Lipid Microenvironment Modulates the Pore-Forming Ability of Polymyxin B
Abstract
1. Introduction
2. Results and Discussion
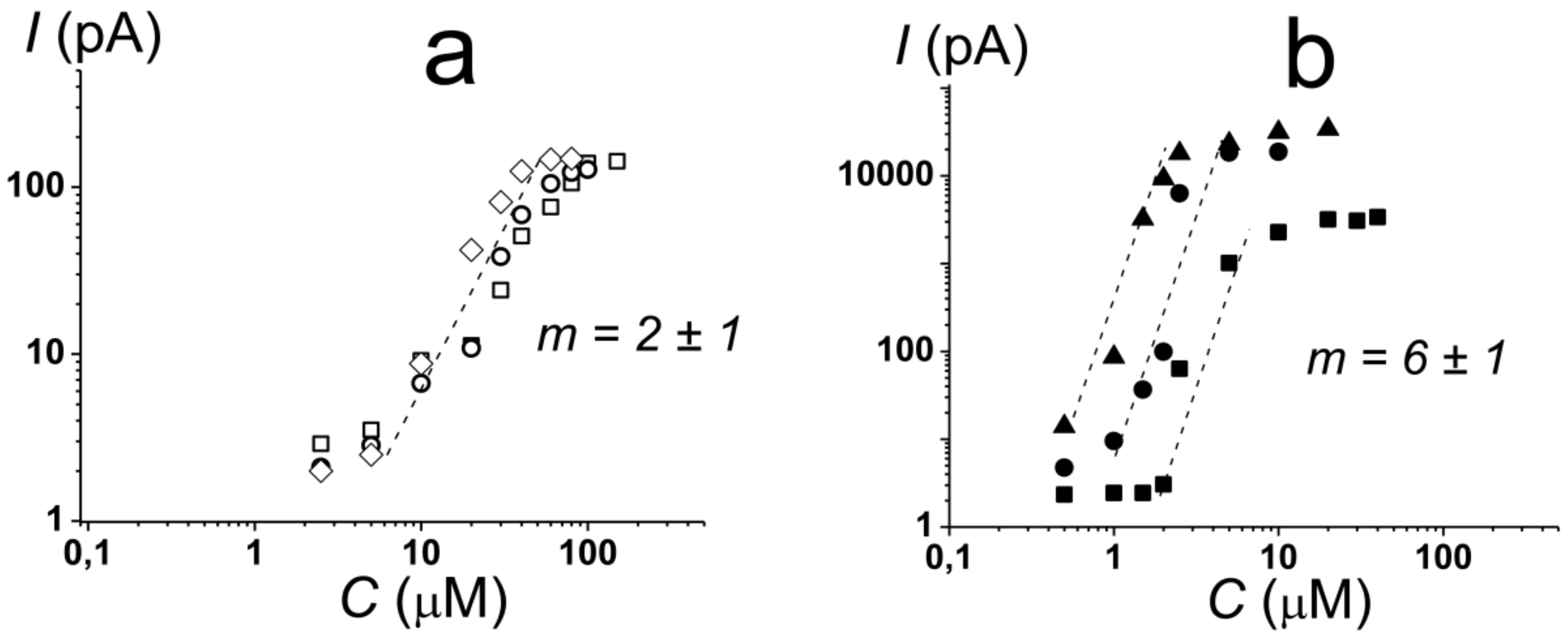
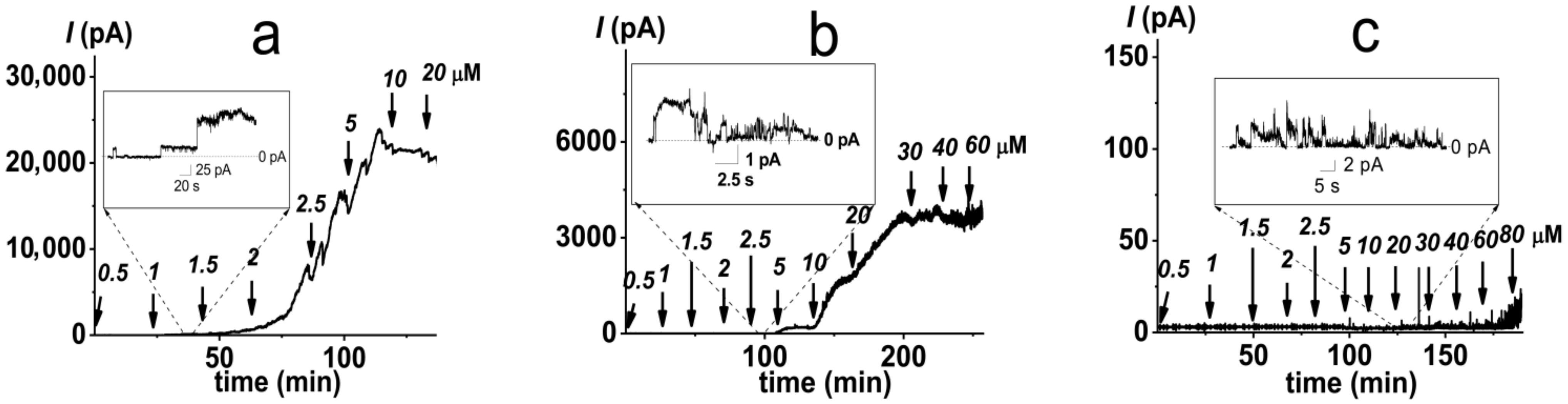
2.1. Pore-Forming Activity of Polymyxin B in Phospholipid Bilayers
2.2. Pore-Forming Activity of Polymyxin B in Lipopolysaccharide-Enriched Model Membranes
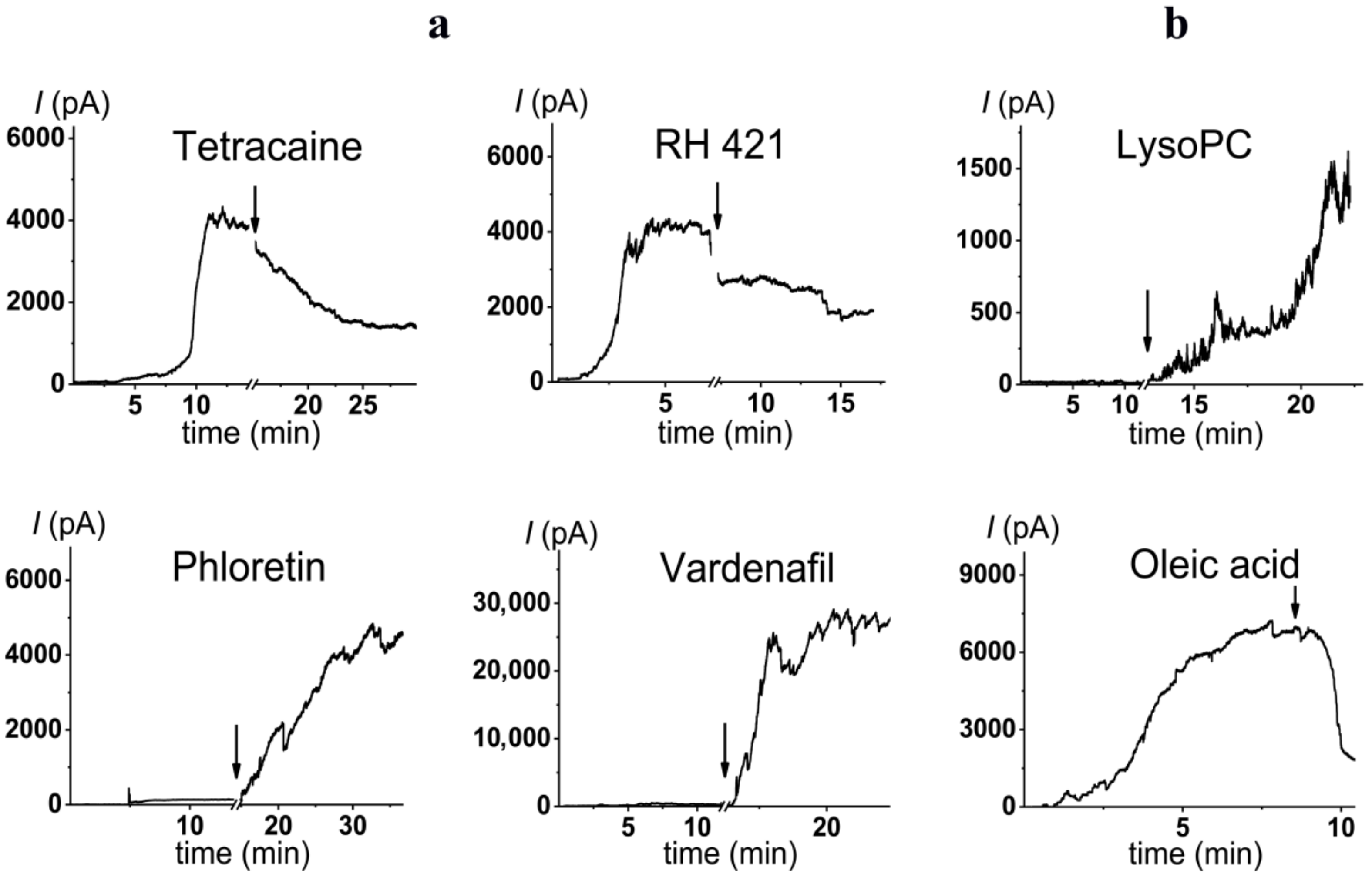
2.3. Alteration in Polymyxin B Pore-Forming Activity in the Presence of Small Molecules Modulating Membrane Physical Properties
3. Materials and Methods
3.1. Chemical Reagents
3.2. Studying PMB Pore-Forming Ability in Planar Lipid Bilayers
3.3. Electrophysiological Measurements of Changes in Membrane Boundary Potential Induced by Agonists and Antagonists of PMB Pore Formation
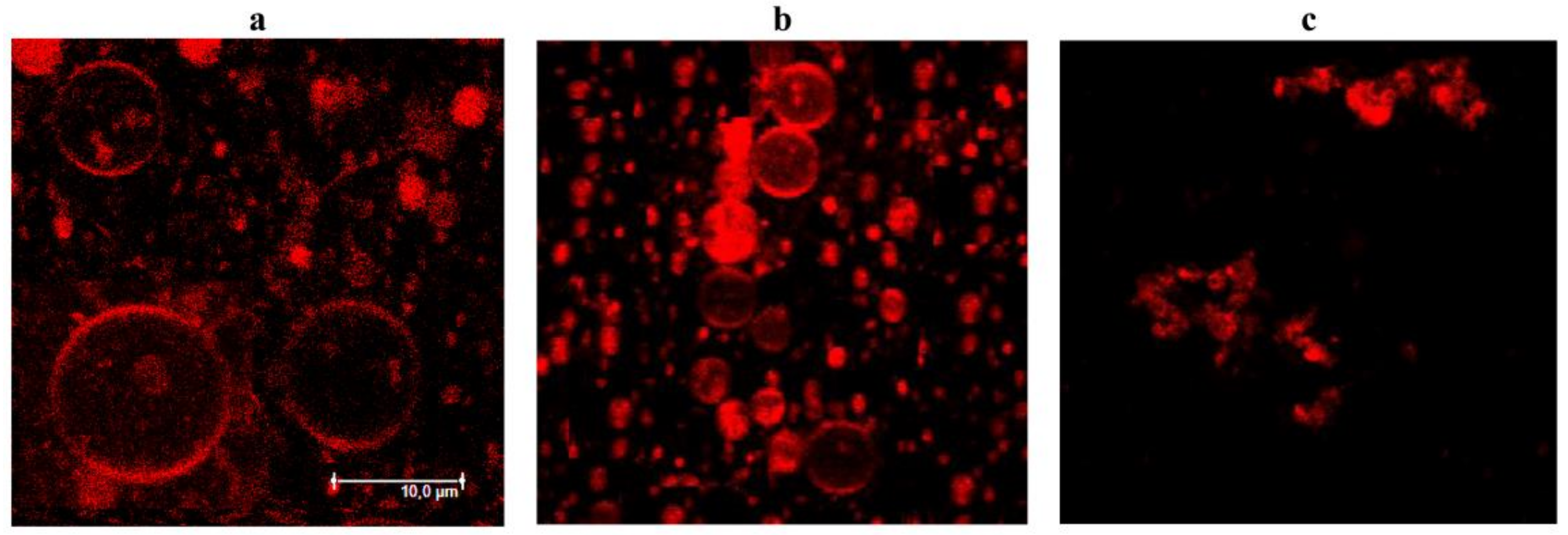
3.4. Confocal Fluorescence Microscopy
4. Conclusions
- (i)
- The type of negatively charged phospholipid (DOPG vs DOPS) does not affect the pore-forming activity of polymyxin B;
- (ii)
- The pore-forming activity of polymyxin B depends on the shape of the membrane lipids;
- (iii)
- Polymyxin B is assumed to produce toroidal lipopeptide-lipid pores;
- (iv)
- Polymyxin pores in DOPG and Kdo2-Lipid A membranes are characterized by different stoichiometries: dimers and hexamers are involved in pore formation in the absence and in the presence of lipopolysaccharides, respectively;
- (v)
- Small molecules diminishing membrane dipole potential and inducing positive curvature stress are agonists of pore formation by polymyxin B.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| PMB | polymyxin B |
| DOPC | 1,2-dioleoyl-sn-glycero-3-phosphocholine |
| DOPG | 1,2-dioleoyl-sn-glycero-3-phospho-(1’-rac-glycerol) |
| DOPS | 1,2-dioleoyl-sn-glycero-3-phospho-L-serine |
| TOCL | 1’,3’-bis[1,2-dioleoyl-sn-glycero-3-phospho]-glycerol |
| DPhPG | 1,2-diphytanoyl-sn-glycero-3-phospho-(1’-rac-glycerol) |
| DPhPC | 1,2-diphytanoyl-sn-glycero-3-phosphocholine |
| Kdo2-Lipid A | di[3-deoxy-D-manno-octulosonyl]-lipid A |
| Lipid A | detoxified lipid A from Salmonella minnesota R595 |
| LysoPC | 1-palmitoyl-2-hydroxy-sn-glycero-3-phosphocholine |
References
- Li, J.; Nation, R.L.; Milne, R.W.; Turnidge, J.D.; Coulthard, K. Evaluation of colistin as an agent against multi-resistant Gram-negative bacteria. Int. J. Antimicrob. Agents 2005, 25, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Landman, D.; Georgescu, C.; Martin, D.A.; Quale, J. Polymyxins revisited. Clin. Microbiol. Rev. 2008, 21, 449–465. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.E.; Feola, D.J.; Rapp, R.P. Polymyxin B sulfate and colistin: Old antibiotics for emerging multiresistant Gram-negative bacteria. Ann. Pharmacother. 1999, 33, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Hermsen, E.D.; Sullivan, C.J.; Rotschafer, J.C. Polymyxins: Pharmacology, pharmacokinetics, pharmacodynamics, and clinical applications. Infect. Dis. Clin. North. Am. 2003, 17, 545–562. [Google Scholar] [CrossRef]
- Gales, A.C.; Jones, R.N.; Sader, H.S. Global assessment of the antimicrobial activity of polymyxin B against 54 731 clinical isolates of Gram-negative bacilli: Report from the SENTRY antimicrobial surveillance programme (2001–2004). Clin. Microbiol. Infect. 2006, 12, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Gales, A.C.; Reis, A.O.; Jones, R.N. Contemporary assessment of antimicrobial susceptibility testing methods for polymyxin B and colistin: Review of available interpretative criteria and quality control guidelines. J. Clin. Microbiol. 2001, 39, 183–190. [Google Scholar] [CrossRef]
- Storm, D.R.; Rosenthal, K.S.; Swanson, P.E. Polymyxin and related peptide antibiotics. Annu. Rev. Biochem. 1977, 46, 723–763. [Google Scholar] [CrossRef] [PubMed]
- Catchpole, C.R.; Andrews, J.M.; Brenwald, N.; Wise, R. A reassessment of the in-vitro activity of colistin sulphomethate sodium. J. Antimicrob. Chemother. 1997, 39, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Landman, D.; Salamera, J.; Quale, J. Irreproducible and uninterpretable Polymyxin B MICs for Enterobacter cloacae and Enterobacter aerogenes. J. Clin. Microbiol. 2013, 51, 4106–4111. [Google Scholar] [CrossRef]
- Warren, G.H.; Gray, J.; Yurchenco, J.A. Effect of polymyxin on the lysis of Neisseria catarrhalis by lysozyme. J. Bacteriol. 1957, 74, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Koike, M.; Iida, K.; Matsuo, T. Electron microscopic studies on mode of action of polymyxin. J. Bacteriol. 1969, 97, 448–452. [Google Scholar] [CrossRef]
- Rosenthal, K.S.; Storm, D.R. Disruption of the Escherichia coli outer membrane permeability barrier by immobilized polymyxin B. J. Antibiot. 1977, 30, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- David, S.A.; Balasubramanian, K.A.; Mathan, V.I.; Balaram, P. Analysis of the binding of polymyxin B to endotoxic lipid A and core glycolipid using a fluorescent displacement probe. Biochim. Biophys. Acta 1992, 1165, 147–152. [Google Scholar] [CrossRef]
- Srimal, S.; Surolia, N.; Balasubramanian, S.; Surolia, A. Titration calorimetric studies to elucidate the specificity of the interactions of polymyxin B with lipopolysaccharides and lipid A. Biochem. J. 1996, 315, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.J.; Gangadhar, B.P.; Surolia, N.; Surolia, A. Kinetics and mechanism of the recognition of endotoxin by polymyxin b. J. Am. Chem. Soc. 1998, 120, 12428–12434. [Google Scholar] [CrossRef]
- Moore, R.A.; Bates, N.C.; Hancock, R.E. Interaction of polycationic antibiotics with Pseudomonas aeruginosa lipopolysaccharide and lipid A studied by using dansyl-polymyxin. Antimicrob. Agents Chemother. 1986, 29, 496–500. [Google Scholar] [CrossRef]
- Thomas, C.J.; Surolia, A. Kinetics of the interaction of endotoxin with polymyxin B and its analogs: A surface plasmon resonance analysis. FEBS Lett. 1999, 445, 420–424. [Google Scholar] [CrossRef]
- Thomas, C.J.; Surolia, N.; Surolia, A. Surface plasmon resonance studies resolve the enigmatic endotoxin neutralizing activity of polymyxin B. J. Biol. Chem. 1999, 274, 29624–29627. [Google Scholar] [CrossRef] [PubMed]
- Mares, J.; Kumaran, S.; Gobbo, M.; Zerbe, O. Interactions of lipopolysaccharide and polymyxin studied by NMR spectroscopy. J. Biol. Chem. 2009, 284, 11498–11506. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.I.; Hu, H.; Moake, M.M.; Churey, J.J.; Whittal, R.; Worobo, R.W.; Vederas, J.C. Isolation, structural characterization, and properties of mattacin (polymyxin M), a cyclic peptide antibiotic produced by Paenibacillus kobensis M. J. Biol. Chem. 2003, 278, 13124–13132. [Google Scholar] [CrossRef]
- Meredith, J.J.; Dufour, A.; Bruch, M.D. Comparison of the structure and dynamics of the antibiotic peptide polymyxin B and the inactive nonapeptide in aqueous trifluoroethanol by NMR spectroscopy. J. Phys. Chem. B 2009, 113, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Pristovsek, P.; Kidric, J. Solution structure of polymyxins B and E and effect of binding to lipopolysaccharide: An NMR and molecular modeling study. J. Med. Chem. 1999, 42, 4604–4613. [Google Scholar] [CrossRef] [PubMed]
- Pristovsek, P.; Kidric, J. The search for molecular determinants of LPS inhibition by proteins and peptides. Curr. Top. Med. Chem. 2004, 4, 1185–1201. [Google Scholar] [CrossRef]
- Santos, D.E.S.; Pol-Fachin, L.; Lins, R.D.; Soares, T.A. Polymyxin binding to the bacterial outer membrane reveals cation displacement and increasing membrane curvature in susceptible but not in resistant lipopolysaccharide chemotypes. J. Chem. Inf. Model. 2017, 57, 2181–2193. [Google Scholar] [CrossRef]
- Hancock, R.E. Antibacterial peptides and the outer membranes of Gram-negative bacilli. J. Med. Microbiol. 1997, 46, 1–3. [Google Scholar] [CrossRef]
- Hancock, R.E.; Lehrer, R. Cationic peptides: A new source of antibiotics. Trends Biotechnol. 1998, 16, 82–88. [Google Scholar] [CrossRef]
- Clausell, A.; Garcia-Subirats, M.; Pujol, M.; Busquets, M.A.; Rabanal, F.; Cajal, Y. Gram-negative outer and inner membrane models: Insertion of cyclic cationic lipopeptides. J. Phys. Chem. B 2007, 111, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.P.; Hancock, R.E. The relationship between peptide structure and antibacterial activity. Peptides 2003, 24, 1681–1691. [Google Scholar] [CrossRef]
- Bruch, M.D.; Cajal, Y.; Koh, J.T.; Jain, M.K. Higher-order structure of polymyxin B: The functional significance of topological flexibility. J. Am. Chem. Soc. 1999, 121, 11993–12004. [Google Scholar] [CrossRef]
- Schröder, G.; Brandenburg, K.; Seydel, U. Polymyxin B induces transient permeability fluctuations in asymmetric planar lipopolysaccharide/phospholipid bilayers. Biochemistry 1992, 31, 631–638. [Google Scholar] [CrossRef]
- Gutsmann, T.; Hagge, S.O.; David, A.; Roes, S.; Böhling, A.; Hammer, M.U.; Seydel, U. Lipid-mediated resistance of Gram-negative bacteria against various pore-forming antimicrobial peptides. J. Endotoxin. Res. 2005, 11, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Champlin, F.R.; Gilleland, H.E., Jr.; Conrad, R.S. Conversion of phospholipids to free fatty acids in response to acquisition of polymyxin resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1983, 24, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Acket, S.; Beaumont, E.; Galez, H.; Duma, L.; Rossez, Y. Colistin treatment affects lipid composition of Acinetobacter baumannii. Antibiotics 2021, 10, 528. [Google Scholar] [CrossRef] [PubMed]
- Imai, M.; Inoue, K.; Nojima, S. Effect of polymyxin B on liposomal membranes derived from Escherichia. coli lipids. Biochim. Biophys. Acta 1975, 375, 130–137. [Google Scholar] [CrossRef]
- Miller, I.R.; Bach, D.; Teuber, M. Effect of polymyxin B on the structure and the stability of lipid layers. J. Membr. Biol. 1978, 39, 49–56. [Google Scholar] [CrossRef]
- Boggs, J.M.; Rangaraj, G. Phase transitions and fatty acid spin label behavior in interdigitated lipid phases induced by glycerol and polymyxin. Biochim. Biophys. Acta 1985, 816, 221–233. [Google Scholar] [CrossRef]
- Fu, L.; Wan, M.; Zhang, S.; Gao, L.; Fang, W. Polymyxin B loosens lipopolysaccharide bilayer but stiffens phospholipid bilayer. Biophys. J. 2020, 118, 138–150. [Google Scholar] [CrossRef]
- Hartmann, W.; Galla, H.J.; Sackmann, E. Polymyxin binding to charged lipid membranes. An example of cooperative lipid-protein interaction. Biochim. Biophys. Acta 1978, 510, 124–139. [Google Scholar] [CrossRef]
- Velkov, T.; Roberts, K.D.; Nation, R.L.; Thompson, P.E.; Li, J. Pharmacology of polymyxins: New insights into an “old” class of antibiotics. Future Microbiol. 2013, 8, 711–724. [Google Scholar] [CrossRef]
- Yu, Z.; Qin, W.; Lin, J.; Fang, S.; Qiu, J. Antibacterial mechanisms of polymyxin and bacterial resistance. Biomed. Res. Int. 2015, 2015, 679109. [Google Scholar] [CrossRef]
- Trimble, M.J.; Mlynarcik, P.; Kolar, M.; Hancock, R.E. Polymyxin: Alternative mechanisms of action and resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a025288. [Google Scholar] [CrossRef] [PubMed]
- Ayoub Moubareck, C. Polymyxins and Bacterial membranes: A review of antibacterial activity and mechanisms of resistance. Membranes 2020, 10, 181. [Google Scholar] [CrossRef]
- Wiegel, J.; Mayer, F. Isolation of lipopolysaccharides and the effect of polymyxin B on the outer membrane of Corynebacterium autotrophicum. Arch Microbiol. 1978, 118(1), 67–69. [Google Scholar] [CrossRef] [PubMed]
- Fernández, L.; Alvarez-Ortega, C.; Wiegand, I.; Olivares, J.; Kocíncová, D.; Lam, J.S.; Martínez, J.L.; Hancock, R.E. Characterization of the polymyxin B resistome of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2013, 57, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Velkov, T.; Thompson, P.E.; Nation, R.L.; Li, J. Structure-activity relationships of polymyxin antibiotics. J. Med. Chem 2010, 53, 1898–1916. [Google Scholar] [CrossRef]
- Cajal, Y.; Rogers, J.; Berg, O.G.; Jain, M.K. Intermembrane molecular contacts by polymyxin B mediate exchange of phospholipids. Biochemistry 1996, 35, 299–308. [Google Scholar] [CrossRef]
- Clausell, A.; Rabanal, F.; Garcia-Subirats, M.; Alsina, M.A.; Cajal, Y. Synthesis and membrane action of polymyxin B analogues. Luminescence 2005, 20, 117–123. [Google Scholar] [CrossRef]
- Clausell, A.; Rabanal, F.; Garcia-Subirats, M.; Asunción Alsina, M.; Cajal, Y. Membrane association and contact formation by a synthetic analogue of polymyxin B and its fluorescent derivatives. J. Phys. Chem. B 2006, 110, 4465–4471. [Google Scholar] [CrossRef]
- Domingues, M.M.; Inácio, R.G.; Raimundo, J.M.; Martins, M.; Castanho, M.A.; Santos, N.C. Biophysical characterization of polymyxin B interaction with LPS aggregates and membrane model systems. Biopolymers 2012, 98, 338–344. [Google Scholar] [CrossRef]
- Simar, S.; Sibley, D.; Ashcraft, D.; Pankey, G. Colistin and polymyxin B minimal inhibitory concentrations determined by eTest found unreliable for Gram-negative bacilli. Ochsner J. 2017, 17, 239–242. [Google Scholar]
- Sheppard, J.D.; Jumarie, C.; Cooper, D.G.; Laprade, R. Ionic channels induced by surfactin in planar lipid bilayer membranes. Biochim. Biophys. Acta 1991, 1064, 13–23. [Google Scholar] [CrossRef]
- Zakharova, A.A.; Efimova, S.S.; Malev, V.V.; Ostroumova, O.S. Fengycin induces ion channels in lipid bilayers mimicking target fungal cell membranes. Sci. Rep. 2019, 9, 16034. [Google Scholar] [CrossRef] [PubMed]
- Abdelraouf, K.; Braggs, K.H.; Yin, T.; Truong, L.D.; Hu, M.; Tam, V.H. Characterization of polymyxin B-induced nephrotoxicity: Implications for dosing regimen design. Antimicrob. Agents Chemother. 2012, 56, 4625–4629. [Google Scholar] [CrossRef]
- Jangra, M.; Randhawa, H.K.; Kaur, M.; Srivastava, A.; Maurya, N.; Patil, P.P.; Jaswal, P.; Arora, A.; Patil, P.B.; Raje, M.; et al. Purification, characterization and in vitro evaluation of polymyxin a from paenibacillus dendritiformis: An underexplored member of the polymyxin family. Front. Microbiol. 2018, 9, 2864. [Google Scholar] [CrossRef]
- Malev, V.V.; Schagina, L.V.; Gurnev, P.A.; Takemoto, J.Y.; Nestorovich, E.M.; Bezrukov, S.M. Syringomycin E channel: A lipidic pore stabilized by lipopeptide? Biophys. J. 2002, 82, 1985–1994. [Google Scholar] [CrossRef]
- Koller, D.; Lohner, K. The role of spontaneous lipid curvature in the interaction of interfacially active peptides with membranes. Biochim. Biophys. Acta 2014, 1838, 2250–2259. [Google Scholar] [CrossRef]
- Efimova, S.S.; Chulkov, E.G.; Ostroumova, O.S. Lipid-mediated mode of action of local anesthetics on lipid pores induced by polyenes, peptides and lipopeptides. Colloids Surf. B. Biointerfaces 2018, 166, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Berglund, N.A.; Piggot, T.J.; Jefferies, D.; Sessions, R.B.; Bond, P.J.; Khalid, S. Interaction of the antimicrobial peptide polymyxin B1 with both membranes of E. coli: A molecular dynamics study. PLoS Comput. Biol. 2015, 11, e1004180. [Google Scholar] [CrossRef]
- Breazeale, S.D.; Ribeiro, A.A.; McClerren, A.L.; Raetz, C.R. A formyltransferase required for polymyxin resistance in Escherichia coli and the modification of lipid A with 4-Amino-4-deoxy-L-arabinose. Identification and function oF UDP-4-deoxy-4-formamido-L-arabinose. J. Biol. Chem. 2005, 280, 14154–14167. [Google Scholar] [CrossRef] [PubMed]
- Gunn, J.S.; Lim, K.B.; Krueger, J.; Kim, K.; Guo, L.; Hackett, M.; Miller, S.I. PmrA-PmrB-regulated genes necessary for 4-aminoarabinose lipid A modification and polymyxin resistance. Mol. Microbiol. 1998, 27, 1171–1182. [Google Scholar] [CrossRef]
- Trent, M.S.; Ribeiro, A.A.; Lin, S.; Cotter, R.J.; Raetz, C.R. An inner membrane enzyme in Salmonella and Escherichia. coli that transfers 4-amino-4-deoxy-L-arabinose to lipid A: Induction on polymyxin-resistant mutants and role of a novel lipid-linked donor. J. Biol. Chem. 2001, 276, 43122–43131. [Google Scholar] [CrossRef] [PubMed]
- Hianik, T.; Fajkus, M.; Tarus, B.; Frangopol, P.T.; Markin, V.S.; Landers, D.F. The electrostriction, surface potential and capacitance relaxation of bilayer lipid membranes induced by tetracaine. Bioelectrochem. Bioenerg. 1998, 46, 1–5. [Google Scholar] [CrossRef]
- Efimova, S.S.; Zakharova, A.A.; Schagina, L.V.; Ostroumova, O.S. Local anesthetics affect gramicidin a channels via membrane electrostatic potentials. J. Membr. Biol. 2016, 249, 781–787. [Google Scholar] [CrossRef]
- Högberg, C.J.; Lyubartsev, A.P. Effect of local anesthetic lidocaine on electrostatic properties of a lipid bilayer. Biophys. J. 2008, 94, 525–531. [Google Scholar] [CrossRef]
- Malkov, D.Y.; Sokolov, V.S. Fluorescent styryl dyes of the RH series affect a potential drop on the membrane/solution boundary. Biochim. Biophys. Acta 1996, 1278, 197–204. [Google Scholar] [CrossRef]
- Efimova, S.S.; Ostroumova, O.S. Effect of dipole modifiers on the magnitude of the dipole potential of sterol-containing bilayers. Langmuir 2012, 28, 9908–9914. [Google Scholar] [CrossRef] [PubMed]
- Andersen, O.S.; Finkelstein, A.; Katz, I.; Cass, A. Effect of phloretin on the permeability of thin lipid membranes. J. Gen. Physiol. 1976, 67, 749–771. [Google Scholar] [CrossRef] [PubMed]
- Franklin, J.C.; Cafiso, D.S. Internal electrostatic potentials in bilayers: Measuring and controlling dipole potentials in lipid vesicles. Biophys. J. 1993, 65, 289–299. [Google Scholar] [CrossRef]
- Zakharova, A.A.; Efimova, S.S.; Ostroumova, O.S. Phosphodiesterase type 5 inhibitors greatly affect physicochemical properties of model lipid membranes. Membranes 2021, 11, 893. [Google Scholar] [CrossRef] [PubMed]
- Sobko, A.A.; Kotova, E.A.; Antonenko, Y.N.; Zakharov, S.D.; Cramer, W.A. Effect of lipids with different spontaneous curvature on the channel activity of colicin E1: Evidence in favor of a toroidal pore. FEBS Lett. 2004, 576, 205–210. [Google Scholar] [CrossRef]
- Zakharov, S.D.; Kotova, E.A.; Antonenko, Y.N.; Cramer, W.A. On the role of lipid in colicin pore formation. Biochim. Biophys. Acta 2004, 1666, 239–249. [Google Scholar] [CrossRef]
- Matsuzaki, K.; Sugishita, K.; Ishibe, N.; Ueha, M.; Nakata, S.; Miyajima, K.; Epand, R.M. Relationship of membrane curvature to the formation of pores by magainin 2. Biochemistry 1998, 37, 11856–11863. [Google Scholar] [CrossRef]
- Ludtke, S.J.; He, K.; Heller, W.T.; Harroun, T.A.; Yang, L.; Huang, H.W. Membrane pores induced by magainin. Biochemistry 1996, 35, 13723–13728. [Google Scholar] [CrossRef]
- Epand, R.M. Lipid polymorphism and protein-lipid interactions. Biochim. Biophys. Acta 1998, 1376, 353–368. [Google Scholar] [CrossRef]
- Shai, Y. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by alpha-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim. Biophys. Acta 1999, 1462, 55–70. [Google Scholar] [CrossRef]
- Chernomordik, L.; Chanturiya, A.; Green, J.; Zimmerberg, J. The hemifusion intermediate and its conversion to complete fusion: Regulation by membrane composition. Biophys. J. 1995, 69, 922–929. [Google Scholar] [CrossRef]
- Fuller, N.; Rand, R.P. The influence of lysolipids on the spontaneous curvature and bending elasticity of phospholipid membranes. Biophys. J. 2001, 81, 243–254. [Google Scholar] [CrossRef]
- Gillams, R.J.; Nylander, T.; Plivelic, T.S.; Dymond, M.K.; Attard, G.S. Formation of inverse topology lyotropic phases in dioleoylphosphatidylcholine/oleic acid and dioleoylphosphatidylethanolamine/oleic acid binary mixtures. Langmuir 2014, 30, 3337–3344. [Google Scholar] [CrossRef] [PubMed]
- Montal, M.; Muller, P. Formation of bimolecular membranes from lipid monolayers and study of their electrical properties. Proc. Nat. Acad. Sci. USA 1972, 65, 3561–3566. [Google Scholar] [CrossRef] [PubMed]



| Potential Target | Proposed Molecular Mechanisms | References |
|---|---|---|
| Outer membrane | Binding to lipopolysaccharides, altering lipid packing, permeabilization and/or self-promoted diffusion | [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,43,44,45] |
| Contacts between outer and inner membranes | Inducing the formation of contacts and lipid exchange | [27,46,47,48] |
| Inner membrane | Binding to acidic phospholipids, altering lipid packing, and formation of pores | [34,35,36,37,38] |
| Small Molecule | Chemical Structure | C (µM) | I∞/I∞0 | Δφb (mV) | Charge # (%) |
|---|---|---|---|---|---|
| phloretin |  | 20 | 28 ± 4 | −75 ± 10 | 23 |
| vardenafil | 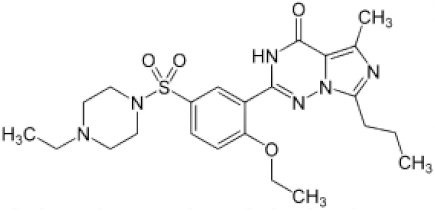 | 100 | 49 ± 8 | −60 ± 25 | 6 |
| RH421 |  | 10 | 0.5 ± 0.1 | 80 ± 40 | 3 |
| tetracaine | 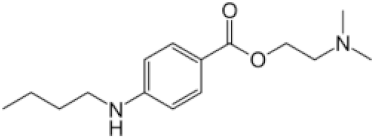 | 500 | 0.3 ± 0.2 | 55 ± 10 | 91 |
| Modifier | Chemical Structure | C (µM) | I∞/I∞0 | R (Å) |
|---|---|---|---|---|
| LysoPC | 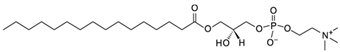 | 7.5 | 32 ± 10 | 68 [77] |
| Oleic acid |  | 30 | 0.4 ± 0.2 | –25.4 * [78] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakharova, A.A.; Efimova, S.S.; Ostroumova, O.S. Lipid Microenvironment Modulates the Pore-Forming Ability of Polymyxin B. Antibiotics 2022, 11, 1445. https://doi.org/10.3390/antibiotics11101445
Zakharova AA, Efimova SS, Ostroumova OS. Lipid Microenvironment Modulates the Pore-Forming Ability of Polymyxin B. Antibiotics. 2022; 11(10):1445. https://doi.org/10.3390/antibiotics11101445
Chicago/Turabian StyleZakharova, Anastasiia A., Svetlana S. Efimova, and Olga S. Ostroumova. 2022. "Lipid Microenvironment Modulates the Pore-Forming Ability of Polymyxin B" Antibiotics 11, no. 10: 1445. https://doi.org/10.3390/antibiotics11101445
APA StyleZakharova, A. A., Efimova, S. S., & Ostroumova, O. S. (2022). Lipid Microenvironment Modulates the Pore-Forming Ability of Polymyxin B. Antibiotics, 11(10), 1445. https://doi.org/10.3390/antibiotics11101445





