Chimeric Peptides Derived from Bovine Lactoferricin and Buforin II: Antifungal Activity against Reference Strains and Clinical Isolates of Candida spp.
Abstract
:1. Introduction
2. Results and Discussion
- (I)
- The C1 and C3 peptides were synthesized and evaluated in order to establish whether the antifungal activity is affected by the position of the RRWQWR or RLLR sequences in the chimera. Chimeras C2 and C4 contained Ahx (6-aminohexanoic acid residue) as a spacer for the two motifs. This is intended to establish whether the inclusion of the Ahx spacer between the two motifs affects the antifungal activity. The inclusion of the Ahx spacer facilitates the synthesis of the chimera and separates the two motifs so that each of them can interact independently with the cell surface of the pathogen.
- (II)
- Chimeras C5, C6, and C7 were synthesized and their antifungal activity was evaluated in order to determine whether partial or total replacement of Arg residues with Lys in RRWQWR and/or RLLR sequences affects their antifungal activity. Replacing Arg residues with Lys has been shown to facilitate and reduce the cost of chimeric synthesis [18].
- (III)
| Antifungal Activity Against C. albicans Strains. µg/mL (µM) | ||||||
|---|---|---|---|---|---|---|
| Group | Code | Sequence | ATCC SC5314 | 256 HUSI-PUJ | ||
| MIC | MFC | MIC | MFC | |||
| Control | LfcinB (20–25) | RRWQWR | 200 (203) | 200 (203) | 200 (203) | 200 (203) |
| BFII (32–35)Pal | RLLRRLLR | >200 (>183) | >200 (>183) | >200 (>183) | >200 (>183) | |
| I | C1 | RRWQWRRLLR | 200 (131) | >200 (>131) | 100 (66) | >200 (>131) |
| C2 | RRWQWR-Ahx-RLLR | 200 (122) | >200 (>122) | 200 (122) | >200(>122) | |
| C3 | RLLRRRWQWR | 100 (66) | 200 (131) | 100 (66) | 200 (131) | |
| C4 | RLLR-Ahx-RRWQWR | >200 (>122) | >200 (>122) | 200 (122) | >200 (>122) | |
| II | C5 | RRWQWR-Ahx-KLLKKLLK | 100 (48) | 200 (97) | 100 (48) | 200 (97) |
| C6 | KKWQWK-Ahx-RLLRRLLR | 50 (24) | 100 (48) | 50 (24) | 100 (48) | |
| C7 | KKWQWK-Ahx-KLLKKLLK | 200 (101) | >200 (>101) | 200 (101) | >200 (>101) | |
| III | C8 | (RRWQWR)2K-Ahx-RLLR | 100 (37) | 100 (37) | 100 (37) | 100 (37) |
| C9 | (RRWQWR)2K-Ahx-RLLRRLLR | 50 (15) | 50 (15) | 50 (15) | 50 (15) | |
2.1. Minimum Inhibitory and Fungicidal Concentration
2.2. Hemolytic Effect
2.3. Antifungal Activity of Chimeric Peptides Mixed with FLC
3. Materials and Methods
3.1. Reagents and Materials
3.2. Peptides
3.3. In Vitro Antifungal Susceptibility Test
3.4. Time-Kill Curves
3.5. Hemolysis Assays
3.6. Checkerboard Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Prim. 2018, 4, 18026. [Google Scholar] [CrossRef]
- Lai, C.C.; Tan, C.K.; Huang, Y.T.; Shao, P.L.; Hsueh, P.R. Current challenges in the management of invasive fungal infections. J. Infect. Chemother. 2008, 14, 77–85. [Google Scholar] [CrossRef]
- de Bedout, C.; Gómez, B.L. Candida y candidiasis invasora: Un reto continuo para su diagnóstico temprano. Infectio 2010, 14, 159–171. [Google Scholar] [CrossRef]
- Ch, G.; De Tema, R.; Gómez Quintero, C.H. Resistencia de levaduras del género Candida al fluconazol Candida yeast’s resistance to fluconazol. Infectio 2010, 14, 172–180. [Google Scholar]
- Basmaciyan, L.; Bon, F.; Paradis, T.; Lapaquette, P.; Dalle, F. Candida Albicans Interactions with the Host: Crossing the Intestinal Epithelial Barrier. Tissue Barriers 2019, 7, 1612661. [Google Scholar] [CrossRef] [Green Version]
- Pfaller, M.A.; Pappas, P.G.; Wingard, J.R. Invasive Fungal Pathogens: Current Epidemiological Trends. Clin. Infect. Dis. 2006, 43 (Suppl. 1), S3–S14. [Google Scholar] [CrossRef]
- Logan, C.; Martin-Loeches, I.; Bicanic, T. Invasive candidiasis in critical care: Challenges and future directions. Intensive Care Med. 2020, 46, 2001–2014. [Google Scholar] [CrossRef]
- de Jong, A.W.; Hagen, F. Attack, Defend and Persist: How the Fungal Pathogen Candida auris was Able to Emerge Globally in Healthcare Environments. Mycopathologia 2019, 184, 353–365. [Google Scholar] [CrossRef] [Green Version]
- Černáková, L.; Roudbary, M.; Brás, S.; Tafaj, S.; Rodrigues, C.F. Candida auris: A quick review on identification, current treatments, and challenges. Int. J. Mol. Sci. 2021, 22, 4470. [Google Scholar] [CrossRef]
- Yapar, N. Epidemiology and risk factors for invasive candidiasis. Ther. Clin. Risk Manag. 2014, 10, 95–105. [Google Scholar] [CrossRef] [Green Version]
- Morio, F.; Jensen, R.H.; Le Pape, P.; Arendrup, M.C. Molecular basis of antifungal drug resistance in yeasts. Int. J. Antimicrob. Agents 2017, 50, 599–606. [Google Scholar] [CrossRef]
- Cortés, J.A.; Ruiz, J.F.; Melgarejo-Moreno, L.N.; Lemos, E.V. Candidemia en Colombia. Biomédica 2020, 40, 195–207. [Google Scholar] [CrossRef] [Green Version]
- Torres, M.D.T.; Sothiselvam, S.; Lu, T.K.; de la Fuente-Nunez, C. Peptide Design Principles for Antimicrobial Applications. J. Mol. Biol. 2019, 431, 3547–3567. [Google Scholar] [CrossRef]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef]
- Bellotti, D.; Remelli, M. Lights and Shadows on the Therapeutic Use of Antimicrobial Peptides. Molecules 2022, 27, 4584. [Google Scholar] [CrossRef]
- Vanzolini, T.; Bruschi, M.; Rinaldi, A.C.; Magnani, M.; Fraternale, A. Multitalented Synthetic Antimicrobial Peptides and Their Antibacterial, Antifungal and Antiviral Mechanisms. Int. J. Mol. Sci. 2022, 23, 545. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, Y.; Song, Z.; Tan, Z.; Cheng, J. Recent advances in design of antimicrobial peptides and polypeptides toward clinical translation. Adv. Drug Deliv. Rev. 2021, 170, 261–280. [Google Scholar] [CrossRef]
- Pineda-Castañeda, H.M.; Huertas-Ortiz, K.A.; Leal-Castro, A.L.; Vargas-Casanova, Y.; Parra-Giraldo, C.M.; García-Castañeda, J.E.; Rivera-Monroy, Z.J. Designing Chimeric Peptides: A Powerful Tool for Enhancing Antibacterial Activity. Chem. Biodivers. 2021, 18, e2000885. [Google Scholar] [CrossRef]
- Barragán-Cárdenas, A.C.; Insuasty-Cepeda, D.S.; Cárdenas-Martínez, K.J.; López-Meza, J.; Ochoa-Zarzosa, A.; Umaña-Pérez, A.; Rivera-Monroy, Z.J.; García-Castañeda, J.E. LfcinB-Derived Peptides: Specific and punctual change of an amino acid in monomeric and dimeric sequences increase selective cytotoxicity in colon cancer cell lines. Arab. J. Chem. 2022, 15, 103998. [Google Scholar] [CrossRef]
- Vargas-Casanova, Y.; Rodríguez-Mayor, A.V.; Cardenas, K.J.; Leal-Castro, A.L.; Muñoz-Molina, L.C.; Fierro-Medina, R.; Rivera-Monroy, Z.J.; García-Castañeda, J.E. Synergistic bactericide and antibiotic effects of dimeric, tetrameric, or palindromic peptides containing the RWQWR motif against Gram-positive and Gram-negative strains. RSC Adv. 2019, 9, 7239–7245. [Google Scholar] [CrossRef] [Green Version]
- Sebastien Farnaud, R.W.E. Lactoferrin—A multifunctional protein with antimicrobial properties. Mol. Immunol. 2003, 40, 395–405. [Google Scholar] [CrossRef]
- Gifford, J.L.; Hunter, H.N.; Vogel, H.J. Lactoferricin: A lactoferrin-derived peptide with antimicrobial, antiviral, antitumor and immunological properties. Cell. Mol. Life Sci. 2005, 62, 2588–2598. [Google Scholar] [CrossRef]
- Yen, C.C.; Shen, C.J.; Hsu, W.H.; Chang, Y.H.; Lin, H.T.; Chen, H.L.; Chen, C.M. Lactoferrin: An iron-binding antimicrobial protein against Escherichia coli infection. BioMetals 2011, 24, 585–594. [Google Scholar] [CrossRef]
- Fernandes, K.E.; Carter, D.A. The antifungal activity of lactoferrin and its derived peptides: Mechanisms of action and synergy with drugs against fungal pathogens. Front. Microbiol. 2017, 8, 2. [Google Scholar] [CrossRef] [Green Version]
- Romo, T.D.; Bradney, L.A.; Greathouse, D.V.; Grossfield, A. Membrane binding of an acyl-lactoferricin B antimicrobial peptide from solid-state NMR experiments and molecular dynamics simulations. Biochim. Biophys. Acta—Biomembr. 2011, 1808, 2019–2030. [Google Scholar] [CrossRef] [Green Version]
- Cho, J.H.; Sung, B.H.; Kim, S.C. Buforins: Histone H2A-derived antimicrobial peptides from toad stomach. In Biochimica et Biophysica Acta—Biomembranes; Elsevier B.V.: Amsterdam, The Netherlands, 2009; Volume 1788, pp. 1564–1569. [Google Scholar]
- Park, C.B.; Yi, K.S.; Matsuzaki, K.; Kim, M.S.; Kim, S.C. Structure-activity analysis of buforin II, a histone H2A-derived antimicrobial peptide: The proline hinge is responsible for the cell-penetrating ability of buforin II. Proc. Natl. Acad. Sci. USA 2000, 97, 8245–8250. [Google Scholar] [CrossRef] [Green Version]
- Tomita, M.; Takase, M.; Bellamy, W.; Shimamura, S. A review: The active peptide of lactoferrin. Pediatr. Int. 1994, 36, 585–591. [Google Scholar] [CrossRef]
- Schibli, D.J.; Hwang, P.M.; Vogel, H.J. The structure of the antimicrobial active center of lactoferricin B bound to sodium dodecyl sulfate micelles. FEBS Lett. 1999, 446, 213–217. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.A.; Kim, H.; Lee, J.Y.; Shin, J.R.; Kim, D.J.; Cho, J.H.; Kim, S.C. Mechanism of action and specificity of antimicrobial peptides designed based on buforin IIb. Peptides 2012, 34, 283–289. [Google Scholar] [CrossRef]
- Vargas Casanova, Y.; Rodríguez Guerra, J.A.; Umaña Pérez, Y.A.; Leal Castro, A.L.; Almanzar Reina, G.; García Castañeda, J.E.; Rivera Monroy, Z.J. Antibacterial Synthetic Peptides Derived from Bovine Lactoferricin Exhibit Cytotoxic Effect against MDA-MB-468 and MDA-MB-231 Breast Cancer Cell Lines. Molecules 2017, 22, 1641. [Google Scholar] [CrossRef]
- Muñoz, A.; Marcos, J.F. Activity and mode of action against fungal phytopathogens of bovine lactoferricin-derived peptides. J. Appl. Microbiol. 2006, 101, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas-Martínez, K.J.; Grueso-Mariaca, D.; Vargas-Casanova, Y.; Bonilla-Velásquez, L.; Estupiñán, S.M.; Parra-Giraldo, C.M.; Leal, A.L.; Rivera-Monroy, Z.J.; García-Castañeda, J.E. Effects of Substituting Arginine by Lysine in Bovine Lactoferricin Derived Peptides: Pursuing Production Lower Costs, Lower Hemolysis, and Sustained Antimicrobial Activity. Int. J. Pept. Res. Ther. 2021, 27, 1751–1762. [Google Scholar] [CrossRef]
- Alves, D.D.N.; Ferreira, A.R.; Duarte, A.B.S.; Melo, A.K.V.; De Sousa, D.P.; Castro, R.D. De Breakpoints for the Classification of Anti- Candida Compounds in Antifungal Screening. Biomed Res. Int. 2021, 2021, 6653311. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, K.E.; Weeks, K.; Carter, D.A. Lactoferrin is broadly active against yeasts and highly synergistic with amphotericin B. Antimicrob. Agents Chemother. 2020, 64, e02284-19. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.K.; Kao, M.C.; Lan, C.Y. Antimicrobial activity of the peptide lfcinb15 against candida albicans. J. Fungi 2021, 7, 519. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.S.; Kim, H.K.; Lee, K.Y.; Kim, S.A.; Han, Y.S.; Lee, I.H. Antifungal activity of synthetic peptide derived from halocidin, antimicrobial peptide from the tunicate, Halocynthia aurantium. FEBS Lett. 2006, 580, 1490–1496. [Google Scholar] [CrossRef] [Green Version]
- Pavia, K.E.; Spinella, S.A.; Elmore, D.E. Novel Histone-Derived Antimicrobial Peptides Use Different Antimicrobial Mechanisms. Biochim. Biophys. Acta 2012, 1818, 869. [Google Scholar] [CrossRef] [Green Version]
- Lemos, A.S.O.; Florêncio, J.R.; Pinto, N.C.C.; Campos, L.M.; Silva, T.P.; Grazul, R.M.; Pinto, P.F.; Tavares, G.D.; Scio, E.; Apolônio, A.C.M.; et al. Antifungal Activity of the Natural Coumarin Scopoletin Against Planktonic Cells and Biofilms from a Multidrug-Resistant Candida tropicalis Strain. Front. Microbiol. 2020, 11, 1525. [Google Scholar] [CrossRef]
- Hassan, Y.; Chew, S.Y.; Than, L.T.L. Candida glabrata: Pathogenicity and resistance mechanisms for adaptation and survival. J. Fungi 2021, 7, 667. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J.; Turnidge, J.D.; Castanheira, M.; Jones, R.N. Twenty Years of the SENTRY Antifungal Surveillance Program: Results for Candida Species From 1997–2016. Open Forum Infect. Dis. 2019, 6, S79–S94. [Google Scholar] [CrossRef] [Green Version]
- Ademe, M.; Girma, F. Candida auris: From Multidrug Resistance to Pan-Resistant Strains. Infect. Drug Resist. 2020, 13, 1287. [Google Scholar] [CrossRef] [PubMed]
- Ježíková, Z.; Pagáč, T.; Víglaš, J.; Pfeiferová, B.; Šoltys, K.; Bujdáková, H.; Černáková, L.; Olejníková, P. Synergy Over Monotherapy. Curr. Microbiol. 2019, 76, 673–677. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Messer, S.A.; Woosley, L.N.; Jones, R.N.; Castanheira, M. Echinocandin and triazole antifungal susceptibility profiles for clinical opportunistic yeast and mold isolates collected from 2010 to 2011: Application of new CLSI clinical breakpoints and epidemiological cutoff values for characterization of geographic. J. Clin. Microbiol. 2013, 51, 2571–2581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvo, B.; Melo, A.S.A.; Perozo-Mena, A.; Hernandez, M.; Francisco, E.C.; Hagen, F.; Meis, J.F.; Colombo, A.L.; Satoh, K.; Makimura, K.; et al. First report of Candida auris in America: Clinical and microbiological aspects of 18 episodes of candidemia. J. Infect. 2016, 73, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Morales-López, S.E.; Parra-Giraldo, C.M.; Ceballos-Garzón, A.; Martínez, H.P.; Rodríguez, G.J.; Álvarez-Moreno, C.A.; Rodríguez, J.Y. Invasive Infections with Multidrug-Resistant Yeast Candida auris, Colombia. Emerg. Infect. Dis. 2017, 23, 162–164. [Google Scholar] [CrossRef] [Green Version]
- Vargas-Casanova, Y.; Carlos Villamil Poveda, J.; Jenny Rivera-Monroy, Z.; Ceballos Garzón, A.; Fierro-Medina, R.; Le Pape, P.; Eduardo García-Castañeda, J.; Marcela Parra Giraldo, C. Palindromic Peptide LfcinB (21–25)Pal Exhibited Antifungal Activity against Multidrug-Resistant Candida. ChemistrySelect 2020, 5, 7236–7242. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. Approved Standard Third Edition; M27-A3; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Pfaller, M.A.; Sheehan, D.J.; Rex, J.H. Determination of Fungicidal Activities against Yeasts and Molds: Lessons Learned from Bactericidal Testing and the Need for Standardization. Clin. Microbiol. Rev. 2004, 17, 268–280. [Google Scholar] [CrossRef] [Green Version]
- Cokol-Cakmak, M.; Bakan, F.; Cetiner, S.; Cokol, M. Diagonal method to measure synergy among any number of drugs. J. Vis. Exp. 2018, 2018, 57713. [Google Scholar] [CrossRef] [Green Version]
- Wei, G.-X.; Campagna, A.N.; Bobek, L.A. Factors affecting antimicrobial activity of MUC7 12-mer, a human salivary mucin-derived peptide. Ann. Clin. Microbiol. Antimicrob. 2007, 6, 14. [Google Scholar] [CrossRef] [Green Version]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Terminology relating to methods for the determination of susceptibility of bacteria to antimicrobial agents. Clin. Microbiol. Infect. 2000, 6, 503–508. [Google Scholar] [CrossRef]
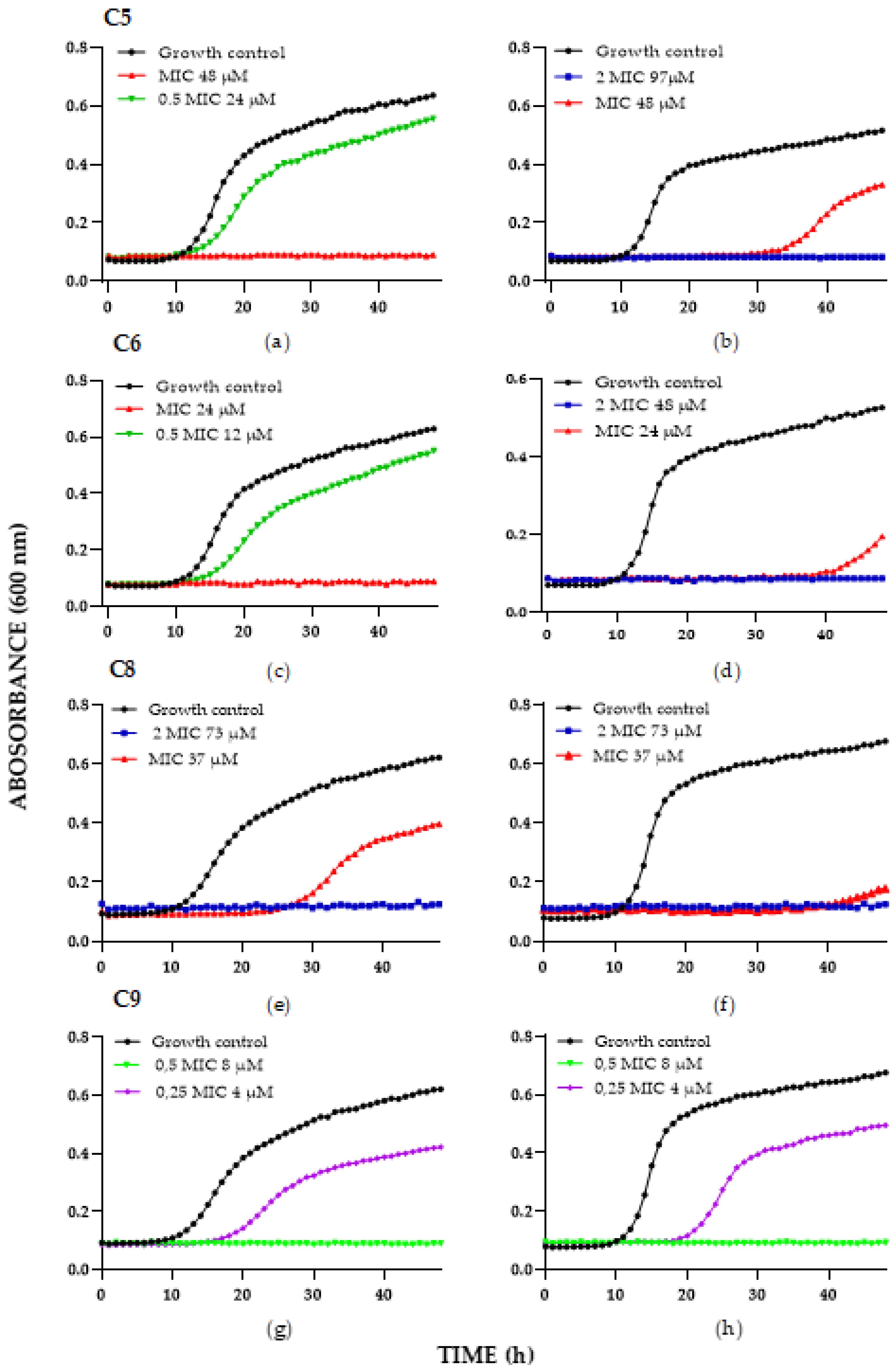
| Time-Kill Result against C. albicans Strains—µg/mL (µM) | ||||
|---|---|---|---|---|
| Peptide | ATCC SC5314 | 256 HUSI-PUJ | ||
| Fungistatic * | Fungicide * | Fungistatic * | Fungicide * | |
| C5 | 50 (24) | 100 (48) | 100 (48) | 200 (97) |
| C6 | 25 (12) | 50 (24) | 50 (24) | 100 (48) |
| C8 | 100 (37) | 200 (73) | 100 (37) | 200 (73) |
| C9 | 13 (4) | 25 (8) | 13 (4) | 25 (8) |
| Hemolytic Activity | ||
|---|---|---|
| Peptide | Concentration (μg/mL) * | % Hemolysis |
| RLLR | 200 | 63 |
| BFII (32–35)Pal | 200 | 2 |
| LfcinB (20–25) | 200 | 1 |
| C1 | 100–200 | 5 |
| C2 | 200 | 3 |
| C3 | 100–200 | 4 |
| C4 | 200 | 5 |
| C5 | 50–200 | 2 |
| C6 | 25–100 | 2 |
| C7 | 200 | 6 |
| C8 | 100 | 2 |
| C9 | 13–50 | 2–11 |
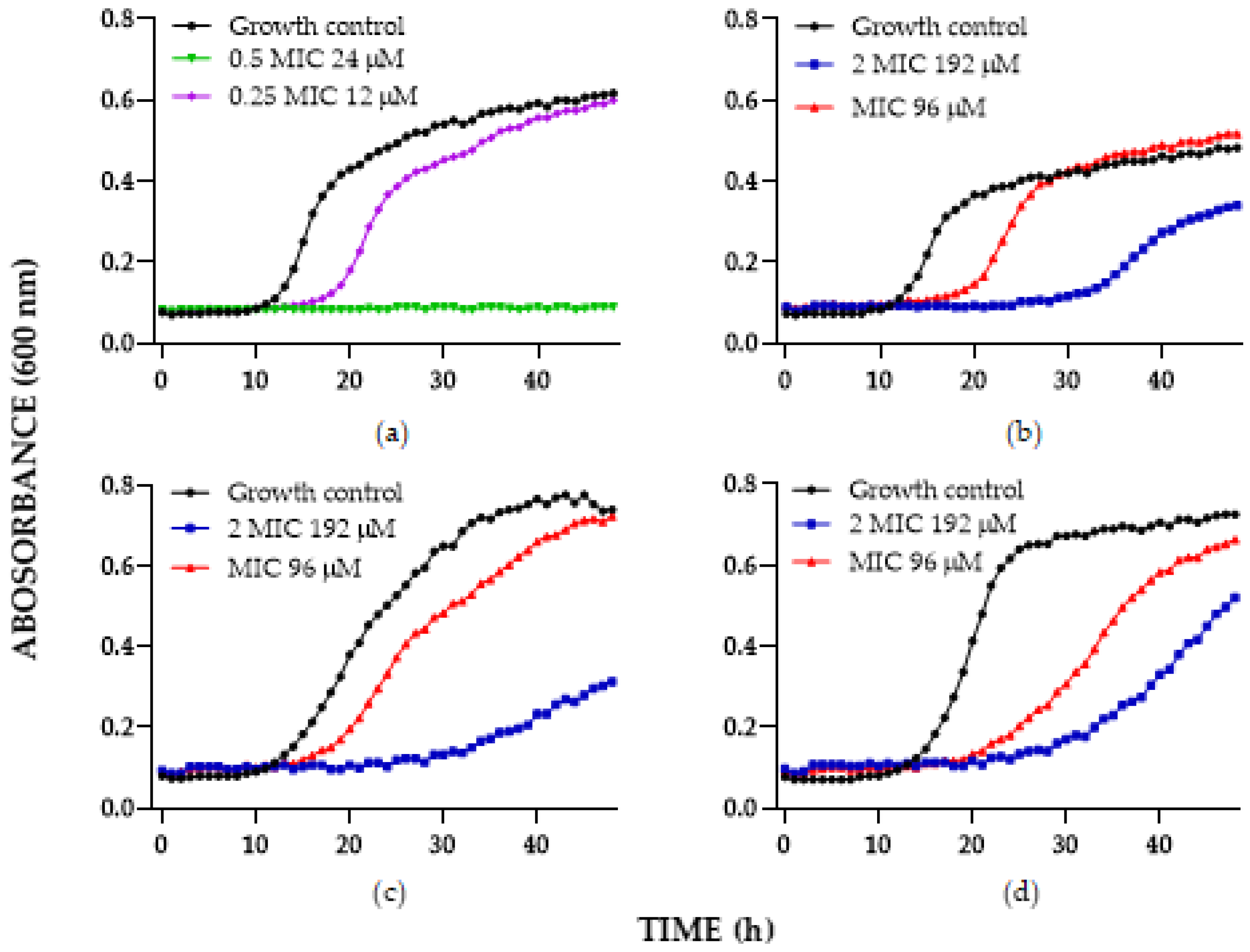
| Synergistic Result C. albicans | ||||||||
|---|---|---|---|---|---|---|---|---|
| C. albicans Strain | Peptide | MICa | MICb | A | B | FICI | MICa/A | MICb/B |
| ATCC SC5314 | C6 | 50 | 1 | 25 | 0.5 | 1 | 2 | 2 |
| C8 | 200 | 0.5 | 25 | 0.13 | 0.38 | 8 | 4 | |
| C9 | 100 | 0.5 | 25 | 0.13 | 0.5 | 4 | 4 | |
| 256 HUSI-PUJ | C6 | 100 | 32 | 3.1 | 32 | 1.03 | 32 | 1 |
| C8 | 100 | 32 | 50 | 32 | 1.5 | 2 | 1 | |
| C9 | 50 | 32 | 25 | 16 | 1 | 2 | 2 | |
| Synergistic Result C. glabrata and C. Auris | |||||||
|---|---|---|---|---|---|---|---|
| Strain | MICa | MICb | A | B | FICI | MICa/A | MICb/B |
| C. glabrata ATCC 2001 | 100 | 0.3 | 50 | 0.03 | 0.6 | 2 | 10 |
| C. glabrata 1875 CHU-PUJ | 400 | 4 | 12.5 | 4 | 1.03 | 32 | 1 |
| C. auris 001 HUSI-PUJ | 400 | 32 | 12.5 | 16 | 0.53 | 32 | 2 |
| C. auris 537 HUSI-PUJ | 400 | 64 | 200 | 32 | 1 | 2 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguirre-Guataqui, K.; Márquez-Torres, M.; Pineda-Castañeda, H.M.; Vargas-Casanova, Y.; Ceballos-Garzon, A.; Rivera-Monroy, Z.J.; García-Castañeda, J.E.; Parra-Giraldo, C.M. Chimeric Peptides Derived from Bovine Lactoferricin and Buforin II: Antifungal Activity against Reference Strains and Clinical Isolates of Candida spp. Antibiotics 2022, 11, 1561. https://doi.org/10.3390/antibiotics11111561
Aguirre-Guataqui K, Márquez-Torres M, Pineda-Castañeda HM, Vargas-Casanova Y, Ceballos-Garzon A, Rivera-Monroy ZJ, García-Castañeda JE, Parra-Giraldo CM. Chimeric Peptides Derived from Bovine Lactoferricin and Buforin II: Antifungal Activity against Reference Strains and Clinical Isolates of Candida spp. Antibiotics. 2022; 11(11):1561. https://doi.org/10.3390/antibiotics11111561
Chicago/Turabian StyleAguirre-Guataqui, Katherine, Mateo Márquez-Torres, Héctor Manuel Pineda-Castañeda, Yerly Vargas-Casanova, Andrés Ceballos-Garzon, Zuly Jenny Rivera-Monroy, Javier Eduardo García-Castañeda, and Claudia Marcela Parra-Giraldo. 2022. "Chimeric Peptides Derived from Bovine Lactoferricin and Buforin II: Antifungal Activity against Reference Strains and Clinical Isolates of Candida spp." Antibiotics 11, no. 11: 1561. https://doi.org/10.3390/antibiotics11111561









