Antibiofilm Effect of Cinnamaldehyde-Chitosan Nanoparticles against the Biofilm of Staphylococcus aureus
Abstract
1. Introduction
2. Results
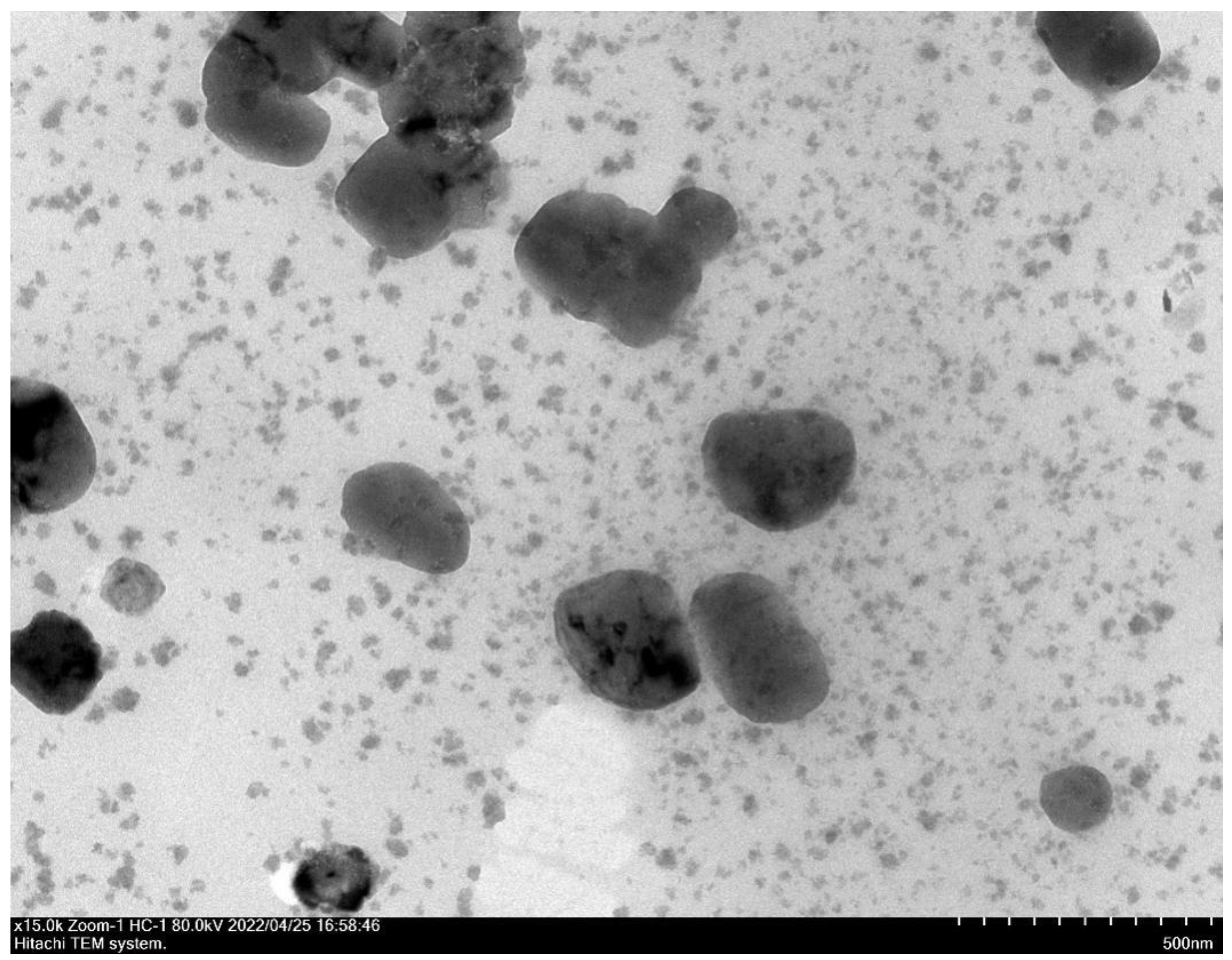
2.1. Characterization of Nanoparticles
2.2. In Vitro Release Studies
2.3. Antibacterial Activity of CA and CSNP-CAs
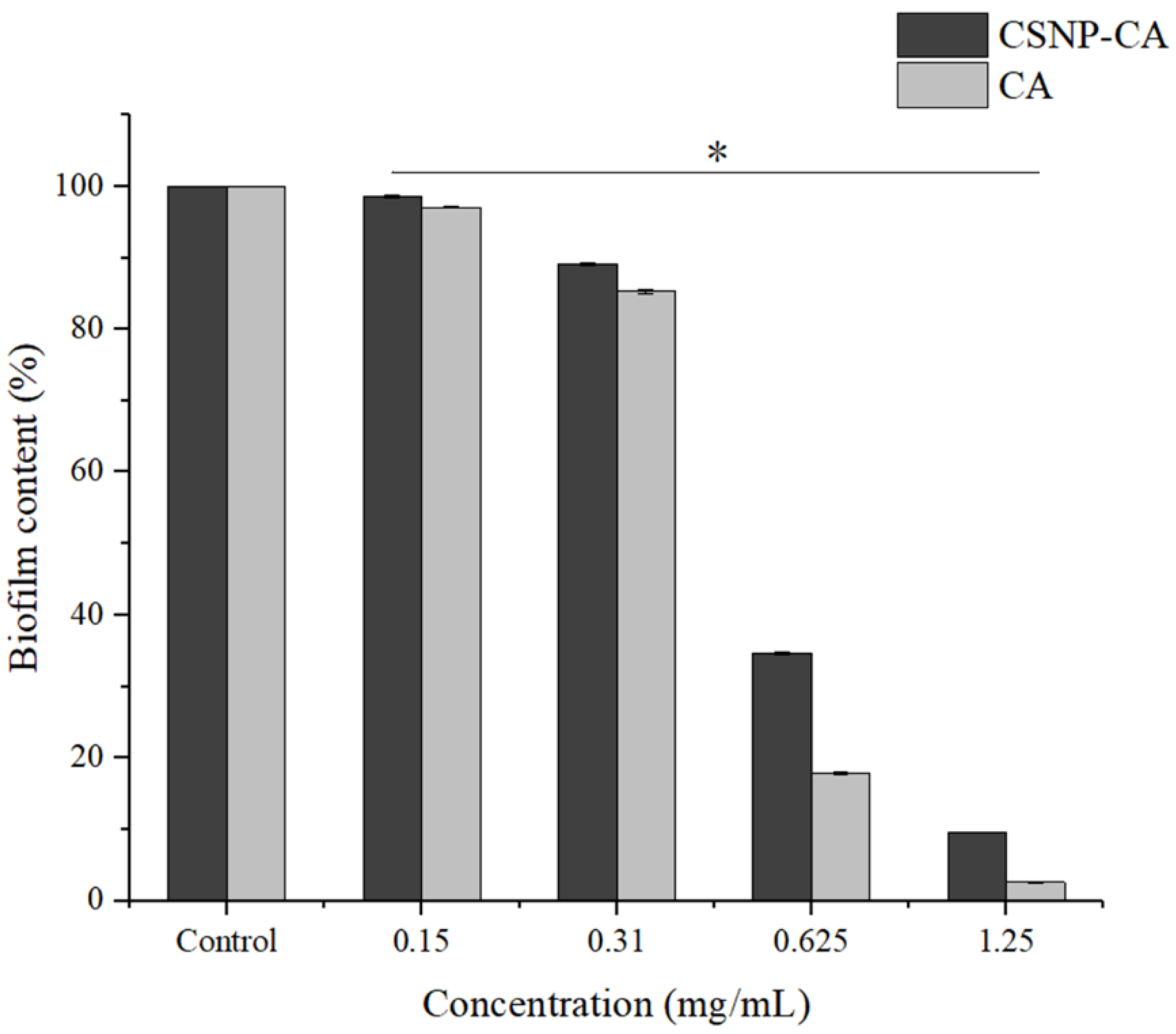
2.4. Inhibition Activity on Biofilm Formation of CA and CSNP-CAs
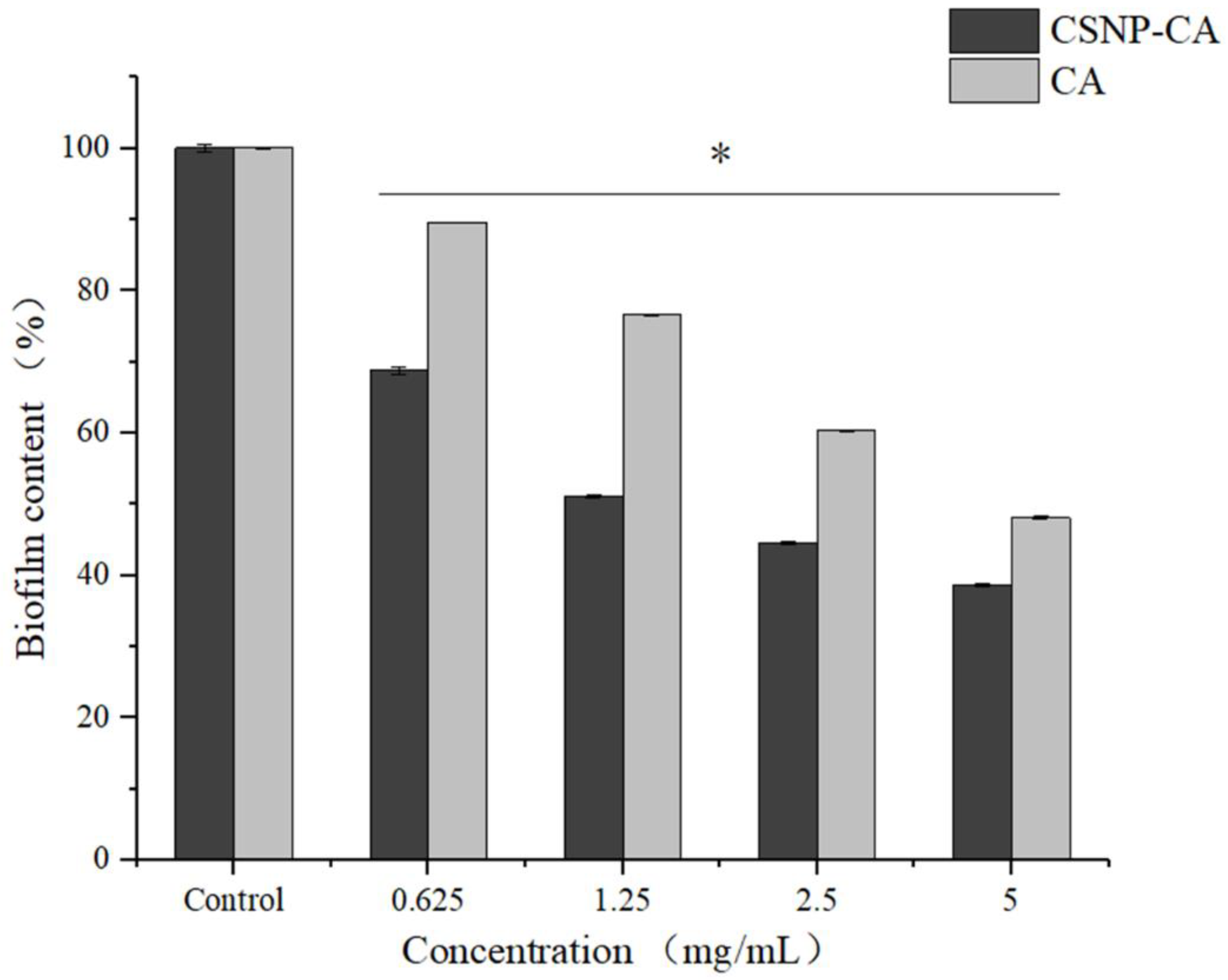
2.5. Antibiofilm Activity on Mature Biofilm of CA and CSNP-CAs
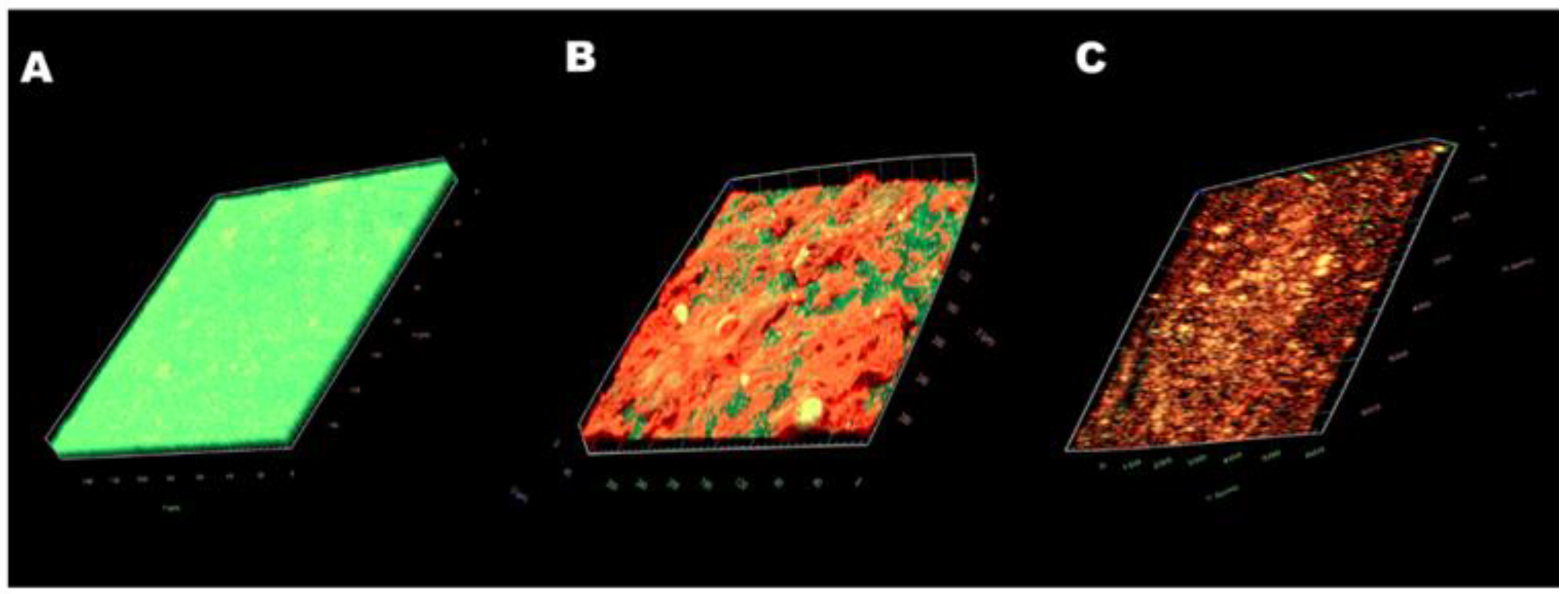
2.6. Antibiofilm Effect of CSNP-CAs on Food-Grade Silicone
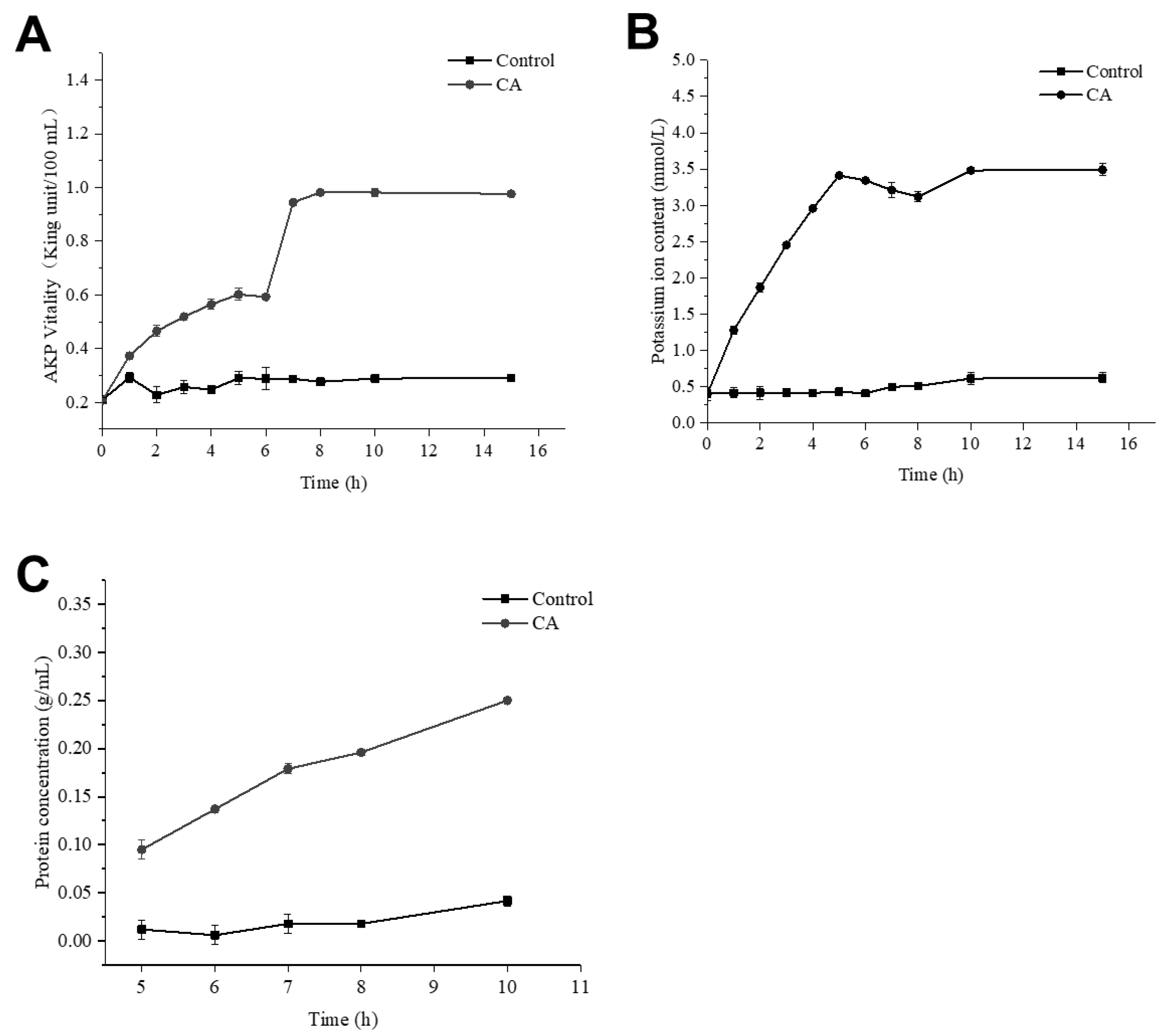
2.7. Effect of CA on Cell-Wall Integrity of S. aureus
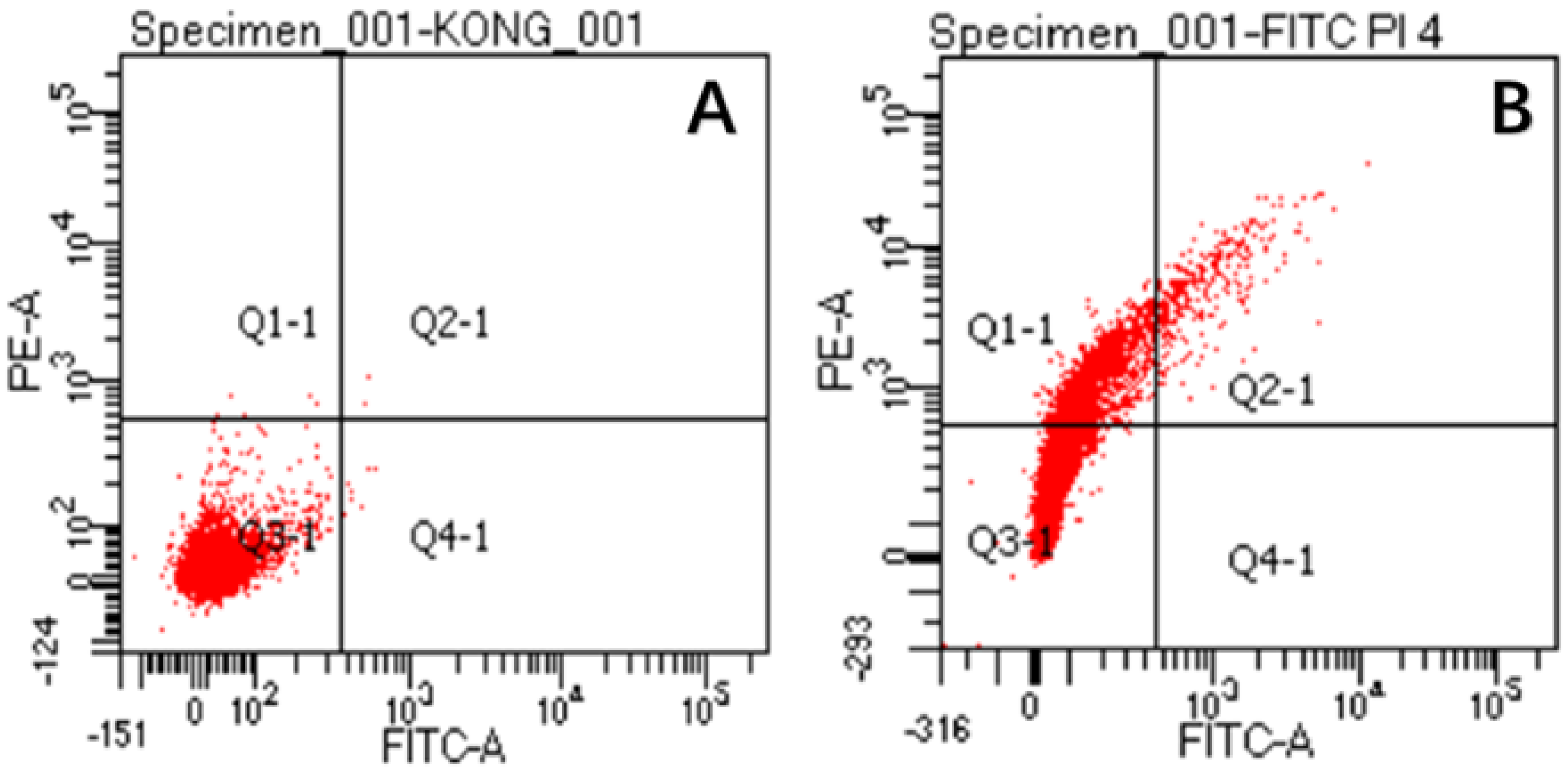
2.8. Effect of CA on Membrane Permeability of S. aureus
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Growth Media
4.2. Preparation of Nanoparticles
4.3. Preparation of CSNP-CAs
4.4. Particle Size and Zeta Potential of NPs
4.5. Drug Encapsulation
4.6. In Vitro Release Test
4.7. Determinations of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
4.8. Growth of Biofilm
4.9. Removal of Biofilm
4.10. Antibiofilm Effect on Food-Grade Silicone Surfaces
4.11. Live/Dead Assay
4.12. Effect of CA on Membrane Permeability of S. aureus
4.13. Effect of CA on Cell Wall Integrity of S. aureus
4.14. The Morphologies of the Bacteria Observed by SEM
4.15. Flow Cytometry Analysis
4.16. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bortolaia, V.; Espinosa-Gongora, C.; Guardabassi, L. Human health risks associated with antimicrobial-resistant enterococci and Staphylococcus aureus on poultry meat. Clin. Microbiol. Infect 2016, 22, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the Natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Akiyoshi, Y.; O’Toole, G.A.; Ogihara, H.; Morinaga, Y. Sugar fatty acid esters inhibit biofilm formation by food-borne pathogenic bacteria. Int. J. Food Microbiol. 2010, 138, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Ma, S.; Leonhard, M.; Moser, D.; Haselmann, G.M.; Wang, J.; Eder, D.; Schneider-Stickler, B. Enhancing antibiofilm activity with functional chitosan nanoparticles targeting biofilm cells and biofilm matrix. Carbohydr Polym. 2018, 200, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Vyas, N.; Wang, Q.X.; Walmsley, A.D. Improved biofilm removal using cavitation from a dental ultrasonic scaler vibrating in carbonated water. Ultrason Sonochem. 2021, 70, 105338. [Google Scholar] [CrossRef]
- Shi, S.F.; Zhang, X.L.; Zhu, C.; Chen, D.S.; Guo, Y.Y. Ultrasonically enhanced rifampin activity against internalized Staphylococcus aureus. Exp. The. Med. 2013, 5, 257–262. [Google Scholar] [CrossRef][Green Version]
- Sadekuzzaman, M.; Yang, S.; Mizan, M.F.R.; Ha, S.D. Current and recent advanced strategies for combating biofilms. Com. Rev. Food Sci. F 2015, 14, 491–509. [Google Scholar] [CrossRef]
- Srinivasan, S.; Harrington, G.W.; Xagoraraki, I.; Goel, R. Factors affecting bulk to total bacteria ratio in drinking water distribution systems. Water Res. 2008, 42, 3393–3404. [Google Scholar] [CrossRef]
- Doyle, A.A.; Stephens, J.C. A review of cinnamaldehyde and its derivatives as antibacterial agents. Fitoterapia 2019, 139, 104405. [Google Scholar] [CrossRef]
- Zhu, R.; Liu, H.; Liu, C.; Wang, L.; Ma, R.; Chen, B.; Li, L.; Niu, J.; Fu, M.; Zhang, D.; et al. Cinnamaldehyde in diabetes: A review of pharmacology, pharmacokinetics and safety. Pharmacol. Res. 2017, 122, 78–89. [Google Scholar] [CrossRef]
- Shreaz, S.; Wani, W.A.; Behbehani, J.M.; Raja, V.; Irshad, M.; Karched, M.; Ali, I.; Siddiqi, W.A.; Hun, L.T. Cinnamaldehyde and its derivatives, a novel class of antifungal agents. Fitoterapia 2016, 112, 116–131. [Google Scholar] [CrossRef] [PubMed]
- Albano, M.; Crulhas, B.P.; Alves, F.C.B.; Pereira, A.F.M.; Andrade, B.F.M.B.; Barbosa, L.N.; Furlanetto, A.; Lyra, L.P.D.S.; Rall, V.L.M.; Júnior, A.F. Antibacterial and anti-biofilm activities of cinnamaldehyde against S. epidermidis. Microb. Pathog. 2019, 126, 231–238. [Google Scholar] [CrossRef]
- Yu, H.H.; Song, Y.J.; Yu, H.S.; Lee, N.K.; Paik, H.D. Investigating the antimicrobial and antibiofilm effects of cinnamaldehyde against Campylobacter spp. using cell surface characteristics. J. Food Sci. 2020, 85, 157–164. [Google Scholar] [CrossRef]
- Kim, J.; Bao, T.H.Q.; Shin, Y.K.; Kim, K.Y. Antifungal activity of magnoflorine against Candida strains. World J. Microb. Biot. 2018, 34, 167. [Google Scholar] [CrossRef]
- Kot, B.; Sytykiewicz, H.; Sprawka, I.; Witeska, M. Effect of trans-cinnamaldehyde on methicillin-resistant Staphylococcus aureus biofilm formation: Metabolic activity assessment and analysis of the biofilm-associated genes expression. Int. J. Mol. Sci. 2020, 21, 102. [Google Scholar] [CrossRef] [PubMed]
- Benjemaa, M.; Neves, M.A.; Falleh, H.; Lsoda, H.; Ksouri, R.; Nakajima, M. Nanoencapsulation of Thymus capitatus essential oil: Formulation process, physical stability characterization and antibacterial efficiency monitoring. Ind. Crop. Prod. 2018, 113, 414–421. [Google Scholar] [CrossRef]
- Taghipour, Y.D.; Hajialyani, M.; Naseri, R.; Hesari, M.; Mohammadi, P.; Stefanucci, A.; Mollica, A.; Farzaei, M.H.; Abdollahi, M. Nanoformulations of natural products for management of metabolic syndrome. Int. J. Nanomed. 2019, 14, 5303–5321. [Google Scholar] [CrossRef]
- Lodhi, G.; Kim, Y.S.; Hwang, J.W.; Kim, S.K.; Jeon, Y.J.; Je, J.Y.; Ahn, C.B.; Moon, S.H.; Jeon, B.T.; Park, P.J. Chitooligosaccharide and its derivatives: Preparation and biological applications. BioMed Res. Int. 2014, 2014, 654913. [Google Scholar] [CrossRef]
- Kashyap, P.L.; Xiang, X.; Heiden, P. Chitosan nanoparticle based delivery systems for sustainable agriculture. Int. J. Biol. Macromol. 2015, 77, 36–51. [Google Scholar] [CrossRef]
- Youssef, A.M.; Abou-Yousef, H.; El-Sayed, S.M.; Kamel, S. Mechanical and antibacterial properties of novel high performance chitosan/nanocomposite films. Int. J. Biol. Macromol. 2015, 76, 25–32. [Google Scholar] [CrossRef]
- Haldorai, Y.; Shim, J.J. Chitosan-Zinc oxide hybrid composite for enhanced dye degradation and antibacterial activity. Compos. Interface 2013, 20, 365–377. [Google Scholar] [CrossRef]
- Liang, J.; Yan, H.; Puligundla, P.; Gao, X.; Zhou, Y.; Wan, X. Applications of chitosan nanoparticles to enhance absorption and bioavailability of tea polyphenols: A review. Food Hydrocolloid. 2017, 69, 286–292. [Google Scholar] [CrossRef]
- Karthik, C.S.; Chethana, M.H.; Manukumar, H.M.; Ananda, A.P.; Sandeep, S.; Nagashree, S.; Mallesha, L.; Mallu, P.; Jayanth, H.S.; Dayananda, B.P. Synthesis and characterization of chitosan silver nanoparticle decorated with benzodioxane coupled piperazine as an effective anti-biofilm agent against MRSA: A validation of molecular docking and dynamics. Int. J. Biol. Macromol. 2021, 181, 540–551. [Google Scholar] [CrossRef]
- Elgadir, M.A.; Uddin, M.S.; Ferdosh, S.; Adam, A.; Chowdhury, A.J.K.; Sarker, M.Z.I. Impact of chitosan composites and chitosan nanoparticle composites on various drug delivery systems: A review. J. Food Drug Anal. 2015, 23, 619–629. [Google Scholar] [CrossRef]
- Zamborini, F.P.; Bao, L.; Dasari, R. Nanoparticles in measurement science. Anal. Chem. 2012, 84, 541–576. [Google Scholar] [CrossRef]
- Tan, Y.; Ma, S.; Leonhard, M.; Moser, D.; Schneider-Stickler, B. β-1,3-glucanase disrupts biofilm formation and increases antifungal susceptibility of Candida albicans DAY185. Int. J. Biol. Macromol. 2018, 108, 942–946. [Google Scholar] [CrossRef]
- Ye, H.; Shen, S.; Xu, J.; Lin, S.; Yuan, Y.; Jones, G.S. Synergistic interactions of cinnamaldehyde in combination with carvacrol against food-borne bacteria. Food Control 2013, 34, 619–623. [Google Scholar] [CrossRef]
- Forier, K.; Raemdonck, K.; De Smedt, S.C.; Demeester, J.; Coenye, T.; Braeckmans, K. Lipid and polymer nanoparticles for drug delivery to bacterial biofilms. J. Control. Release 2014, 190, 607–623. [Google Scholar] [CrossRef] [PubMed]
- Bellich, B.; D’Agostino, I.; Semeraro, S.; Gamini, A.; Cesaro, A. “The good, the bad and the ugly” of chitosans. Mar. Drugs 2016, 14, 99. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An update on potential biomedical and pharmaceutical applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef]
- Mu, H.; Guo, F.; Niu, H.; Liu, Q.; Wang, S.; Duan, J. Chitosan improves anti-biofilm efficacy of gentamicin through facilitating antibiotic penetration. Int. J. Mol. Sci. 2014, 15, 22296–22308. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Deng, Y.; Yeh, C.K.; Sun, Y. Quaternized chitosans bind onto preexisting biofilms and eradicate pre-attached microorganisms. J. Mater Chem. B 2014, 2, 8518–8527. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Hamblin, M.R.; Kishen, A. Characterization of a conjugate between Rose Bengal and chitosan for targeted antibiofilm and tissue stabilization effects as a potential treatment of infected dentin. Antimicrob. Agents Ch. 2012, 56, 4876–4884. [Google Scholar] [CrossRef]
- Inoue, Y.; Shiraishi, A.; Hada, T.; Hirose, K.; Hamashima, H.; Shimada, J. The antibacterial effects of terpene alcohols on Staphylococcus aureus and their mode of action. FEMS Microbiol. Lett. 2006, 237, 325–331. [Google Scholar] [CrossRef]
- Frohling, A.; Baier, M.; Ehlbeck, J.; Knorr, D.; Schlüter, O. Atmospheric pressure plasma treatment of Listeria innocua and Escherichia coli at polysaccharide surfaces: Inactivation kinetics and flow cytometric characterization. Innov. Food Sci. Emerg. 2012, 13, 142–150. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Chandra, J.; Kuhn, D.M.; Ghannoum, M.A. Mechanism of fluconazole resistance in Candida albicans biofilms: Phase-specific role of efflux pumps and membrane sterols. Infect. Immun. 2003, 71, 4333–4340. [Google Scholar] [CrossRef] [PubMed]
- Borezée-Durant, E.; Hiron, A.; Piard, J.C.; Juillard, V. Dual role of the oligopeptide permease opp3 during growth of staphylococcus aureus in milk. Appl. Environ. Microb. 2009, 75, 3355–3357. [Google Scholar] [CrossRef]
- Wesson, C.A.; Liou, L.E.; Todd, K.M.; Bohach, G.A.; Trumble, W.R.; Bayles, K.W. Staphylococcus aureus agr and sar global regulators influence internalization and induction of apoptosis. Infect. Immun. 1998, 66, 5238–5243. [Google Scholar] [CrossRef]
- Klinger-Strobel, M.; Ernst, J.; Lautenschlaeger, C.; Pletz, M.W.; Fischer, D.; Makarewicz, O. A blue fluorescent labeling technique utilizing micro-and nanoparticles for tracking in Live/Dead® stained pathogenic biofilms of Staphylococcus aureus and Burkholderia cepacia. Int. J. Nanomed. 2016, 11, 575–583. [Google Scholar] [CrossRef][Green Version]
- Ji, D.B.; Zhang, L.Y.; Li, C.L.; Ye, J.; Zhu, H.B. Effect of Hydroxysafflor yellow A on human umbilical vein endothelial cells under hypoxia. Vasc. Pharmacol. 2009, 50, 137–145. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Lin, Q.; Sheng, M.; Ding, T.; Li, B.; Gao, Y.; Tan, Y. Antibiofilm Effect of Cinnamaldehyde-Chitosan Nanoparticles against the Biofilm of Staphylococcus aureus. Antibiotics 2022, 11, 1403. https://doi.org/10.3390/antibiotics11101403
Xu J, Lin Q, Sheng M, Ding T, Li B, Gao Y, Tan Y. Antibiofilm Effect of Cinnamaldehyde-Chitosan Nanoparticles against the Biofilm of Staphylococcus aureus. Antibiotics. 2022; 11(10):1403. https://doi.org/10.3390/antibiotics11101403
Chicago/Turabian StyleXu, Jiaman, Quan Lin, Maokun Sheng, Ting Ding, Bing Li, Yan Gao, and Yulong Tan. 2022. "Antibiofilm Effect of Cinnamaldehyde-Chitosan Nanoparticles against the Biofilm of Staphylococcus aureus" Antibiotics 11, no. 10: 1403. https://doi.org/10.3390/antibiotics11101403
APA StyleXu, J., Lin, Q., Sheng, M., Ding, T., Li, B., Gao, Y., & Tan, Y. (2022). Antibiofilm Effect of Cinnamaldehyde-Chitosan Nanoparticles against the Biofilm of Staphylococcus aureus. Antibiotics, 11(10), 1403. https://doi.org/10.3390/antibiotics11101403





