant Escherichia coli indicates low propensity for clonal spread. J. Antimicrob. Chemother. 2013, 68, 1974–1977. [Google Scholar] [CrossRef] [PubMed]

| Mecillinam | Trimethoprim | Nitrofurantoin | Multi-resistance | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | Cases | Controls | Cases | Controls | Cases | Control | ||
| n = 1853 | n = 39,083 | n = 10,767 | n = 30,189 | n = 886 | n = 37,571 | n = 72 | n = 26,854 | ||
| Age [Median, (IQR *)] | 80 (73–87) | 78 (71–85) | 82 (75–88) | 78 (71–85) | 82 (75–88) | 78 (71–85) | 83 (76–90) | 78 (71–85) | |
| Female (%) | 80 | 84 | 83 | 84 | 72 | 84 | 74 | 84 | |
| Time since last prescription [n (%)] | No exposure | 715 (38.6) | 22,055 (56.4) | 4451 (41.3) | 18,324 (60.7) | 251 (28.3) | 21,025 (56.0) | 16 (22.2) | 16,463 (61.3) |
| 8–30 days | 708 (38.2) | 9204 (23.6) | 3784 (35.1) | 6137 (20.3) | 388 (43.8) | 8999 (24.0) | 37 (51.4) | 5310 (19.8) | |
| 31–60 days | 262 (14.1) | 4681 (12.0) | 1585 (14.7) | 3362 (11.1) | 170 (19.2) | 4496 (12.0) | 14 (19.4) | 2967 (11.1) | |
| 61–90 days | 168 (9.1) | 3143 (8.0) | 947 (8.8) | 2366 (7.8) | 77 (8.7) | 3051 (8.1) | 5 (6.9) | 2114 (7.9) | |
| Number of prescriptions [n (%)] | 0 | 715 (38.6) | 22,055 (56.4) | 4451 (41.3) | 18,324 (60.7) | 251 (28.3) | 21,025 (56.0) | 16 (22.2) | 16,463 (61.3) |
| 1 | 494 (26.7) | 9170 (23.5) | 2750 (25.5) | 6916 (22.9) | 215 (24.3) | 8899 (23.7) | 11 (15.3) | 6137 (22.9) | |
| 2 | 286 (15.4) | 4258 (10.9) | 1623 (15.1) | 2928 (9.7) | 159 (18.0) | 4114 (11.0) | 16 (22.2) | 2542 (9.5) | |
| ≥3 | 358 (19.3) | 3600 (9.2) | 1943 (18.1) | 2021 (6.7) | 261 (29.5) | 3533 (9.4) | 29 (40.3) | 1712 (6.4) | |
| Number of DDD [n (%)] | 0 | 715 (38.6) | 22,055 (56.4) | 4451 (41.3) | 18,324 (60.7) | 251 (28.3) | 21,025 (56.0) | 16 (22.2) | 16,463 (61.3) |
| >0–33.3 percentile ** | 261 (14.1) | 5800 (14.8) | 1594 (14.8) | 4470 (14.8) | 89 (10.1) | 5624 (15.0) | 6 (8.3) | 4004 (14.9) | |
| >33.3–66.6 percentile | 362 (19.6) | 5678 (14.5) | 1820 (16.9) | 4226 (14.0) | 151 (17.0) | 5547 (14.8) | 11 (15.3) | 3723 (13.9) | |
| >66.6 percentile | 515 (27.8) | 5550 (14.2) | 2902 (27.0) | 3169 (10.5) | 395 (44.6) | 5375 (14.3) | 39 (54.2) | 2664 (9.9) | |
| Exposure within 90 days to the following agents [n (%)] | |||||||||
| Phenoxymethylpenicillin or dicloxacillin | 279 (15.1) | 2899 (7.4) | 964 (9.0) | 2219 (7.4) | 87 (9.8) | 2928 (7.8) | 6 (8.3) | 1916 (7.1) | |
| Pivmecillinam | 631 (34.1) | 8705 (22.3) | 3088 (28.7) | 6251 (20.7) | 305 (34.4) | 8494 (22.6) | 37 (51.4) | 5487 (20.4) | |
| Amoxicillin | 95 (95.1) | 986 (2.5) | 353 (3.3) | 728 (2.4) | 18 (2.0) | 998 (2.7) | - *** | - | |
| Amoxicillin + β lactamase inhibitor | 43 (2.3) | 428 (1.1) | 165 (1.5) | 306 (1.0) | 16 (1.8) | 430 (1.1) | 7 (9.7) | 258 (1.0) | |
| Trimethoprim | 155 (8.4) | 2157 (5.5) | 1673 (15.5) | 640 (2.1) | 85 (9.6) | 2130 (5.7) | 16 (22.2) | 575 (2.1) | |
| Sulfamethizole | 176 (9.5) | 3001 (7.7) | 1081 (10.0) | 2102 (7.0) | 80 (9.0) | 2954 (7.9) | 7 (9.72) | 1892 (7.1) | |
| Nitrofurantoin | 187 (10.1) | 2323 (5.9) | 1118 (10.4) | 1392 (4.6) | 356 (40.2) | 2023 (5.4) | 23 (31.9) | 1090 (4.1) | |
| Macrolides | 47 (2.5) | 1087 (2.8) | 350 (3.3) | 782 (2.6) | 23 (2.6) | 1036 (2.8) | - | - | |
| Quinolones | 70 (3.8) | 1194 (3.1) | 644 (6.0) | 621 (2.1) | 68 (7.7) | 1156 (3.1) | 5 (6.9) | 551 (2.1) | |
| Others | 185 (10.0) | 2004 (5.1) | 837 (7.8) | 1358 (4.5) | 74 (8.4) | 2012 (5.4) | 11 (15.3) | 1183 (4.4) | |
| Number of admissions [n (%)] | 0 **** | 1344 (72.5) | 31,970 (81.8) | 8452 (78.5) | 24,870 (82.4) | 686 (77.4) | 30,612 (81.4) | 57 (79.2) | 22,218 (82.7) |
| 1 | 249 (13.4) | 4271 (10.9) | 1346 (12.5) | 3179 (10.5) | 114 (12.9) | 4117 (11.0) | 8 (11.1) | 2786 (10.4) | |
| ≥2 | 260 (14.0) | 2842 (7.3) | 969 (9) | 2140 (7.1) | 86 (9.7) | 2842 (7.6) | 7 (9.7) | 1850 (6.9) | |
| Number of admission days [n (%)] | 0 **** | 1393 (75.2) | 32,904 (84.2) | 8718 (81.0) | 25,585 (84.8) | 716 (80.8) | 31,507 (83.9) | 59 (81.9) | 22,852 (85.1) |
| 1–7 days | 223 (12.0) | 3379 (8.7) | 1091 (10.1) | 2519 (8.3) | 97 (11.0) | 3279 (8.7) | 8 (11.1) | 2201 (8.2) | |
| ≥1 week | 237 (12.8) | 2800 (7.2) | 958 (8.9) | 2085 (6.9) | 73 (8.2) | 2785 (7.4) | 5 (6.9) | 1801 (6.7) | |
| Number of admissions, infection related [n (%)] | 0 | 1705 (92.0) | 37,587 (96.2) | 10,196 (94.7) | 29,111 (96.4) | 845 (95.4) | 36,069 (96.0) | 69 (95.8) | 25,929 (96.7) |
| 1 | 105 (5.7) | 1202 (3.1) | 456 (4.24) | 854 (2.8) | 29 (3.3) | 1193 (32) | 3 (4.2) | 139 (2.8) | |
| ≥2 | 43 (2.3) | 294 (0.8) | 115 (1.07) | 224 (0.7) | 12 (1.4) | 309 (0.8) | 0 | 186 (0.7) | |
| Number of admission days, infection related [n (%)] | 0 | 1714 (92.5) | 37,715 (96.5) | 10,246 (95.1) | 29,198 (96.7) | 852 (96.2) | 36,194 (96.3) | - | - |
| 1–7 days | 86 (4.6) | 855 (2.2) | 322 (3.0) | 623 (2.1) | 21 (2.4) | 852 (2.3) | - | - | |
| ≥1 week | 53 (2.9) | 513 (1.3) | 199 (1.9) | 368 (1.2) | 13 (1.5) | 525 (1.4) | - | - | |
| Nursing home residency [n (%)] | Yes | 42 (2.3) | 807 (2.1) | 249 (2.3) | 604 (2.0) | 18 (2.0) | 781 (2.1) | - | - |
| Mecillinam | Trimethoprim | Nitrofurantoin | Multi-Resistance | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR * | CI95% ** | OR | CI95% | OR | CI95% | OR | CI95% | ||
| Number of prescriptions | 0 | Ref | Ref | Ref | Ref | ||||
| 1 | 1.32 | (1.15;1.53) | 1.40 | (1.32;1.50) | 1.25 | (0.97;1.61) | 1.35 | (0.59;3.08) | |
| 2 | 1.82 | (1.59;2.07) | 1.66 | (1.56;1.77) | 2.10 | (1.69;2.60) | 2.86 | (1.45;5.63) | |
| ≥3 | 2.47 | (2.18;2.80) | 3.11 | (2.92;3.31) | 4.53 | (3.76;5.46) | 11.53 | (6.37;20.85) | |
| Number of DDD *** | 0 | Ref | Ref | Ref | Ref | ||||
| >0–33.3 percentile | 1.56 | (1.39;1.76) | 1.54 | (1.46;1.63) | 1.84 | (1.51;2.23) | 1.34 | (0.59;3.05) | |
| >33.3–6.66 percentile | 1.88 | (1.63;2.18) | 2.03 | (1.89;2.17) | 2.59 | (2.07;3.24) | 2.29 | (1.07;4.95) | |
| >66.6 percentile | 2.60 | (2.26;2.99) | 3.22 | (2.99;3.46) | 4.49 | (3.65;5.51) | 10.21 | (5.78;18.02) | |
| Time since last prescription | No exposure | Ref | Ref | Ref | Ref | ||||
| 8–30 days | 2.13 | (1.91;2.38) | 2.20 | (2.09;2.32) | 2.83 | (2.38;3.37) | 5.81 | (3.37;10.03) | |
| 31–60 days | 1.53 | (1.32;1.78) | 1.71 | (1.60;1.83) | 2.51 | (2.00;3.14) | 3.72 | (1.93;7.16) | |
| 61–90 days | 1.51 | (1.27;1.79) | 1.49 | (1.38;1.62) | 1.81 | (1.37;2.40) | 2.15 | (0.85;5.40) | |
| Mecillinam | Trimethoprim | Nitrofurantoin | Multi-Resistance | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR * | CI95% ** | OR | CI95% | OR | CI95% | OR | CI95% | ||
| Exposure to specific drugs within 8–90 days (reference: no exposure) | |||||||||
| Phenoxymethylpenicillin or dicloxacillin *** | Yes | 2.01 | (1.75;2.31) | 1.18 | (1.09;1.28) | 1.28 | (1;1.64) | 1.06 | (0.45;2.49) |
| Pivmecillinam | Yes | 1.62 | (1.46;1.8) | 1.36 | (1.29;1.43) | 1.37 | (1.16;1.63) | 3.2 | (2.07;4.95) |
| Amoxicillin | Yes | 1.8 | (1.43;2.27) | 1.25 | (1.09;1.42) | 0.84 | (0.49;1.44) | - | - |
| Amoxicillin + β lactamase inhibitor | Yes | 1.75 | (1.25;2.47) | 1.31 | (1.08;1.59) | 1.48 | (0.84;2.59) | 10.18 | (4.27;24.27) |
| Trimethoprim | Yes | 1.43 | (1.19;1.71) | 6.48 | (5.93;7.09) | 1.05 | (0.71;1.55) | 8.67 | (4.62;16.26) |
| Sulfamethizole | Yes | 1.25 | (1.07;1.47) | 1.41 | (1.31;1.53) | 1.03 | (0.78;1.35) | 1.45 | (0.74;2.83) |
| Nitrofurantoin | Yes | 1.56 | (1.32;1.84) | 1.85 | (1.7;2.02) | 8.64 | (7.26;10.29) | 9.11 | (5.47;15.16) |
| Macrolides | Yes | 0.87 | (0.64;1.18) | 1.18 | (1.03;1.34) | 1.09 | (0.71;1.68) | - | - |
| Quinolones | Yes | 0.92 | (0.69;1.22) | 2.14 | (1.91;2.4) | 1.68 | (1.16;2.43) | 2.54 | (0.86;7.52) |
| Others | Yes | 1.76 | (1.48;2.08) | 2.25 | (2.01;2.51) | 1.21 | (0.87;1.69) | 3.16 | (1.66;6.01) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jensen, M.L.V.; Siersma, V.; Søes, L.M.; Nicolaisdottir, D.; Bjerrum, L.; Holzknecht, B.J. Prior Antibiotic Use Increases Risk of Urinary Tract Infections Caused by Resistant Escherichia coli among Elderly in Primary Care: A Case-Control Study. Antibiotics 2022, 11, 1382. https://doi.org/10.3390/antibiotics11101382
Jensen MLV, Siersma V, Søes LM, Nicolaisdottir D, Bjerrum L, Holzknecht BJ. Prior Antibiotic Use Increases Risk of Urinary Tract Infections Caused by Resistant Escherichia coli among Elderly in Primary Care: A Case-Control Study. Antibiotics. 2022; 11(10):1382. https://doi.org/10.3390/antibiotics11101382
Chicago/Turabian StyleJensen, Maria L. V., Volkert Siersma, Lillian M. Søes, Dagny Nicolaisdottir, Lars Bjerrum, and Barbara J. Holzknecht. 2022. "Prior Antibiotic Use Increases Risk of Urinary Tract Infections Caused by Resistant Escherichia coli among Elderly in Primary Care: A Case-Control Study" Antibiotics 11, no. 10: 1382. https://doi.org/10.3390/antibiotics11101382
APA StyleJensen, M. L. V., Siersma, V., Søes, L. M., Nicolaisdottir, D., Bjerrum, L., & Holzknecht, B. J. (2022). Prior Antibiotic Use Increases Risk of Urinary Tract Infections Caused by Resistant Escherichia coli among Elderly in Primary Care: A Case-Control Study. Antibiotics, 11(10), 1382. https://doi.org/10.3390/antibiotics11101382







