In Vitro Antifungal Activity of Chimeric Peptides Derived from Bovine Lactoferricin and Buforin II against Cryptococcus neoformans var. grubii
Abstract
:1. Introduction
2. Results
2.1. Antifungal Activity of Chimeric Peptides Derived from Bovine Lactoferricin and Buforin II against Cryptococcus neoformans var. grubii
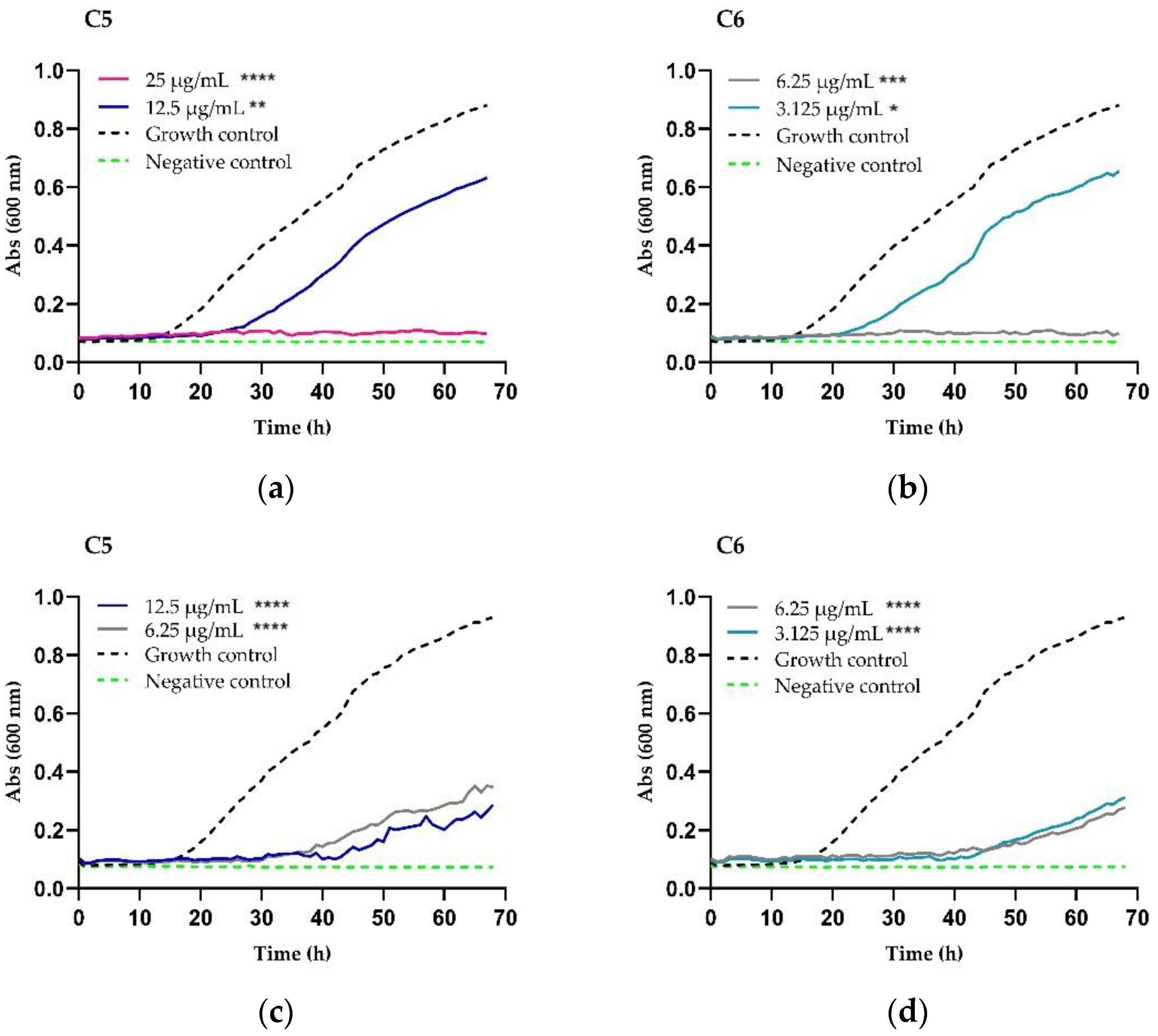
2.2. Growth Inhibition and Killing Kinetics
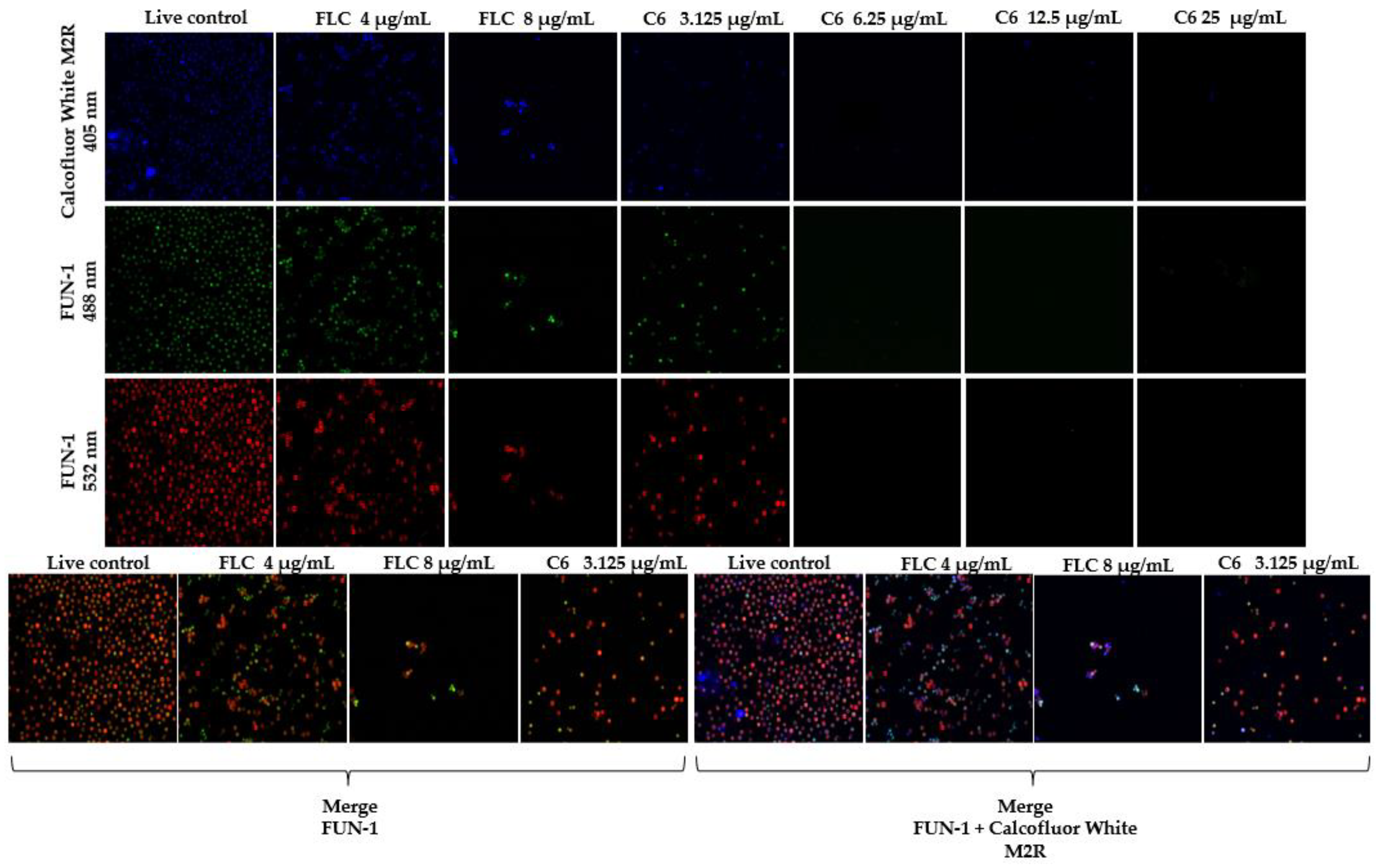
2.3. Fluorescent Staining for Yeast Viability
2.4. Checkerboard Assay
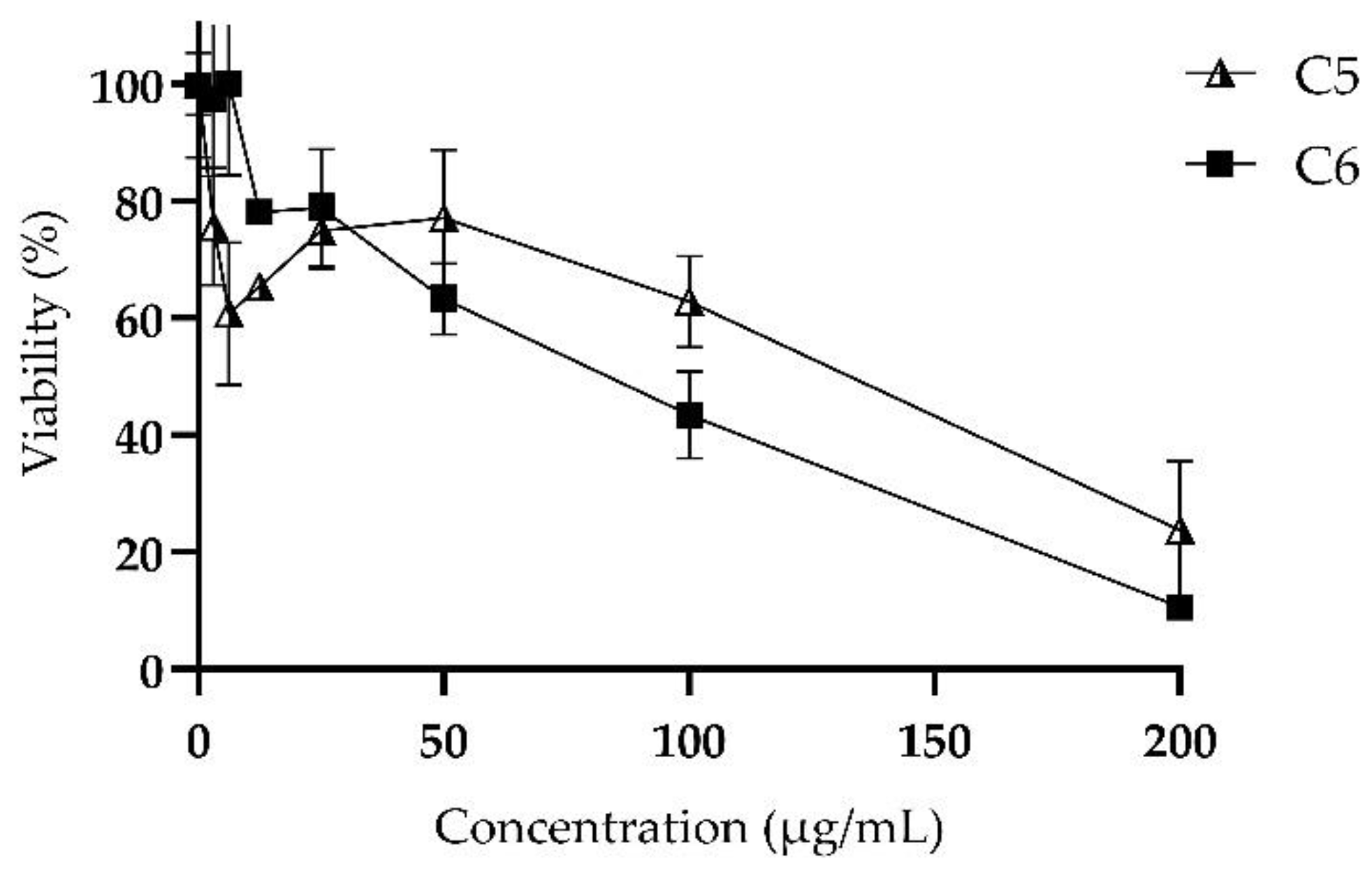
2.5. Cytotoxicity: MTT Assay
3. Discussion
4. Materials and Methods
4.1. Fungal Isolates and Culture Conditions
4.2. Compounds
4.3. Minimum Inhibitory and Fungicidal Concentration Assays
4.4. Growth Inhibition and Killing Kinetics
4.5. Fluorescent Staining for Yeast Viability
4.6. Checkerboard Assay
4.7. Cytotoxicity: MTT Assay
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, Q.; Ding, Y.; Zhou, W.; Li, H.; Zhang, M.; Shi, Y.; Su, X. Clinical features of pulmonary cryptococcosis among patients with different levels of peripheral blood CD4+ T lymphocyte counts. BMC Infect. Dis. 2017, 17, 768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.Y.; Shiau, S.; Fang, C.T. Risk factors for invasive Cryptococcus neoformans diseases: A case-control study. PLoS ONE 2015, 10, e0119090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmiedel, Y.; Zimmerli, S. Common invasive fungal diseases: An overview of invasive candidiasis, aspergillosis, cryptococcosis, and Pneumocystis pneumonia. Swiss Med. Wkly. 2016, 146, w14281. [Google Scholar] [CrossRef] [Green Version]
- Park, B.J.; Wannemuehler, K.A.; Marston, B.J.; Govender, N.; Pappas, P.G.; Chiller, T.M. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. Aids 2009, 23, 525–530. [Google Scholar] [CrossRef] [PubMed]
- May, R.C.; Stone, N.R.H.; Wiesner, D.L.; Bicanic, T.; Nielsen, K. Cryptococcus: From environmental saprophyte to global pathogen. Nat. Rev. Microbiol. 2016, 14, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Firacative, C.; Trilles, L.; Meyer, W. Recent advances in cryptococcus and cryptococcosis. Microorganisms 2022, 10, 13. [Google Scholar] [CrossRef]
- Cogliati, M. Global Molecular Epidemiology of Cryptococcus neoformans and Cryptococcus gattii: An Atlas of the Molecular Types. Scientifica 2013, 2013, 675213. [Google Scholar] [CrossRef] [Green Version]
- Rajasingham, R.; Smith, R.M.; Park, B.J.; Jarvis, J.N.; Govender, N.P.; Chiller, T.M.; Denning, D.W.; Loyse, A.; Boulware, D.R. Global burden of disease of HIV-associated cryptococcal meningitis: An updated analysis. Lancet Infect. Dis. 2017, 17, 873–881. [Google Scholar] [CrossRef] [Green Version]
- Escandón, P.; Lizarazo, J.; Agudelo, C.I.; Castañeda, E. Cryptococcosis in Colombia: Compilation and analysis of data from laboratory-based surveillance. J. Fungi. 2018, 4, 32. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.L.; Xiang, Z.J.; Yang, J.H.; Wang, W.J.; Xu, Z.C.; Xiang, R.L. Adverse Effects Associated With Currently Commonly Used Antifungal Agents: A Network Meta-Analysis and Systematic Review. Front. Pharmacol. 2021, 12, 697330. [Google Scholar] [CrossRef]
- Felton, T.; Troke, P.F.; Hope, W.W. Tissue penetration of antifungal agents. Clin. Microbiol. Rev. 2014, 27, 68–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashley, E.D. Antifungal drugs: Special problems treating central nervous system infections. J. Fungi. 2019, 5, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loyse, A.; Thangaraj, H.; Easterbrook, P.; Ford, N.; Roy, M.; Chiller, T.; Govender, N.; Harrison, T.S.; Bicanic, T. Cryptococcal meningitis: Improving access to essential antifungal medicines in resource-poor countries. Lancet Infect. Dis. 2013, 13, 629–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mondon, P.; Petter, R.; Amalfitano, G.; Luzzati, R.; Concia, E.; Polacheck, I.; Kwon-Chung, K.J. Heteroresistance to fluconazole and voriconazole in Cryptococcus neoformans. Antimicrob. Agents Chemother. 1999, 43, 1856–1861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayab, S.; Aslam, M.A.; Rahman, S.U.; Sindhu, Z.U.D.; Sajid, S.; Zafar, N.; Razaq, M.; Kanwar, R. Amanullah. A Review of Antimicrobial Peptides: Its Function, Mode of Action and Therapeutic Potential. Int. J. Pept. Res. Ther. 2022, 28, 46. [Google Scholar] [CrossRef]
- Vorland, L.H.; Ulvatne, H.; Andersen, J.; Haukland, H.H.; Rekdal, Ø.; Svendsen, J.S.; Gutteberg, T.J. Lactoferricin of bovine origin is more active than lactoferricins of human, murine and caprine origin. Scand. J. Infect. Dis. 1998, 30, 513–517. [Google Scholar]
- Park, C.B.; Yi, K.S.; Matsuzaki, K.; Kim, M.S.; Kim, S.C. Structure-activity analysis of buforin II, a histone H2A-derived antimicrobial peptide: The proline hinge is responsible for the cell-penetrating ability of buforin II. Proc. Natl. Acad. Sci. USA 2000, 97, 8245–8250. [Google Scholar] [CrossRef] [Green Version]
- Pineda-Castañeda, H.M.; Huertas-Ortiz, K.A.; Leal-Castro, A.L.; Vargas-Casanova, Y.; Parra-Giraldo, C.M.; García-Castañeda, J.E.; Rivera-Monroy, Z.J. Designing Chimeric Peptides: A Powerful Tool for Enhancing Antibacterial Activity. Chem. Biodivers. 2021, 18, e2000885. [Google Scholar] [CrossRef]
- Aguirre-Guataqui, K.; Márquez-Torres, M.; Pineda-Castañeda, H.M.; Vargas-Casanova, Y.; Ceballos-Garzon, A.; Rivera-Monroy, Z.J.; García-Castañeda, J.E.; Parra-Giraldo, C.M. Chimeric Peptides Derived from Bovine Lactoferricin and Buforin II: Antifungal Activity against Reference Strains and Clinical Isolates of Candida spp. Antibiotics 2022, 11, 1561. [Google Scholar] [CrossRef]
- Yang, S.T.; Shin, S.Y.; Lee, C.W.; Kim, Y.C.; Hahm, K.S.; Kim, J.I. Selective cytotoxicity following Arg-to-Lys substitution in tritrpticin adopting a unique amphipathic turn structure. FEBS Lett. 2003, 540, 229–233. [Google Scholar] [CrossRef]
- Cárdenas-Martínez, K.J.; Grueso-Mariaca, D.; Vargas-Casanova, Y.; Bonilla-Velásquez, L.; Estupiñán, S.M.; Parra-Giraldo, C.M.; Leal, A.L.; Rivera-Monroy, Z.J.; García-Castañeda, J.E. Effects of Substituting Arginine by Lysine in Bovine Lactoferricin Derived Peptides: Pursuing Production Lower Costs, Lower Hemolysis, and Sustained Antimicrobial Activity. Int. J. Pept. Res. Ther. 2021, 27, 1751–1762. [Google Scholar] [CrossRef]
- Sun, C.; Li, Y.; Cao, S.; Wang, H.; Jiang, C.; Pang, S.; Hussain, M.A.; Hou, J. Antibacterial Activity and Mechanism of Action of Bovine Lactoferricin Derivatives with Symmetrical Amino Acid Sequences. Int. J. Mol. Sci. 2018, 19, 2951. [Google Scholar] [CrossRef] [Green Version]
- Tomita, M.; Takase, M.; Bellamy, W.; Shimamura, S. A review: The active peptide of lactoferrin. Acta Paediatr. Jpn. 1994, 36, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Arias, M.; Piga, K.B.; Hyndman, M.E.; Vogel, H.J. Improving the activity of trp-rich antimicrobial peptides by Arg/Lys substitutions and changing the length of cationic residues. Biomolecules 2018, 8, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Vorobyov, I.; Allen, T.W. The different interactions of lysine and arginine side chains with lipid membranes. J. Phys. Chem. 2003, 117, 11906–11920. [Google Scholar] [CrossRef] [PubMed]
- Scocchi, M.; Mardirossian, M.; Runti, G.; Benincasa, M. Non-Membrane Permeabilizing Modes of Action of Antimicrobial Peptides on Bacteria. Curr. Top. Med. Chem. 2015, 16, 76–88. [Google Scholar] [CrossRef]
- Park, C.B.; Kim, H.S.; Kim, S.C. Mechanism of action of the antimicrobial peptide buforin II: Buforin II kills microorganisms by penetrating the cell membrane and inhibiting cellular functions. Biochem. Biophys. Res. Commun. 1998, 244, 253–257. [Google Scholar] [CrossRef] [Green Version]
- Pulido, X.C.; Royo, M.; Albericio, F.; Rodríguez, H. Cell Penetrating Peptides as potential drug carriers. Bionatura 2016, 1, 208–216. [Google Scholar]
- Markowska, A.; Markowski, A.R.; Jarocka-Karpowicz, I. The importance of 6-aminohexanoic acid as a hydrophobic, flexible structural element. Int. J. Mol. Sci. 2021, 22, 12122. [Google Scholar] [CrossRef]
- Ardila-Chantré, N.; Hernández-Cardona, A.K.; Pineda-Castañeda, H.M.; Estupiñan-Torres, S.M.; Leal-Castro, A.L.; Fierro-Medina, R.; Rivera-Monroy, Z.J.; García-Castaneda, J.E. Short peptides conjugated to non-peptidic motifs exhibit antibacterial activity. RSC Adv. 2020, 10, 29580–29586. [Google Scholar] [CrossRef]
- Arastehfar, A.; Gabaldón, T.; Garcia-Rubio, R.; Jenks, J.D.; Hoenigl, M.; Salzer, H.J.F.; Ilkit, M.; Lass-Flörl, C.; Perlin, D.S. Drug-resistant fungi: An emerging challenge threatening our limited antifungal armamentarium. Antibiotics 2020, 9, 877. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Ingavale, S.S.; Bien, C.; Espenshade, P.; Kwon-Chung, K.J. Conservation of the sterol regulatory element-binding protein pathway and its pathobiological importance in Cryptococcus neoformans. Eukaryot. Cell 2009, 8, 1770–1779. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Bien, C.M.; Lee, H.; Espenshade, P.J.; Kwon-Chung, K.J. Sre1p, a regulator of oxygen sensing and sterol homeostasis, is required for virulence in Cryptococcus neoformans. Mol. Microbiol. 2007, 64, 614–629. [Google Scholar] [CrossRef] [PubMed]
- Jordá, T.; Puig, S. Regulation of Ergosterol Biosynthesis in Saccharomyces cerevisiae. Genes 2020, 11, 795. [Google Scholar] [CrossRef]
- Sionov, E.; Chang, Y.C.; Garraffo, H.M.; Dolan, M.A.; Ghannoum, M.A.; Kwon-Chung, K.J. Identification of a Cryptococcus neoformans cytochrome P450 lanosterol 14α-demethylase (Erg11) residue critical for differential susceptibility between fluconazole/voriconazole and itraconazole/posaconazole. Antimicrob. Agents Chemother. 2012, 56, 1162–1169. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Park, S.C.; Noh, G.; Kim, H.; Yoo, S.H.; Kim, I.R.; Lee, J.R.; Jang, M.K. Antifungal effect of a chimeric peptide HN-MC against pathogenic fungal strains. Antibiotics 2020, 9, 454. [Google Scholar] [CrossRef]
- Mader, J.S.; Salsman, J.; Conrad, D.M.; Hoskin, D.W. Bovine lactoferricin selectively induces apoptosis in human leukemia and carcinoma cell lines. Mol. Cancer Ther. 2005, 4, 612–624. [Google Scholar] [CrossRef] [Green Version]
- Takeshima, K.; Chikushi, A.; Lee, K.K.; Yonehara, S.; Matsuzaki, K. Translocation of analogues of the antimicrobial peptides magainin and buforin across human cell membranes. J. Biol. Chem. 2003, 278, 1310–1315. [Google Scholar] [CrossRef] [Green Version]
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution. Ref Method Broth Dilution Antifung Susceptibility Test Yeasts Approv Stand, 3rd ed.; M27-A3; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2008. [Google Scholar]
- Gil-Alonso, S.; Jauregizar, N.; Cantón, E.; Eraso, E.; Quindós, G. Comparison of the in vitro activity of echinocandins against Candida albicans, Candida dubliniensis, and Candida africana by time-kill curves. Diagn. Microbiol. Infect. Dis. 2015, 82, 57–61. [Google Scholar] [CrossRef]
- Kwolek-Mirek, M.; Zadrag-Tecza, R. Comparison of methods used for assessing the viability and vitality of yeast cells. FEMS Yeast Res. 2014, 14, 1068–1079. [Google Scholar] [CrossRef] [Green Version]
- Mor, V.; Rella, A.; Farnoud, A.M.; Singh, A.; Munshi, M.; Bryan, A.; Naseem, S.; Konopka, J.B.; Ojima, I.; Bullesbach, E.; et al. Identification of a New Class of Antifungals Targeting the Synthesis of fungal sphingolipids. mBio 2015, 6, e00647. [Google Scholar] [CrossRef] [PubMed]

| Code | Sequence | MIC/MFC µg/mL (µM) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| H99 | 2807 | 3279 | 2643 | ||||||
| MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | ||
| C5 | RRWQWR-Ahx-KLLKKLLK | 12.5 (6) | 12.5 (6) | 12.5 (6) | 12.5 (6) | 12.5 (6) | 12.5 (6) | 12.5 (6) | 12.5 (6) |
| C6 | KKWQWK-Ahx-RLLRRLLR | 6.25 (3) | 6.25 (3) | 6.25 (3) | 6.25 (3) | 6.25 (3) | 12.5 (6) | 6.25 (3) | 6.25 (3) |
| LfcinB (20–25) | RRWQWR | >25 (>25) | >25 (>25) | >25 (>25) | >25 (>25) | >25 (>25) | >25 (>25) | >25 (>25) | >25 (>25) |
| BFII (32–35)Pal | RLLRRLLR | >50 (>46) | >50 (>46) | >50 (>46) | >50 (>46) | >50 (>46) | >50 (>46) | >50 (>46) | >50 (>46) |
| [K]-LfcinB (20–25) | KKWQWK | >100 (111) | >100 (111) | >100 (111) | >100 (111) | ND | ND | ND | ND |
| [K]-BFII (32–35)Pal | KLLKKLLK | >100 (114) | >100 (114) | >100 (114) | >100 (114) | ND | ND | ND | ND |
| FLC | - | 4 | - | 16 | - | 16 | - | 8 | - |
| Code | Sequence | MIC/MFC µg/mL (µM) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CNAG_04693 | CNAG_01580 | CNAG_04804 | CNAG_06925 | CNAG_02050 | CNAG_02430 | CNAG_06348 | CNAG_00730 | CNAG_05150 | CNAG_02341 | CNAG_02915 | |||||||||||||
| MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | ||
| C5 | RRWQWR-Ahx-KLLKKLLK | 6.25 (3) | 25 (12) | ≤3.125 (≤2) | 3.125 (2) | 3.125 (2) | 3.125 (2) | 6.25 (3) | 6.25 (3) | 6.25 (3) | 6.25 (3) | 6.25 (3) | 6.25 (3) | 6.25 (3) | 6.25 (3) | 6.25 (3) | 6.25 (3) | 12.5 (6) | 12.5 (6) | 12.5 (6) | 12.5 (6) | ≤3.125 (≤2) | 3.125 (2) |
| C6 | KKWQWK-Ahx-RLLRRLLR | 6.25 (3) | 12.5 (6) | 6.25 (3) | 6.25 (3) | 6.25 (3) | 6.25 (3) | 6.25 (3) | 6.25 (3) | 6.25 (3) | 6.25 (3) | 6.25 (3) | 6.25 (3) | 6.25 (3) | 12.5 (6) | 3.125 (1.5) | 6.25 (3) | 6.25 (3) | 12.5 (6) | 6.25 (3) | 6.25 (3) | 3.125 (2) | 6.25 (3) |
| LfcinB (20–25) | RRWQWR | >25 (>25) | >25 (>25) | >25 (>25) | >25 (>25) | >25 (>25) | >25 (>25) | >50 (>51) | >50 (>51) | >50 (>51) | >50 (>51) | >50 (>51) | >50 (>51) | >50 (>51) | >50 (>51) | >50 (>51) | >50 (>51) | >25 (>25) | >25 (>25) | >25 (>25) | >25 (>25) | >25 (>25) | >25 (>25) |
| BFII (32–35)Pal | RLLRRLLR | >50 (>46) | >50 (>46) | >50 (>46) | >50 (>46) | >50 (>46) | >50 (>46) | >50 (>46) | >50 (>46) | >50 (>46) | >50 (>46) | >50 (>46) | >50 (>46) | >50 (>46) | >50 (>46) | >50 (>46) | >50 (>46) | >50 (>46) | >50 (>46) | >50 (>46) | >50 (>46) | >50 (>46) | >50 (>46) |
| FLC | - | 1 | 0.25 | 1 | 1 | 1 | 4 | 4 | 0.75 | 2 | 8 | 1.5 | |||||||||||
| Chimera | Strain | MIC µg/mL (µM) | Effect µg/mL (µM) | |
|---|---|---|---|---|
| Fungistatic | Fungicide | |||
| C5 RRWQWR-Ahx-KLLKKLLK | H99 | 12.5 (6) | 12.5 (6) | 25 (12) |
| 2807 | 12.5 (6) | 6.25 (3) | >12.5 (>6) | |
| 3279 | 12.5 (6) | 12.5 (6) | 25 (12) | |
| 2643 | 12.5 (6) | 6.25 (3) | >12.5 (>6) | |
| C6 KKWQWK-Ahx-RLLRRLLR | H99 | 6.25 (3) | 3.125 (1.5) | 6.25 (3) |
| 2807 | 6.25 (3) | 3.125 (1.5) | >6.25 (>3) | |
| 3279 | 6.25 (3) | 6.25 (3) | >12.5 (>6) | |
| 2643 | 6.25 (3) | 6.25 (3) | >12.5 (>6) | |
| Synergistic Effect of Chimera/FLC Combination against C. neoformans var. grubii | ||||||||
|---|---|---|---|---|---|---|---|---|
| Strain | Mixture | MICa | MICb | A | B | FICI | MICa/A | MICb/B |
| H99 | C5 + FLC | 12.5 | 4 | 6.25 | 2 | 1 | 2 | 2 |
| 2807 | 12.5 | 16 | 0.8 | 4 | 0.3 | 16 | 4 | |
| H99 | C6 + FLC | 6.25 | 4 | 1.56 | 2 | 0.8 | 4 | 2 |
| 2807 | 6.25 | 16 | 0.4 | 4 | 0.3 | 16 | 4 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvajal, S.K.; Vargas-Casanova, Y.; Pineda-Castañeda, H.M.; García-Castañeda, J.E.; Rivera-Monroy, Z.J.; Parra-Giraldo, C.M. In Vitro Antifungal Activity of Chimeric Peptides Derived from Bovine Lactoferricin and Buforin II against Cryptococcus neoformans var. grubii. Antibiotics 2022, 11, 1819. https://doi.org/10.3390/antibiotics11121819
Carvajal SK, Vargas-Casanova Y, Pineda-Castañeda HM, García-Castañeda JE, Rivera-Monroy ZJ, Parra-Giraldo CM. In Vitro Antifungal Activity of Chimeric Peptides Derived from Bovine Lactoferricin and Buforin II against Cryptococcus neoformans var. grubii. Antibiotics. 2022; 11(12):1819. https://doi.org/10.3390/antibiotics11121819
Chicago/Turabian StyleCarvajal, Silvia Katherine, Yerly Vargas-Casanova, Héctor Manuel Pineda-Castañeda, Javier Eduardo García-Castañeda, Zuly Jenny Rivera-Monroy, and Claudia Marcela Parra-Giraldo. 2022. "In Vitro Antifungal Activity of Chimeric Peptides Derived from Bovine Lactoferricin and Buforin II against Cryptococcus neoformans var. grubii" Antibiotics 11, no. 12: 1819. https://doi.org/10.3390/antibiotics11121819
APA StyleCarvajal, S. K., Vargas-Casanova, Y., Pineda-Castañeda, H. M., García-Castañeda, J. E., Rivera-Monroy, Z. J., & Parra-Giraldo, C. M. (2022). In Vitro Antifungal Activity of Chimeric Peptides Derived from Bovine Lactoferricin and Buforin II against Cryptococcus neoformans var. grubii. Antibiotics, 11(12), 1819. https://doi.org/10.3390/antibiotics11121819








