Antibiotic Resistance of Selected Bacteria after Treatment of the Supragingival Biofilm with Subinhibitory Chlorhexidine Concentrations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Passaging of Supragingival Plaque with Subinhibitory Concentrations of CHX and Isolation of Bacteria
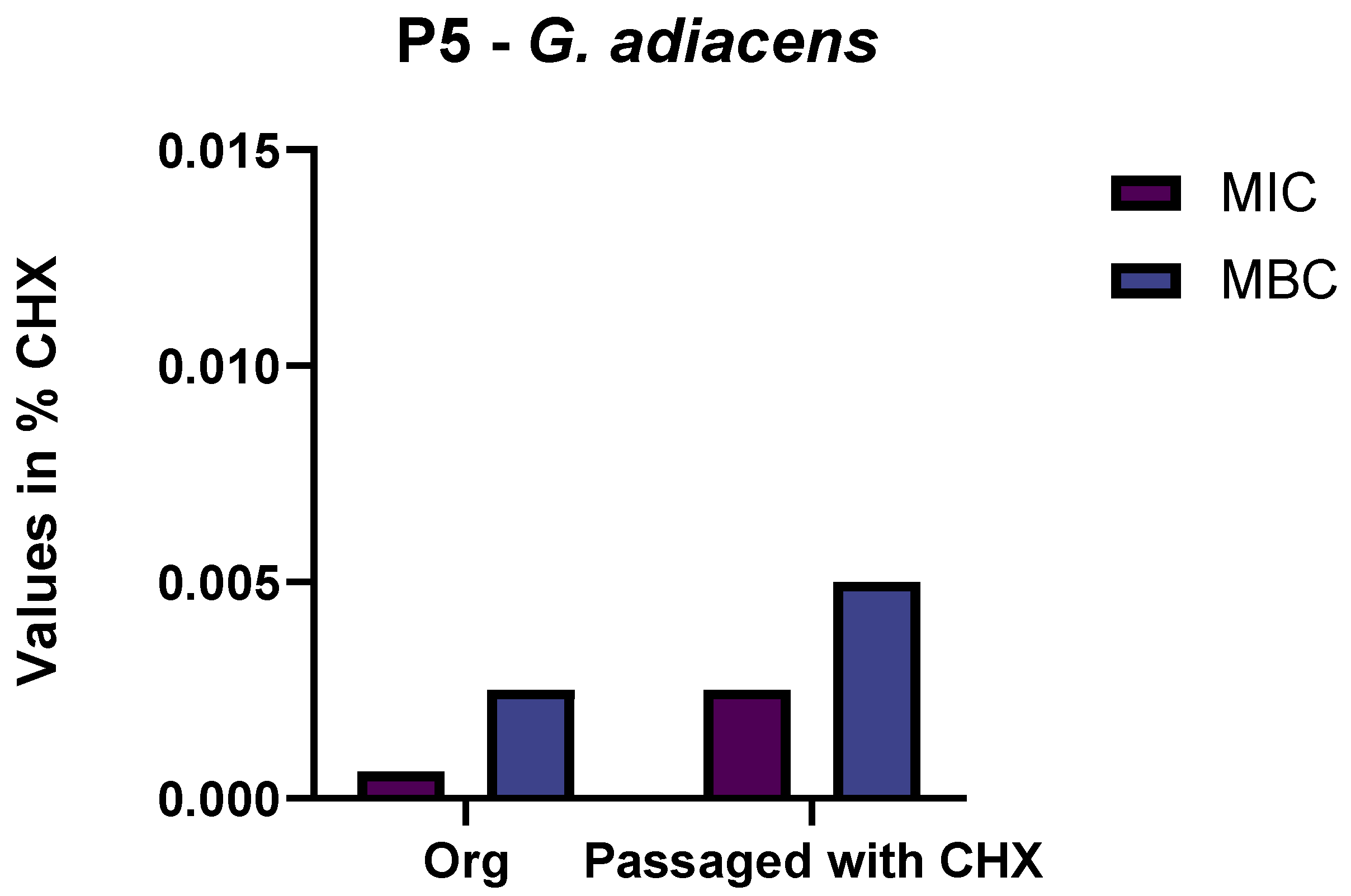
2.3. Minimum Inhibitory Concentrations (MICs) of Bacterial Isolates before and after Passaging with CHX
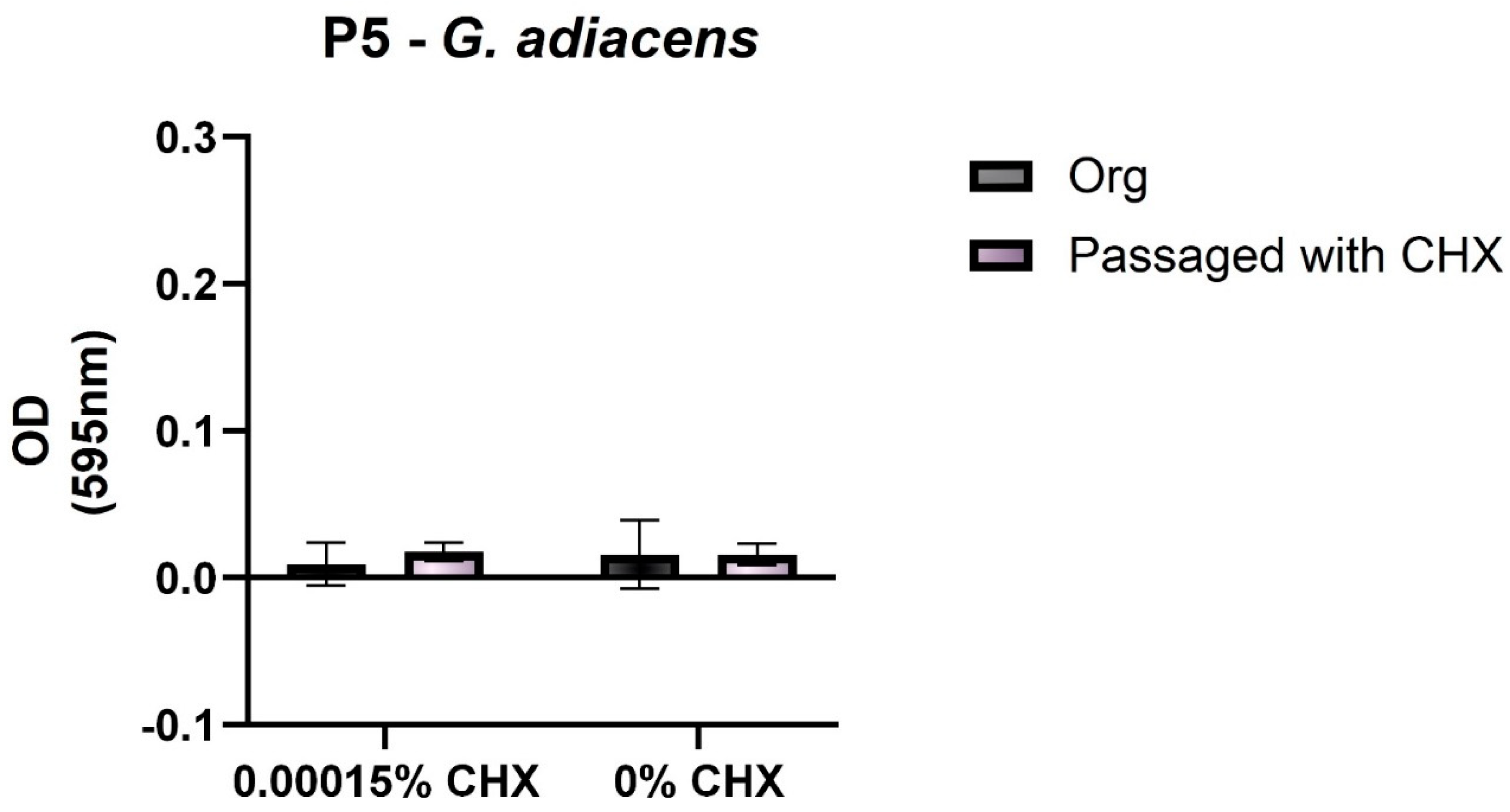
2.4. Biofilm Forming Capacity
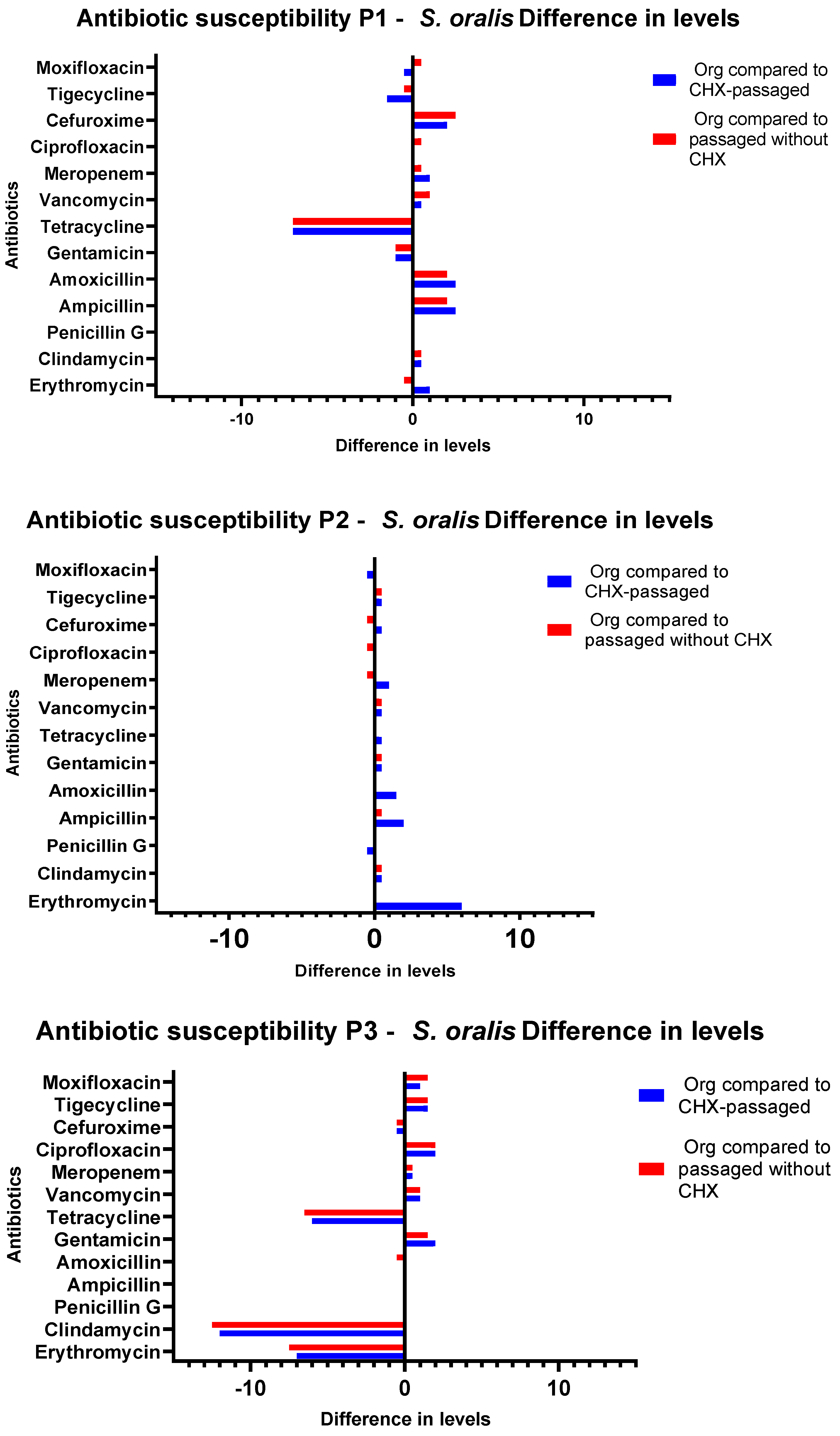
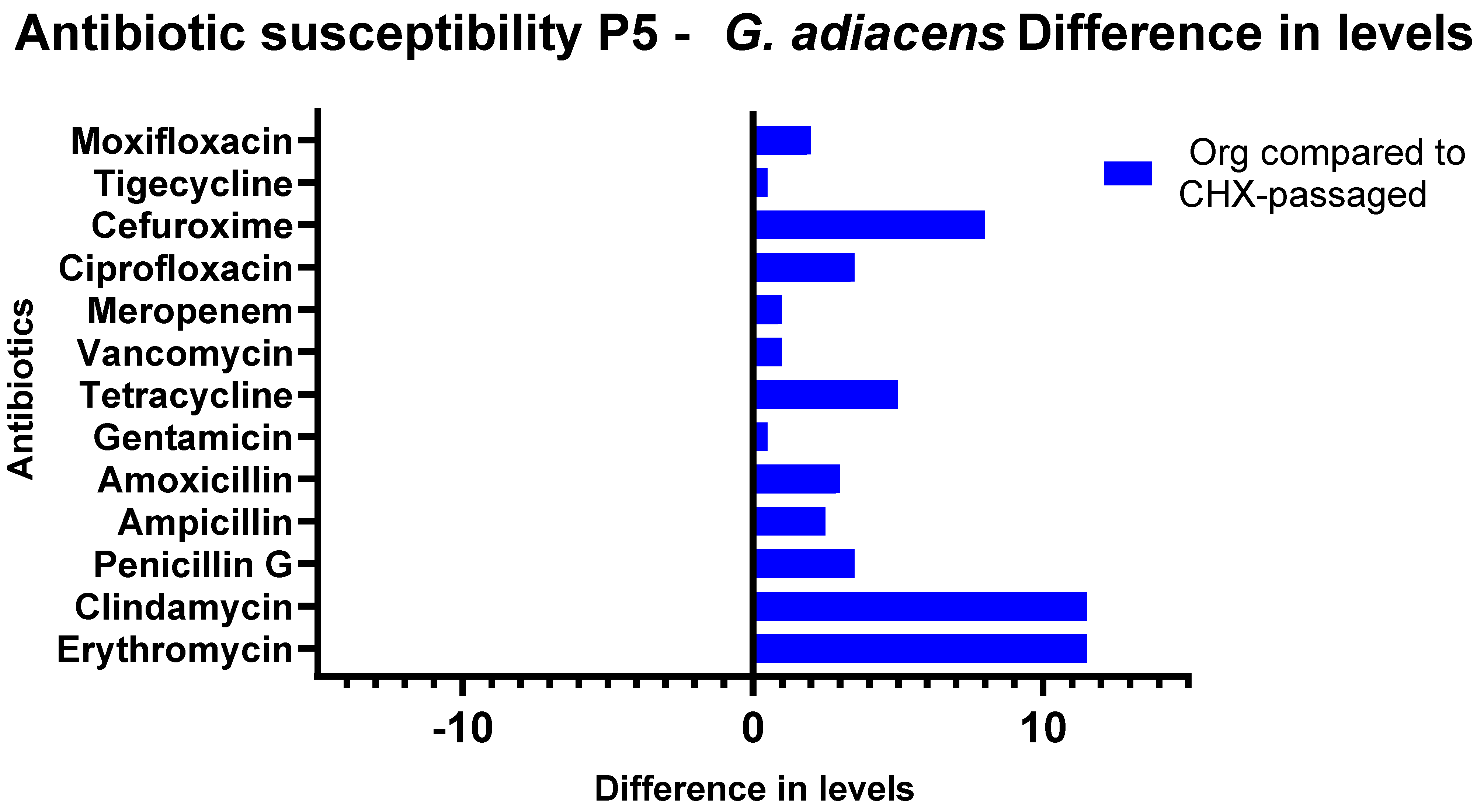
2.5. Antibiotic Susceptibility Testing
2.6. Statistical Analysis
3. Results
3.1. Selection of Isolates by Passaging in Sub-Inhibitory Concentrations of CHX
3.2. Biofilm-Forming Capacity
3.3. Antibiotic Susceptibility Testing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antimicrobial Resistance: Global Report on Surveillance. Available online: https://www.who.int/publications-detail-redirect/9789241564748 (accessed on 10 February 2022).
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. 2016. Available online: https://books.google.co.in/books/about/Tackling_Drug_resistant_Infections_Globa.html?id=aa6lAQAACAAJ (accessed on 12 February 2022).
- D’Costa, V.M.; King, C.E.; Kalan, L.; Morar, M.; Sung, W.W.L.; Schwarz, C.; Froese, D.; Zazula, G.; Calmels, F.; Debruyne, R.; et al. Antibiotic resistance is ancient. Nature 2011, 477, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Kaoutar, B.; Joly, C.; L’Heriteau, F.; Barbut, F.; Robert, J.; Denis, M.; Espinasse, F.; Merrer, J.; Doit, C.; Costa, Y.; et al. Nosocomial infections and hospital mortality: A multicentre epidemiological study. J. Hosp. Infect. 2004, 58, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Wand, M.E.; Bock, L.J.; Bonney, L.C.; Sutton, J.M. Mechanisms of Increased Resistance to Chlorhexidine and Cross-Resistance to Colistin following Exposure of Klebsiella pneumoniae Clinical Isolates to Chlorhexidine. Antimicrob. Agents Chemother. 2017, 61, e01162-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, X.; Auer, D.L.; Buchalla, W.; Hiller, K.-A.; Maisch, T.; Hellwig, E.; Al-Ahmad, A.; Cieplik, F. Cetylpyridinium Chloride: Mechanism of Action, Antimicrobial Efficacy in Biofilms, and Potential Risks of Resistance. Antimicrob. Agents Chemother. 2020, 64, e00576-20. [Google Scholar] [CrossRef]
- Muehler, D.; Mao, X.; Czemmel, S.; Geißert, J.; Engesser, C.; Hiller, K.-A.; Widbiller, M.; Maisch, T.; Buchalla, W.; Al-Ahmad, A.; et al. Transcriptomic Stress Response in Streptococcus mutans following Treatment with a Sublethal Concentration of Chlorhexidine Digluconate. Microorganisms 2022, 10, 561. [Google Scholar] [CrossRef] [PubMed]
- Cieplik, F.; Jakubovics, N.S.; Buchalla, W.; Maisch, T.; Hellwig, E.; Al-Ahmad, A. Resistance Toward Chlorhexidine in Oral Bacteria—Is There Cause for Concern? Front. Microbiol. 2019, 10, 587. [Google Scholar] [CrossRef] [Green Version]
- Verspecht, T.; Rodriguez Herrero, E.; Khodaparast, L.; Khodaparast, L.; Boon, N.; Bernaerts, K.; Quirynen, M.; Teughels, W. Development of antiseptic adaptation and cross-adapatation in selected oral pathogens in vitro. Sci. Rep. 2019, 9, 8326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langsrud, S.; Sundheim, G.; Holck, A.L. Cross-resistance to antibiotics of Escherichia coli adapted to benzalkonium chloride or exposed to stress-inducers. J. Appl. Microbiol. 2004, 96, 201–208. [Google Scholar] [CrossRef]
- Kim, M.; Hatt, J.K.; Weigand, M.R.; Krishnan, R.; Pavlostathis, S.G.; Konstantinidis, K.T. Genomic and Transcriptomic Insights into How Bacteria Withstand High Concentrations of Benzalkonium Chloride Biocides. Appl. Environ. Microbiol. 2018, 84, e00197-18. [Google Scholar] [CrossRef] [Green Version]
- Figuero, E.; Herrera, D.; Tobías, A.; Serrano, J.; Roldán, S.; Escribano, M.; Martín, C. Efficacy of adjunctive anti-plaque chemical agents in managing gingivitis: A systematic review and network meta-analyses. J. Clin. Periodontol. 2019, 46, 723–739. [Google Scholar] [CrossRef]
- ten Cate, J.M. The Need for Antibacterial Approaches to Improve Caries Control. Adv. Dent. Res. 2009, 21, 8–12. [Google Scholar] [CrossRef]
- Jones, C.G. Chlorhexidine: Is it still the gold standard? Periodontology 2000 1997, 15, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Auer, D.L.; Mao, X.; Anderson, A.C.; Muehler, D.; Wittmer, A.; von Ohle, C.; Wolff, D.; Frese, C.; Hiller, K.-A.; Maisch, T.; et al. Phenotypic Adaptation to Antiseptics and Effects on Biofilm Formation Capacity and Antibiotic Resistance in Clinical Isolates of Early Colonizers in Dental Plaque. Antibiotics 2022, 11, 688. [Google Scholar] [CrossRef]
- Jakubovics, N.S.; Goodman, S.D.; Mashburn-Warren, L.; Stafford, G.P.; Cieplik, F. The dental plaque biofilm matrix. Periodontology 2000 2021, 86, 32–56. [Google Scholar] [CrossRef] [PubMed]
- Madsen, J.S.; Burmølle, M.; Hansen, L.H.; Sørensen, S.J. The interconnection between biofilm formation and horizontal gene transfer. FEMS Immunol. Med. Microbiol. 2012, 65, 183–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowler, P.; Murphy, C.; Wolcott, R. Biofilm exacerbates antibiotic resistance: Is this a current oversight in antimicrobial stewardship? Antimicrob. Resist. Infect. Control. 2020, 9, 162. [Google Scholar] [CrossRef]
- Mao, X.; Hiergeist, A.; Auer, D.L.; Scholz, K.J.; Muehler, D.; Hiller, K.-A.; Maisch, T.; Buchalla, W.; Hellwig, E.; Gessner, A.; et al. Ecological Effects of Daily Antiseptic Treatment on Microbial Composition of Saliva-Grown Microcosm Biofilms and Selection of Resistant Phenotypes. Front. Microbiol. 2022, 13, 934525. [Google Scholar] [CrossRef]
- Kampf, G. Antiseptic Stewardship: Biocide Resistance and Clinical Implications; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-98784-2. [Google Scholar]
- Vijayakumar, R.; Sandle, T. A review on biocide reduced susceptibility due to plasmid-borne antiseptic-resistant genes—Special notes on pharmaceutical environmental isolates. J. Appl. Microbiol. 2019, 126, 1011–1022. [Google Scholar] [CrossRef] [Green Version]
- Baker, P.J.; Coburn, R.A.; Genco, R.J.; Evans, R.T. Structural Determinants of Activity of Chlorhexidine and Alkyl Bisbiguanides against the Human Oral Flora. J. Dent. Res. 1987, 66, 1099–1106. [Google Scholar] [CrossRef]
- Souza-Júnior, S.; Castro-Prado, M. Chlorhexidine digluconate induces mitotic recombination in diploid cells of Aspergillus nidulans: The recombinogenic effect of CLX. Oral Dis. 2005, 11, 146–150. [Google Scholar] [CrossRef]
- Suessmuth, R.; Ackermann, B.; Lingens, F. Mutagenic effect of 1.1′-hexamethylene-bis-[(5-p-chlorophenyl)-biguanide]. Chem. Biol. Interact. 1979, 28, 249–258. [Google Scholar] [CrossRef]
- Sakagami, Y.; Yamazaki, H.; Ogasawara, N.; Yokoyama, H.; Ose, Y.; Sato, T. The evaluation of genotoxic activities of disinfectants and their metabolites by umu test. Mutat. Res. Lett. 1988, 209, 155–160. [Google Scholar] [CrossRef]
- Anderson, A.C.; Sanunu, M.; Schneider, C.; Clad, A.; Karygianni, L.; Hellwig, E.; Al-Ahmad, A. Rapid species-level identification of vaginal and oral lactobacilli using MALDI-TOF MS analysis and 16S rDNA sequencing. BMC Microbiol. 2014, 14, 312. [Google Scholar] [CrossRef] [Green Version]
- Weinstein, M.P.; Patel, J.B. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically: M07-A11, 11th ed.; Documents/Clinical and Laboratory Standards Institute; Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2018; ISBN 978-1-56238-836-2. [Google Scholar]
- Bernardi, S.; Anderson, A.; Macchiarelli, G.; Hellwig, E.; Cieplik, F.; Vach, K.; Al-Ahmad, A. Subinhibitory Antibiotic Concentrations Enhance Biofilm Formation of Clinical Enterococcus faecalis Isolates. Antibiotics 2021, 10, 874. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahmad, A.; Ameen, H.; Pelz, K.; Karygianni, L.; Wittmer, A.; Anderson, A.C.; Spitzmüller, B.; Hellwig, E. Antibiotic Resistance and Capacity for Biofilm Formation of Different Bacteria Isolated from Endodontic Infections Associated with Root-filled Teeth. J. Endod. 2014, 40, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.C.; Jonas, D.; Huber, I.; Karygianni, L.; Wölber, J.; Hellwig, E.; Arweiler, N.; Vach, K.; Wittmer, A.; Al-Ahmad, A. Enterococcus faecalis from Food, Clinical Specimens, and Oral Sites: Prevalence of Virulence Factors in Association with Biofilm Formation. Front. Microbiol. 2016, 6, 1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitagawa, H.; Izutani, N.; Kitagawa, R.; Maezono, H.; Yamaguchi, M.; Imazato, S. Evolution of resistance to cationic biocides in Streptococcus mutans and Enterococcus faecalis. J. Dent. 2016, 47, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.R.; Hirsch, S.; Hiergeist, A.; Kirschneck, C.; Muehler, D.; Hiller, K.-A.; Maisch, T.; Al-Ahmad, A.; Gessner, A.; Buchalla, W.; et al. Limited antimicrobial efficacy of oral care antiseptics in microcosm biofilms and phenotypic adaptation of bacteria upon repeated exposure. Clin. Oral Investig. 2021, 25, 2939–2950. [Google Scholar] [CrossRef]
- Keller, M.; Zengler, K. Tapping into microbial diversity. Nat. Rev. Microbiol. 2004, 2, 141–150. [Google Scholar] [CrossRef]
- Aas, J.A.; Paster, B.J.; Stokes, L.N.; Olsen, I.; Dewhirst, F.E. Defining the Normal Bacterial Flora of the Oral Cavity. J. Clin. Microbiol. 2005, 43, 5721–5732. [Google Scholar] [CrossRef]
- Stewart, E.J. Growing Unculturable Bacteria. J. Bacteriol. 2012, 194, 4151–4160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wieser, A.; Schneider, L.; Jung, J.; Schubert, S. MALDI-TOF MS in microbiological diagnostics-identification of microorganisms and beyond (mini review). Appl. Microbiol. Biotechnol. 2012, 93, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Fox, L.J.; Kelly, P.P.; Humphreys, G.J.; Waigh, T.A.; Lu, J.R.; McBain, A.J. Assessing the risk of resistance to cationic biocides incorporating realism-based and biophysical approaches. J. Ind. Microbiol. Biotechnol. 2022, 49, kuab074. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.S. Biocide resistance mechanisms. Int. Biodeterior. Biodegrad. 2003, 51, 133–138. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Hans, A.; Ruikar, K.; Guan, Z.; Palmer, K.L. Reduced Chlorhexidine and Daptomycin Susceptibility in Vancomycin-Resistant Enterococcus faecium after Serial Chlorhexidine Exposure. Antimicrob. Agents Chemother. 2018, 62, e01235-17. [Google Scholar] [CrossRef] [Green Version]
- Nyvad, B.; Kilian, M. Microflora associated with experimental root surface caries in humans. Infect. Immun. 1990, 58, 1628–1633. [Google Scholar] [CrossRef] [Green Version]
- Do, T.; Jolley, K.A.; Maiden, M.C.J.; Gilbert, S.C.; Clark, D.; Wade, W.G.; Beighton, D. Population structure of Streptococcus oralis. Microbiology 2009, 155, 2593. [Google Scholar] [CrossRef] [Green Version]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival Mechanisms of Clinically Relevant Microorganisms. Clin. Microbiol. Rev. 2002, 15, 27. [Google Scholar] [CrossRef] [Green Version]
- Willcox, M.D.; Drucker, D.B. Surface structures, co-aggregation and adherence phenomena of Streptococcus oralis and related species. Microbios 1989, 59, 19–29. [Google Scholar]
- Busscher, H.J.; Der Mei, R.B.H.C. Initial microbial adhesion is a determinant for the strength of biofilm adhesion. FEMS Microbiol. Lett. 1995, 128, 229–234. [Google Scholar] [CrossRef]
- Karched, M.; Bhardwaj, R.G.; Asikainen, S.E. Coaggregation and biofilm growth of Granulicatella spp. with Fusobacterium nucleatum and Aggregatibacter actinomycetemcomitans. BMC Microbiol. 2015, 15, 114. [Google Scholar] [CrossRef] [PubMed]
- Whiley, R.A.; Beighton, D. Current classification of the oral streptococci. Oral Microbiol. Immunol. 1998, 13, 195–216. [Google Scholar] [CrossRef] [PubMed]
- Alberti, M.O.; Hindler, J.A.; Humphries, R.M. Antimicrobial Susceptibilities of Abiotrophia defectiva, Granulicatella adiacens, and Granulicatella elegans. Antimicrob. Agents Chemother. 2016, 60, 1411–1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wisplinghoff, H.; Reinert, R.R.; Cornely, O.; Seifert, H. Molecular Relationships and Antimicrobial Susceptibilities of Viridans Group Streptococci Isolated from Blood of Neutropenic Cancer Patients. J. Clin. Microbiol. 1999, 37, 1876–1880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciofu, O.; Moser, C.; Jensen, P.Ø.; Høiby, N. Tolerance and resistance of microbial biofilms. Nat. Rev. Microbiol. 2022, 20, 621–635. [Google Scholar] [CrossRef]
- Roberts, M.C. Environmental macrolide–lincosamide–streptogramin and tetracycline resistant bacteria. Front. Microbiol. 2011, 2, 40. [Google Scholar] [CrossRef] [Green Version]
- Reflection Paper on the Use of Aminopenicillins and Their Beta-Lactamase Inhibitor Combinations in Animals in the European Union: Development of Resistance and Impact on Human and Animal Health. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-use-aminopenicillins-their-beta-lactamase-inhibitor-combinations-animals-european_en.pdf (accessed on 17 February 2022).
- Weiger, R.; Netuschil, L.; von Ohle, C.; Schlagenhauf, U.; Brecx, M. Microbial generation time during the early phases of supragingival dental plaque formation. Oral Microbiol. Immunol. 1995, 10, 93–97. [Google Scholar] [CrossRef]
- Burchard, T.; Karygianni, L.; Hellwig, E.; Wittmer, A.; Al-Ahmad, A. Microbial Composition of Oral Biofilms after Visible Light and Water-Filtered Infrared a Radiation (VIS+wIRA) in Combination with Indocyanine Green (ICG) as Photosensitizer. Antibiotics 2020, 9, 532. [Google Scholar] [CrossRef]
- Karygianni, L.; Al-Ahmad, A.; Argyropoulou, A.; Hellwig, E.; Anderson, A.C.; Skaltsounis, A.L. Natural Antimicrobials and Oral Microorganisms: A Systematic Review on Herbal Interventions for the Eradication of Multispecies Oral Biofilms. Front. Microbiol. 2016, 6, 1529. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Früh, R.; Anderson, A.; Cieplik, F.; Hellwig, E.; Wittmer, A.; Vach, K.; Al-Ahmad, A. Antibiotic Resistance of Selected Bacteria after Treatment of the Supragingival Biofilm with Subinhibitory Chlorhexidine Concentrations. Antibiotics 2022, 11, 1420. https://doi.org/10.3390/antibiotics11101420
Früh R, Anderson A, Cieplik F, Hellwig E, Wittmer A, Vach K, Al-Ahmad A. Antibiotic Resistance of Selected Bacteria after Treatment of the Supragingival Biofilm with Subinhibitory Chlorhexidine Concentrations. Antibiotics. 2022; 11(10):1420. https://doi.org/10.3390/antibiotics11101420
Chicago/Turabian StyleFrüh, Robin, Annette Anderson, Fabian Cieplik, Elmar Hellwig, Annette Wittmer, Kirstin Vach, and Ali Al-Ahmad. 2022. "Antibiotic Resistance of Selected Bacteria after Treatment of the Supragingival Biofilm with Subinhibitory Chlorhexidine Concentrations" Antibiotics 11, no. 10: 1420. https://doi.org/10.3390/antibiotics11101420
APA StyleFrüh, R., Anderson, A., Cieplik, F., Hellwig, E., Wittmer, A., Vach, K., & Al-Ahmad, A. (2022). Antibiotic Resistance of Selected Bacteria after Treatment of the Supragingival Biofilm with Subinhibitory Chlorhexidine Concentrations. Antibiotics, 11(10), 1420. https://doi.org/10.3390/antibiotics11101420






