Investigating the Antibacterial Activity and Safety of Zinc Oxide Nanoparticles versus a Commercial Alcohol-Based Hand-Sanitizer: Can Zinc Oxide Nanoparticles Be Useful for Hand Sanitation?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of ZnO-NPs
Experimental Design and Statistical Analysis by a Full Factorial Design
2.2.2. Characterization of ZnO-NPs
UV-Visible Spectral Analysis
Particle Size and Zeta-Potential
Transmission Electron Microscopy (TEM)
Thermogravimetry and Differential Scanning Calorimeter (TGA/DSC)
Fourier Transformer Infra-Red (FTIR) Analysis
X-ray Diffraction (XRD) Analysis
2.2.3. Antibacterial Study
Susceptibility Test
Determination of Minimum Inhibitory Concentration (MIC)
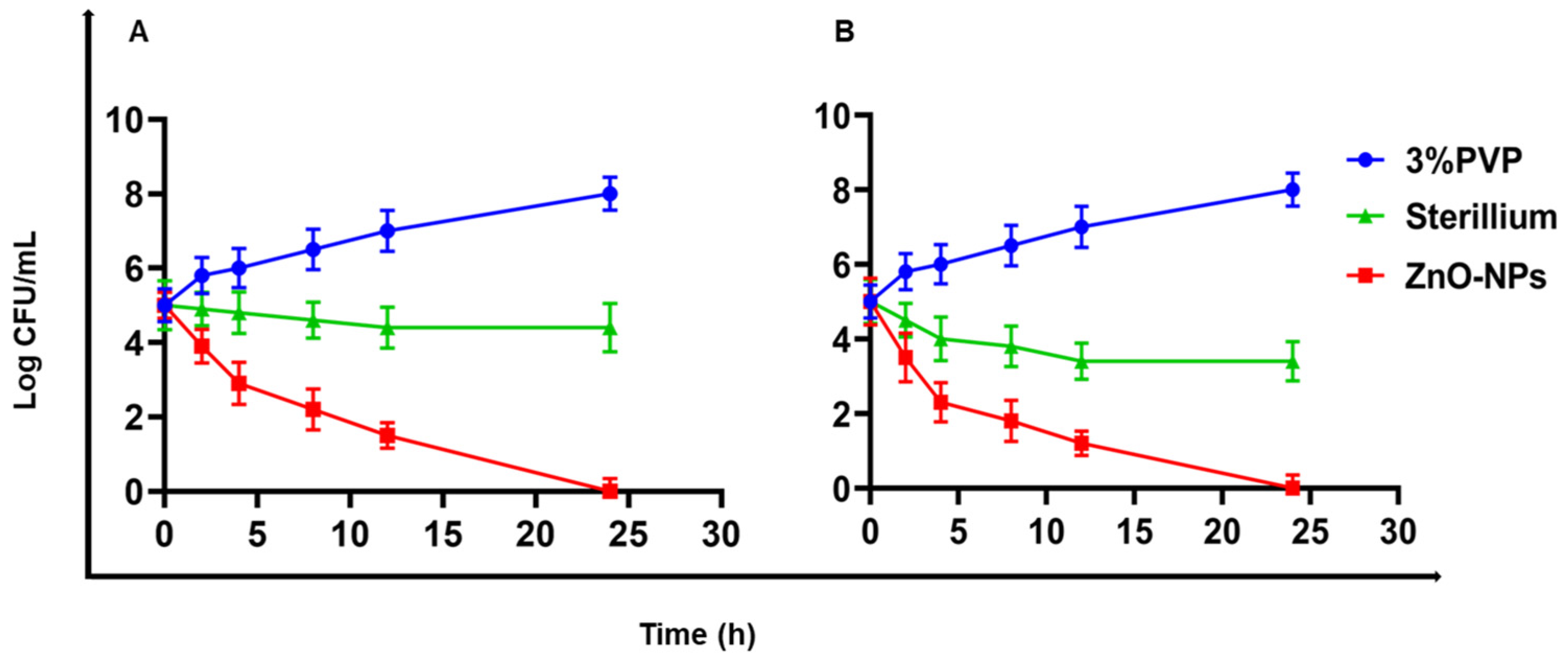
Time–Kill Curve Study of ZnO-NPs Solution
Transmission Electron Microscopy Study
2.2.4. Cytotoxicity Assay
Cell Culture
2.2.5. In Vivo Study
Animals
Experimental Design
Histopathological and Immunohistochemical Evaluations
Biochemical Analysis
Hematological Examination
2.2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterization of ZnO-NPs
3.1.1. Full Factorial Design Analysis
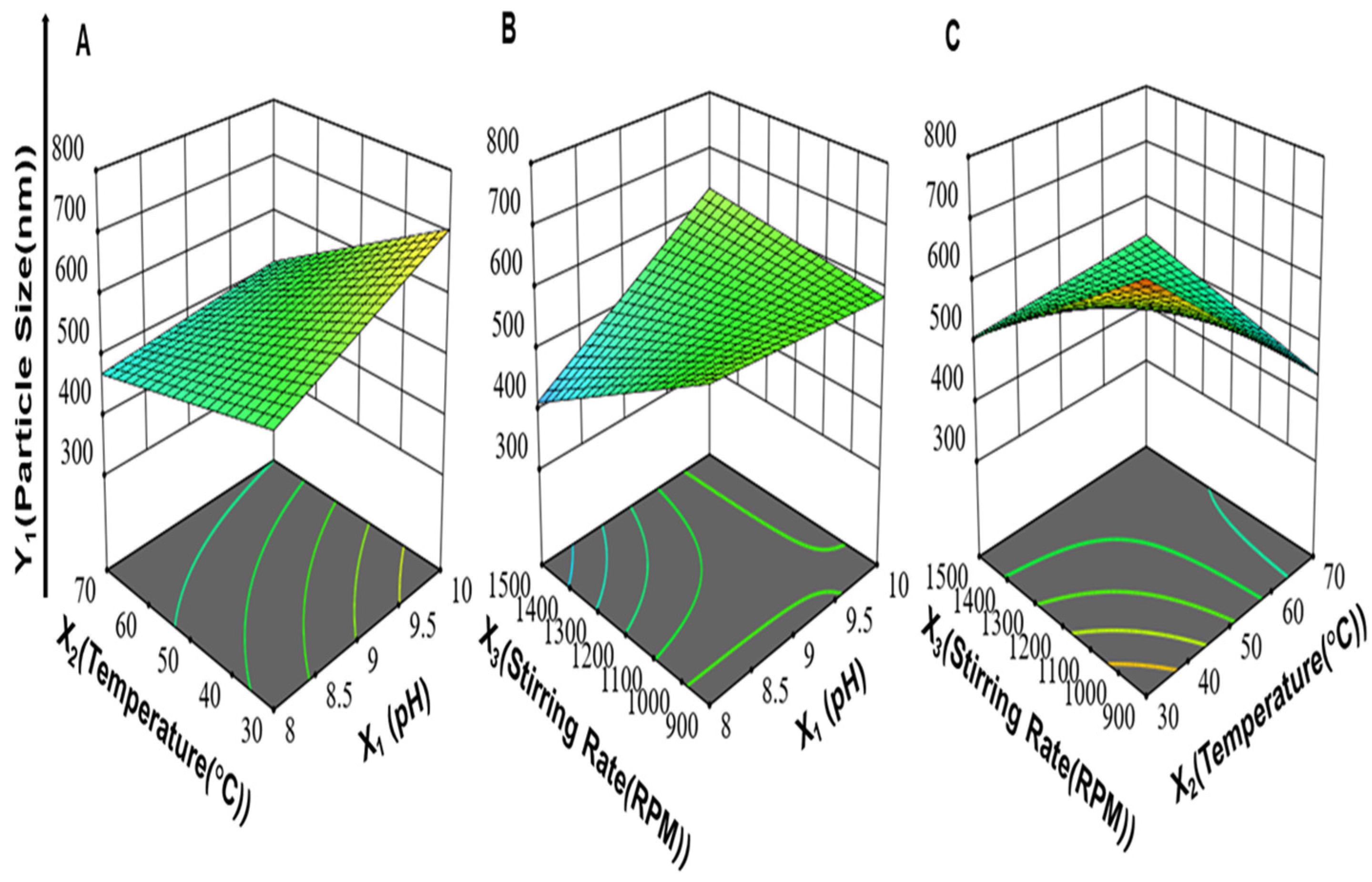
3.1.2. Effect of Synthesis Process Variables on Particle Size of ZnO-NPs (Y1)
3.1.3. Effect of Synthesis Process Variables on Homogeneity of ZnO-NPs (Y2)
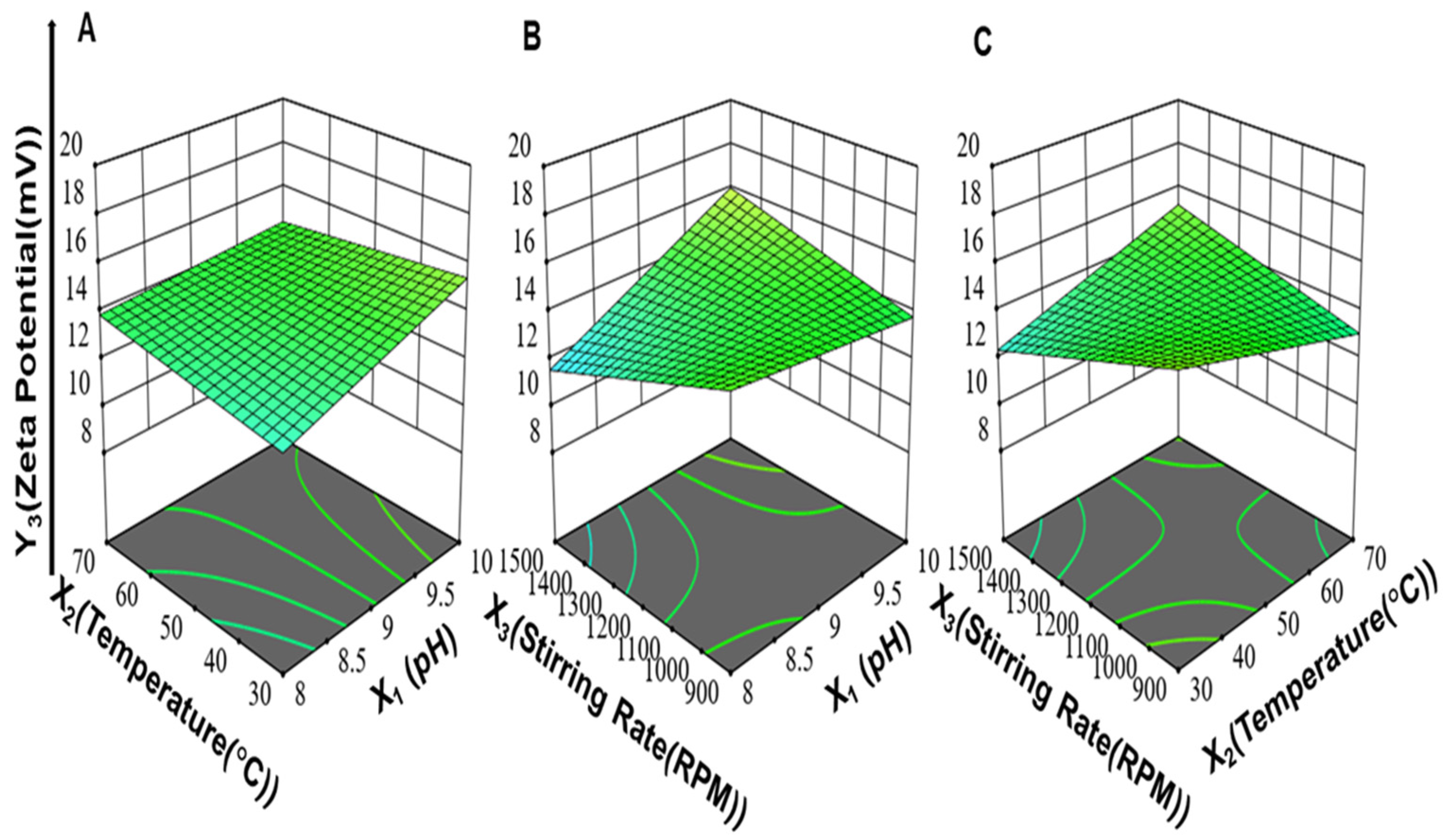
3.1.4. Effect of Synthesis Process Variables on Zeta Potential of ZnO-NPs (Y3)
3.1.5. Optimization of the Synthesized ZnO-NPs
3.1.6. Transmission Electron Microscopy (TEM)
3.2. Antibacterial Study
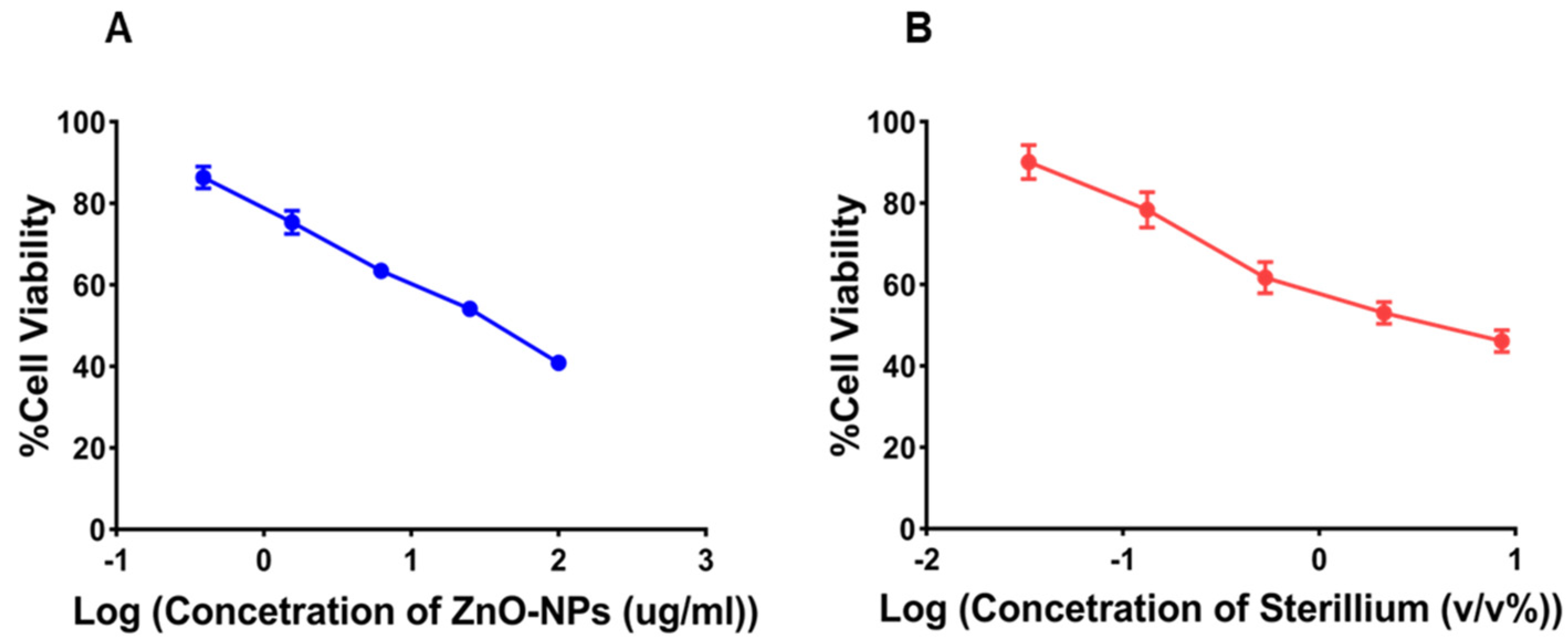
3.3. Cell Viability
3.4. In Vivo Study
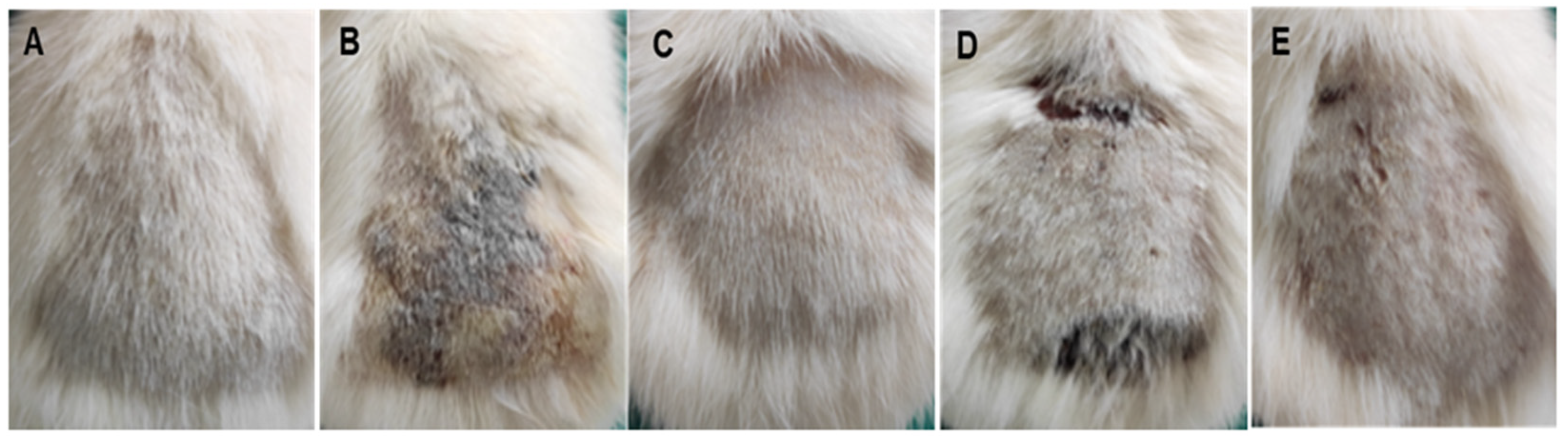
3.4.1. Histopathological Findings
3.4.2. Hematological Examination
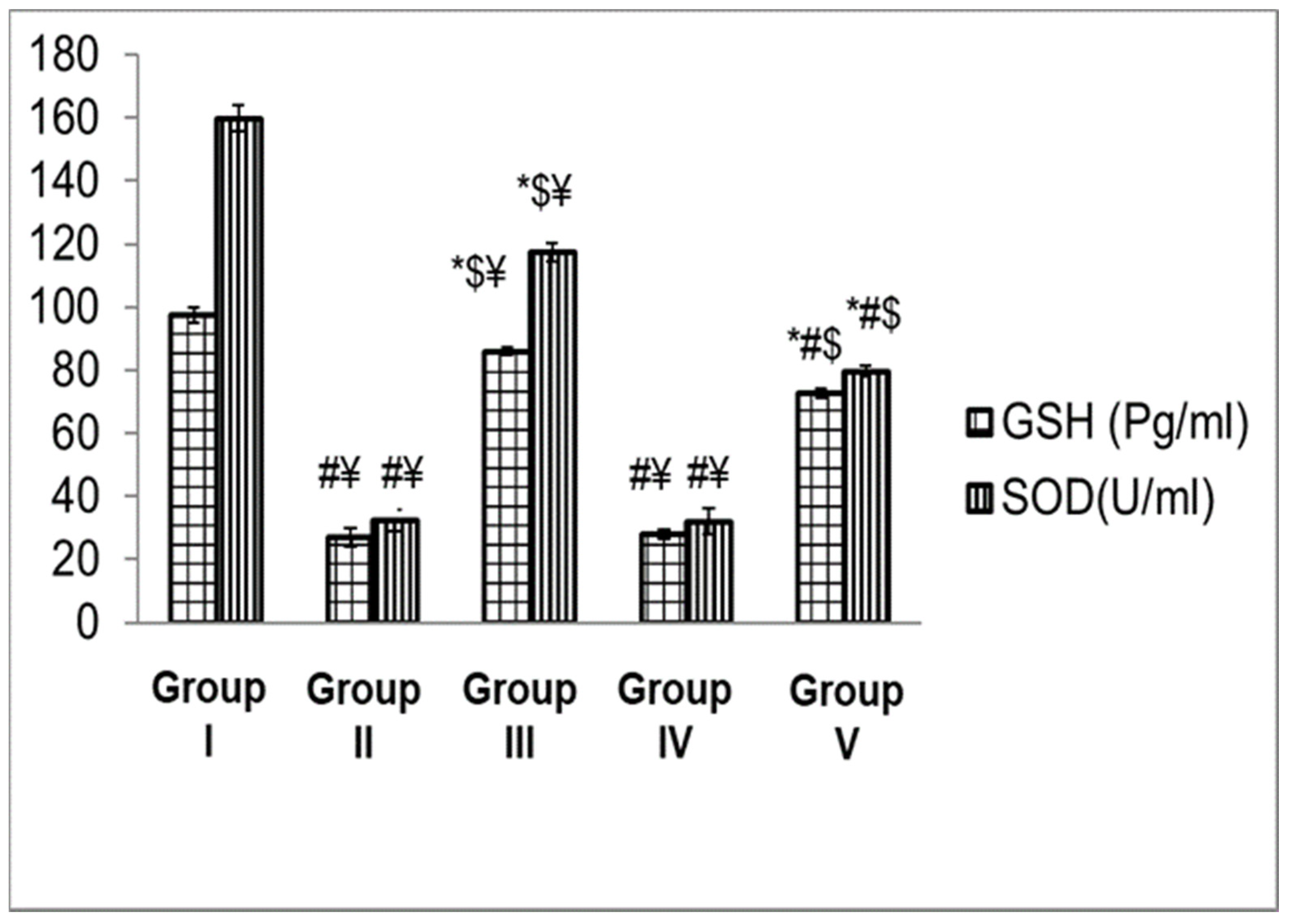
3.4.3. Biochemical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haque, M.; McKimm, J.; Sartelli, M.; Dhingra, S.; Labricciosa, F.M.; Islam, S.; Jahan, D.; Nusrat, T.; Chowdhury, T.S.; Coccolini, F.; et al. Strategies to Prevent Healthcare-Associated Infections: A Narrative Overview. Risk Manag. Healthc. Policy 2020, 13, 1765–1780. [Google Scholar] [CrossRef]
- Abo-zeid, Y.; Bakkar, M.R.; Elkhouly, G.E.; Raya, N.R.; Zaafar, D. Rhamnolipid Nano-Micelles versus Alcohol-Based Hand Sanitizer: A Comparative Study for Antibacterial Activity against Hospital-Acquired Infections and Toxicity Concerns. Antibiotics 2022, 11, 605. [Google Scholar] [CrossRef]
- Balkrishna, A.; Singh, K.; Singh, H.; Haldar, S.; Varshney, A. GermiX: A Skin Friendly Hand Sanitizer with Prolonged Effectivity against Pathogenic Bacteria. AMB Express 2020, 10, 210. [Google Scholar] [CrossRef]
- Hamam, S.; Sakr, A.; Zahran, W.; El Kholy, R.; Kasemy, Z.; Ibrahem, R.; Sakr, M.; Younis, F. Health Care-Associated Infections at an Egyptian Tertiary Care Hospital: A 2-Year Prospective Study. Menoufia Med. J. 2021, 34, 514. [Google Scholar] [CrossRef]
- Pittet, D.; Allegranzi, B.; Sax, H.; Dharan, S.; Pessoa-Silva, C.L.; Donaldson, L.; Boyce, J.M. Evidence-Based Model for Hand Transmission during Patient Care and the Role of Improved Practices. Lancet Infect. Dis. 2006, 6, 641–652. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Bakkar, M.R.; Faraag, A.H.I.; Soliman, E.R.S.; Fouda, M.S.; Sarguos, A.M.M.; McLean, G.R.; Hebishy, A.M.S.; Elkhouly, G.E.; Raya, N.R.; Abo-zeid, Y. Rhamnolipids Nano-Micelles as a Potential Hand Sanitizer. Antibiotics 2021, 10, 751. [Google Scholar] [CrossRef]
- Onyedibe, K.I.; Shehu, N.Y.; Pires, D.; Isa, S.E.; Okolo, M.O.; Gomerep, S.S.; Ibrahim, C.; Igbanugo, S.J.; Odesanya, R.U.; Olayinka, A.; et al. Assessment of Hand Hygiene Facilities and Staff Compliance in a Large Tertiary Health Care Facility in Northern Nigeria: A Cross Sectional Study. Antimicrob. Resist. Infect. Control 2020, 9, 30. [Google Scholar] [CrossRef] [Green Version]
- Chiang, W.L.; Chen, T.W.; Liu, M.Y.; Hsu, C.J. Application and Robust H Control of PDC Fuzzy Controller for Nonlinear Systems with External Disturbance. J. Mar. Sci. Technol. 2001, 9, 84–90. [Google Scholar] [CrossRef]
- Emami, A.; Javanmardi, F.; Keshavarzi, A.; Pirbonyeh, N. Hidden Threat Lurking behind the Alcohol Sanitizers in COVID-19 Outbreak. Dermatol. Ther. 2020, 33, e13627. [Google Scholar] [CrossRef]
- Mahmood, A.; Eqan, M.; Pervez, S.; Ahmed, H.; Bari, A. COVID-19 and Frequent Use of Hand Sanitizers; Human Health and Environmental Hazards by Exposure Pathways. Sci. Total Environ. 2020, 742, 140561. [Google Scholar] [CrossRef]
- Vogel, L. Hand Sanitizers May Increase Norovirus Risk. CMAJ 2011, 183, 799–800. [Google Scholar] [CrossRef] [Green Version]
- Blaney, D.D.; Daly, E.R.; Kirkland, K.B.; Tongren, J.E.; Kelso, P.T.; Talbot, E.A. Use of Alcohol-Based Hand Sanitizers as a Risk Factor for Norovirus Outbreaks in Long-Term Care Facilities in Northern New England: December 2006 to March 2007. Am. J. Infect. Control 2011, 39, 296–301. [Google Scholar] [CrossRef]
- Pidot, S.J.; Gao, W.; Buultjens, A.H.; Monk, I.R.; Guerillot, R.; Carter, G.P.; Lee, J.Y.H.; Lam, M.M.C.; Grayson, M.L.; Ballard, S.A.; et al. Increasing Tolerance of Hospital Enterococcus Faecium to Handwash Alcohols. Sci. Transl. Med. 2018, 10, eaar6115. [Google Scholar] [CrossRef] [Green Version]
- Hayat, A.; Munnawar, F. Antibacterial Effectiveness of Commercially Available Hand Sanitizers. Int. J. Biol. Biotech 2016, 13, 427–431. [Google Scholar]
- Szunerits, S.; Barras, A.; Khanal, M.; Pagneux, Q.; Boukherroub, R. Nanostructures for the Inhibition of Viral Infections. Molecules 2015, 20, 14051–14081. [Google Scholar] [CrossRef]
- Abo-zeid, Y.; Williams, G.R.; Touabi, L.; Mclean, G.R. An Investigation of Rhinovirus Infection on Cellular Uptake of Poly (Glycerol-Adipate) Nanoparticles. Int. J. Pharm. 2020, 589, 119826. [Google Scholar] [CrossRef]
- Hillaireau, H.; Le Doan, T.; Appel, M.; Couvreur, P. Hybrid Polymer Nanocapsules Enhance in Vitro Delivery of Azidothymidine-Triphosphate to Macrophages. J. Control. Release 2006, 116, 346–352. [Google Scholar] [CrossRef]
- Abo-zeid, Y.; Mantovani, G.; Irving, W.L.; Garnett, M.C. Synthesis of Nucleoside-Boronic Esters Hydrophobic pro-Drugs: A Possible Route to Improve Hydrophilic Nucleoside Drug Loading into Polymer Nanoparticles. J. Drug Deliv. Sci. Technol. 2018, 46, 354–364. [Google Scholar] [CrossRef]
- Hashim, F.; El-Ridy, M.; Nasr, M.; Abdallah, Y. Preparation and Characterization of Niosomes Containing Ribavirin for Liver Targeting. Drug Deliv. 2010, 17, 282–287. [Google Scholar] [CrossRef]
- Lembo, D.; Swaminathan, S.; Donalisio, M.; Civra, A.; Pastero, L.; Aquilano, D.; Vavia, P.; Trotta, F.; Cavalli, R. Encapsulation of Acyclovir in New Carboxylated Cyclodextrin-Based Nanosponges Improves the Agent’s Antiviral Efficacy. Int. J. Pharm. 2013, 443, 262–272. [Google Scholar] [CrossRef]
- Abo-zeid, Y.; Garnett, M.C. Polymer Nanoparticle as a Delivery System for Ribavirin: Do Nanoparticle Avoid Uptake by Red Blood Cells? J. Drug Deliv. Sci. Technol. 2020, 56, 101552. [Google Scholar] [CrossRef]
- Sobhy, Y.; Mady, M.; Mina, S.; Abo-zeid, Y. Phytochemical and Pharmacological Values of Two Major Constituents of Asparagus Species and Their Nano Formulations: A Review. J. Adv. Pharm. Res. 2022, 6, 94–106. [Google Scholar] [CrossRef]
- Burgess, K.; Li, H.; Abo-Zeid, Y.; Fatimah; Williams, G.R. The Effect of Molecular Properties on Active Ingredient Release from Electrospun Eudragit Fibers. Pharmaceutics 2018, 10, 103. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, Z.; Abo-zeid, Y.; Bear, J.C.; Davies, G.; Lei, X.; Williams, G.R. SiO2-Coated Layered Gadolinium Hydroxides for Simultaneous Drug Delivery and Magnetic Resonance Imaging. J. Solid State Chem. 2020, 286, 121291. [Google Scholar] [CrossRef]
- Ali, A.M.; Hill, H.J.; Elkhouly, G.E.; Bakkar, M.R.; Raya, N.R.; Stamataki, Z.; Abo-zeid, Y. Rhamnolipid Nano-Micelles Inhibit SARS-CoV-2 Infection and Have No Dermal or Eye Toxic Effects in Rabbits. Antibiotics 2022, 11, 1556. [Google Scholar] [CrossRef]
- Abo-Zeid, Y.; Ismail, N.S.; McLean, G.R.; Hamdy, N.M. A Molecular Docking Study Repurposes FDA Approved Iron Oxide Nanoparticles to Treat and Control COVID-19 Infection. Eur. J. Pharm. Sci. 2020, 153, 105465. [Google Scholar] [CrossRef]
- Abo-zeid, Y.; Urbanowicz, R.A.; Thomsonb, B.J.; William, L.; Irvingb, A.W.T.; Garnett, M.C. Enhanced Nanoparticle Uptake into Virus Infected Cells: Could Nanoparticles Be Useful in Antiviral Therapy? Int. J. Pharm. 2018, 547, 572–581. [Google Scholar] [CrossRef]
- Chintagunta, A.D.; Krishna, M.S.; Nalluru, S.; Kumar N. S., . Nanotechnology: An Emerging Approach to Combat COVID-19. Emergent Mater. 2021, 4, 119–130. [Google Scholar] [CrossRef]
- Rangayasami, A.; Kannan, K.; Murugesan, S.; Radhika, D.; Sadasivuni, K.K.; Reddy, K.R.; Raghu, A.V. Influence of Nanotechnology to Combat against COVID-19 for Global Health Emergency: A Review. Sens. Int. 2021, 2, 100079. [Google Scholar] [CrossRef]
- Abo-zeid, Y.; Amer, A.; El-Houssieny, B.; Mahmoud, M.; Sakran, W. Overview on Bacterial Resistance and Nanoparticles to Overcome Bacterial Resistance. J. Adv. Pharm. Res. 2021, 5, 312–326. [Google Scholar] [CrossRef]
- Abo-zeid, Y.; Williams, G.R. The Potential Anti-Infective Applications of Metal Oxide Nanoparticles: A Systematic Review. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1592. [Google Scholar] [CrossRef]
- Raghunath, A.; Perumal, E. Metal Oxide Nanoparticles as Antimicrobial Agents: A Promise for the Future. Int. J. Antimicrob. Agents 2017, 49, 137–152. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Pi, J.; Cai, J. The Advancing of Zinc Oxide Nanoparticles for Biomedical Applications. Bioinorg. Chem. Appl. 2018, 2018, 1062562. [Google Scholar] [CrossRef]
- Rasmussen, J.W.; Martinez, E.; Louka, P.; Wingett, D.G. Zinc Oxide Nanoparticles for Selective Destruction of Tumor Cells and Potential for Drug Delivery Applications. Expert Opin. Drug Deliv. 2010, 7, 1063–1077. [Google Scholar] [CrossRef] [Green Version]
- Bedi, P.S.; Kaur, A. An Overview On Uses of Zinc Oxide Nanoparticles. World J. Pharm. Pharm. Sci. 2015, 4, 1177–1196. [Google Scholar]
- Elgaied, A.M.A.; Nofal, A.M.Z.; Kasemy, Z.B.A.; Elaziz, M.M.A.; El Nasr, I.A.S. Influence of Different Scrubbing Methods of Surgical Team on Surgical Site Infection in Cesarean Section. Egypt. J. Hosp. Med. 2021, 83, 1082–1087. [Google Scholar] [CrossRef]
- Hassan, R.; El-Gilany, A.H.; Abd elaal, A.M.; El-Mashad, N.; Azim, D.A. An Overview of Healthcare-Associated Infections in a Tertiary Care Hospital in Egypt. Infect. Prev. Pract. 2020, 2, 100059. [Google Scholar] [CrossRef]
- Saied, T.; Elkholy, A.; Hafez, S.F.; Basim, H.; Wasfy, M.O.; El-Shoubary, W.; Samir, A.; Pimentel, G.; Talaat, M. Antimicrobial Resistance in Pathogens Causing Nosocomial Bloodstream Infections in University Hospitals in Egypt. Am. J. Infect. Control 2011, 39, e61–e65. [Google Scholar] [CrossRef]
- Abo safe, F.; Salah, N.; Rashed, M.; Saad, S. Susceptibility of Aminoglycoside Resistant Acinetobacter Baumannii to Antibiotic Combinations. Egypt. J. Microbiol. 2019, 54, 137–147. [Google Scholar] [CrossRef] [Green Version]
- Sudha, M.; Senthilkumar, S.; Hariharan, R.; Suganthi, A.; Rajarajan, M. Synthesis, Characterization and Study of Photocatalytic Activity of Surface Modified ZnO Nanoparticles by PEG Capping. J. Sol-Gel Sci. Technol. 2013, 65, 301–310. [Google Scholar] [CrossRef]
- Habib, B.A.; Abd El-Samiae, A.S.; El-Houssieny, B.M.; Tag, R. Formulation, Characterization, Optimization, and in-Vivo Performance of Febuxostat Self-Nano-Emulsifying System Loaded Sublingual Films. Drug Deliv. 2021, 28, 1321–1333. [Google Scholar] [CrossRef] [PubMed]
- El Naggar, A.M.A.; Nassar, I.M.; Gobara, H.M. Enhanced Hydrogen Production from Water via a Photo-Catalyzed Reaction Using Chalcogenide d-Element Nanoparticles Induced by UV Light. Nanoscale 2013, 5, 9994–9999. [Google Scholar] [CrossRef] [PubMed]
- Winiarski, J.; Tylus, W.; Winiarska, K.; Szczygieł, I.; Szczygieł, B. XPS and FT-IR Characterization of Selected Synthetic Corrosion Products of Zinc Expected in Neutral Environment Containing Chloride Ions. J. Spectrosc. 2018, 2018, 2079278. [Google Scholar] [CrossRef] [Green Version]
- Devaraj, N.K.; Han, T.C.; Low, P.L.; Ong, B.H.; Sin, Y.K. Synthesis and Characterisation of Zinc Oxide Nanoparticles for Thermoelectric Application. Mater. Res. Innov. 2014, 18, S6-350–S6-353. [Google Scholar] [CrossRef]
- CLSI Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing Supplement M100S; CLSI Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2016; pp. 19087. ISBN 1-56238-923-8. [Google Scholar]
- Bancroft, D.J.; Churchill, M.G. ; Theory and Practice of Histological Techniques; Churchill Livingstone: Chatswood, Australia, 2008; Volume 36, ISBN 9780443102790. [Google Scholar]
- Hirasawa, Y.; Ohtsu, S.; Matsui, Y.; Nagase, T.; Shimizu, M.; Masaaki, O.; Kyuki, K.; Takahashi, T.; Takahashi, K. Assessing Effects of a Product Containing Crude Drugs Including Scutellaria Root, Phellodendron Bark, Coptis Rhizome and Product Containing Crude Drugs Including Lithospermum Root, Japanese Angelica Root, Sesame Oil in Atopic Dermatitis NC/Nga Mice. Pharmacometrics 2000, 59, 123–134. [Google Scholar]
- Ellman, G.L. Tissue Sulfhydryl Groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- MARKLUND, S.; MARKLUND, G. Involvement of the Superoxide Anion Radical in the Autoxidation of Pyrogallol and a Convenient Assay for Superoxide Dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Ghamsari, M.S.; Alamdari, S.; Han, W.; Park, H.H. Impact of Nanostructured Thin ZnO Film in Ultraviolet Protection. Int. J. Nanomed. 2017, 12, 207–216. [Google Scholar] [CrossRef] [Green Version]
- Akhil, K.; Jayakumar, J.; Gayathri, G.; Khan, S.S. Effect of Various Capping Agents on Photocatalytic, Antibacterial and Antibiofilm Activities of ZnO Nanoparticles. J. Photochem. Photobiol. B Biol. 2016, 160, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Spoială, A.; Ilie, C.-I.; TrusCă, R.-D.; Oprea, O.-C.; Surdu, V.-A.; Vasile, S.; Ficai, A.; Ficai, D.; Andronescu, E.; Ditu, L.-M. Zinc Oxide Nanoparticles for Water Purification. Materials 2021, 14, 4747. [Google Scholar] [CrossRef] [PubMed]
- Dilshad, M.R.; Islam, A.; Sabir, A.; Shafiq, M.; Butt, M.T.Z.; Ijaz, A.; Jamil, T. Fabrication and Performance Characterization of Novel Zinc Oxide Filled Cross-Linked PVA/PEG 600 Blended Membranes for CO2/N2 Separation. J. Ind. Eng. Chem. 2017, 55, 65–73. [Google Scholar] [CrossRef]
- Kajbafvala, A.; Zanganeh, S.; Kajbafvala, E.; Zargar, H.R.; Bayati, M.R.; Sadrnezhaad, S.K. Microwave-Assisted Synthesis of Narcis-like Zinc Oxide Nanostructures. J. Alloys Compd. 2010, 497, 325–329. [Google Scholar] [CrossRef]
- Varughese, A.; Kaur, R.; Singh, P. Green Synthesis and Characterization of Copper Oxide Nanoparticles Using Psidium Guajava Leaf Extract. IOP Conf. Ser. Mater. Sci. Eng. 2020, 961, 012011. [Google Scholar] [CrossRef]
- Xu, X.; He, Z.; Lu, S.; Guo, D.; Yu, J. Enhanced Thermal and Mechanical Properties of Lignin/Polypropylene Wood-Plastic Composite by Using Flexible Segment-Containing Reactive Compatibilizer. Macromol. Res. 2014, 22, 1084–1089. [Google Scholar] [CrossRef]
- Tai, M.F.; Lai, C.W.; Abdul Hamid, S.B. Facile Synthesis Polyethylene Glycol Coated Magnetite Nanoparticles for High Colloidal Stability. J. Nanomater. 2016, 2016, 8612505. [Google Scholar] [CrossRef] [Green Version]
- Devi, R.S.; Gayathri, R. Green Synthesis of Zinc Oxide Nanoparticles by Using Hibiscus Rosa-Sinensis. Int. J. Curr. Eng. Technol. 2014, 4, 2444–2446. [Google Scholar]
- Alias, S.S.; Ismail, A.B.; Mohamad, A.A. Effect of PH on ZnO Nanoparticle Properties Synthesized by Sol-Gel Centrifugation. J. Alloys Compd. 2010, 499, 231–237. [Google Scholar] [CrossRef]
- Ikono, R.; Akwalia, R.; Siswanto, W.; Bambang, W.; Sukarto, A.; Rochman, N.T. Effect of PH Variation on Particle Size and Purity of Nano Zinc Oxide Synthesized by Sol-Gel Method. Int. J. Eng. Technol. IJET-IJENS 2012, 12, 5. [Google Scholar]
- Shaba, E.Y.; Jacob, J.O.; Tijani, J.O.; Suleiman, M.A.T. A Critical Review of Synthesis Parameters Affecting the Properties of Zinc Oxide Nanoparticle and Its Application in Wastewater Treatment; Springer International Publishing: Cham, Switzerland, 2021; Volume 11, ISBN 0123456789. [Google Scholar]
- Siswanto; Rochman, N.T.; Akwalia, P.R. Fabrication and Characterization of Zinc Oxide (ZnO) Nanoparticle by Sol-Gel Method. J. Phys. Conf. Ser. 2017, 853, 012041. [Google Scholar] [CrossRef] [Green Version]
- Kumari, M.; Mishra, A.; Pandey, S.; Singh, S.P.; Chaudhry, V.; Mudiam, M.K.R.; Shukla, S.; Kakkar, P.; Nautiyal, C.S. Physico-Chemical Condition Optimization during Biosynthesis Lead to Development of Improved and Catalytically Efficient Gold Nano Particles. Sci. Rep. 2016, 6, 27575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saxena, J.; Sharma, P.K.; Sharma, M.M.; Singh, A. Process Optimization for Green Synthesis of Silver Nanoparticles by Sclerotinia Sclerotiorum MTCC 8785 and Evaluation of Its Antibacterial Properties. Springerplus 2016, 5, 861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Zhou, S.; Hou, P.; Yang, Y.; Weng, J.; Li, X.; Li, M. Synthesis and Characterization of Biocompatible Fe3O4 Nanoparticles Jing. J. Biomed. Mater. Res. Part A 2007, 80, 333–341. [Google Scholar] [CrossRef]
- Nidhin, M.; Indumathy, R.; Sreeram, K.J.; Nair, B.U. Synthesis of Iron Oxide Nanoparticles of Narrow Size Distribution on Polysaccharide Templates. Bull. Mater. Sci. 2008, 31, 93–96. [Google Scholar] [CrossRef]
- Kalliola, S.; Repo, E.; Sillanpää, M.; Singh Arora, J.; He, J.; John, V.T. The Stability of Green Nanoparticles in Increased PH and Salinity for Applications in Oil Spill-Treatment. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 493, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Hussain, Z.; Sahudin, S. Preparation, Characterisation and Colloidal Stability of Chitosan-Tripolyphosphate Nanoparticles: Optimisation of Formulation and Process Parameters. Int. J. Pharm. Pharm. Sci. 2016, 8, 297–308. [Google Scholar]
- Lau, C.P.; Abdul-Wahab, M.F.; Jaafar, J.; Chan, G.F.; Rashid, N.A.A. Effect of PH and Biological Media on Polyvinylpyrrolidone-Capped Silver Nanoparticles. AIP Conf. Proc. 2016, 1756, 1–8. [Google Scholar] [CrossRef]
- Nurdin, I.; Ridwan; Satriananda. The Effect of PH and Time on the Stability of Superparamagnetic Maghemite Nanoparticle Suspensions. MATEC Web Conf. 2016, 39, 3–6. [Google Scholar] [CrossRef] [Green Version]
- Das, P.K. Effect of Temperature on Zeta Potential of Functionalized Gold Nanorod. Microfluid. Nanofluid. 2017, 21, 95. [Google Scholar] [CrossRef]
- Zohri, M.; Javar, H.A.; Gazori, T.; Khoshayand, M.R.; Aghaee-Bakhtiari, S.H.; Ghahremani, M.H. Response Surface Methodology for Statistical Optimization of Chitosan/Alginate Nanoparticles as a Vehicle for Recombinant Human Bone Morphogenetic Protein-2 Delivery. Int. J. Nanomed. 2020, 15, 8345–8356. [Google Scholar] [CrossRef] [PubMed]
- El-Sahrigy, S.A.F.; Shouman, M.G.; Ibrahim, H.M.; Rahman, A.M.O.A.; Habib, S.A.; Khattab, A.A.; Gomaa, H.E.; Helmy, N.A. Prevalence and Anti-Microbial Susceptibility of Hospital Acquired Infections in Two Pediatric Intensive Care Units in Egypt. Open Access Maced. J. Med. Sci. 2019, 7, 1744–1749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tayel, A.A.; El-Tras, W.F.; Moussa, S.; El-Baz, A.F.; Mahrous, H.; Salem, M.F.; Brimer, L. Antibacterial Action of Zinc Oxide Nanoparticles against Foodborne Pathogens. J. Food Saf. 2011, 31, 211–218. [Google Scholar] [CrossRef]
- Reddy, M.; Feris, K.; Bell, J.; Wingett, G.D.; Hanley, C.; Punnoose, A. Selective Toxicity of Zinc Oxide Nanoparticles to Prokaryotic and Eukaryotic Systems. Appl. Phys. Lett. 2007, 90, 213902-1–213902-3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Hu, C.; Shao, L. The Antimicrobial Activity of Nanoparticles: Present Situation and Prospects for the Future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [Green Version]
- De Freitas Ferreira, J.; Vieira, E.A.; Nitschke, M. The Antibacterial Activity of Rhamnolipid Biosurfactant Is PH Dependent. Food Res. Int. 2019, 116, 737–744. [Google Scholar] [CrossRef]
- Pati, R.; Mehta, R.K.; Mohanty, S.; Padhi, A.; Sengupta, M.; Vaseeharan, B.; Goswami, C.; Sonawane, A. Topical Application of Zinc Oxide Nanoparticles Reduces Bacterial Skin Infection in Mice and Exhibits Antibacterial Activity by Inducing Oxidative Stress Response and Cell Membrane Disintegration in Macrophages. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1195–1208. [Google Scholar] [CrossRef]
- Silva, B.L.d.; Abuçafy, M.P.; Manaia, E.B.; Junior, J.A.O.; Chiari-Andréo, B.G.; Pietro, R.C.L.R.; Chiavacci, L.A. Relationship between Structure and Antimicrobial Activity of Zinc Oxide Nanoparticles: An Overview. Int. J. Nanomed. 2019, 14, 9395–9410. [Google Scholar] [CrossRef] [Green Version]
- Akbar, A.; Anal, K. Zinc Oxide Nanoparticles Loaded Active Packaging, a Challenge Study against Salmonells Typhimurium and Staphylococcus Aureus in Ready-to-Eat-Poultry Meat. Food Control 2014, 38, 88–95. [Google Scholar] [CrossRef]
- Tiwari, V.; Mishra, N.; Gadani, K.; Solanki, P.S.; Shah, N.A.; Tiwari, M. Mechanism of Anti-Bacterial Activity of Zinc Oxide Nanoparticle against Carbapenem-Resistant Acinetobacter Baumannii. Front. Microbiol. 2018, 9, 1218. [Google Scholar] [CrossRef] [Green Version]
- Hamouda, R.A.; Yousuf, W.E.; Mohammed, A.B.A.; Mohammed, R.S.; Darwish, D.B.; Abdeen, E.E. Comparative Study between Zinc Oxide Nanoparticles Synthesis by Biogenic and Wet Chemical Methods in Vivo and in Vitro against Staphylococcus Aureus. Microb. Pathog. 2020, 147, 104384. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, H.; Menon, S.; Venkat Kumar, S.; Rajeshkumar, S. Mechanistic Study on Antibacterial Action of Zinc Oxide Nanoparticles Synthesized Using Green Route. Chem. Biol. Interact. 2018, 286, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.L.J.; Yi, T.P.; Bose, R.J.C.; McCarthy, J.R.; Tharmalingam, N.; Madheswaran, T. Hand Sanitizers: A Review on Formulation Aspects, Adverse Effects, and Regulations. Int. J. Environ. Res. Public Health 2020, 17, 3326. [Google Scholar] [CrossRef] [PubMed]
- Suchomel, M.; Rotter, M. Ethanol in Pre-Surgical Hand Rubs: Concentration and Duration of Application for Achieving European Norm EN 12791. J. Hosp. Infect. 2011, 77, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Fred, T.; Sophia, K.; Alex, S.; Emmanuel, B.; Tom, L.; Lucas, A. Comparison of Antibacterial Efficacy of Locally Produced Alcohol Based Hand Sanitizer and Commonly Available Commercial Hand Sanitizer Used in Healthcare Facilities in Uganda. OALib 2020, 7, 1–13. [Google Scholar] [CrossRef]
- Ryu, H.J.; Seo, M.Y.; Jung, S.K.; Maeng, E.H.; Lee, S.Y.; Jang, D.H.; Lee, T.J.; Jo, K.Y.; Kim, Y.R.; Cho, K.B.; et al. Zinc Oxide Nanoparticles: A 90-Day Repeated-Dose Dermal Toxicity Study in Rats. Int. J. Nanomed. 2014, 9, 137–144. [Google Scholar] [CrossRef]
- Gaupp, R.; Ledala, N.; Somerville, G.A. Staphylococcal Response to Oxidative Stress. Front. Cell. Infect. Microbiol. 2012, 2, 33. [Google Scholar] [CrossRef] [Green Version]
- Stark, L. Staphylococcus Aureus- Aspects of Pathogenesis and Molecular Epidemiology; Scandinavian Society for Prehistoric Art, Tanums Hällristningsmuseum Underslös: Tanumshed, Sweden, 2013; ISBN 9789175195681. [Google Scholar]
- Backx, M.; Healy, B. Serious Staphylococcal Infections. Clin. Med. J. R. Coll. Phys. Lond. 2008, 8, 535–538. [Google Scholar] [CrossRef]
- Lagler, H.; Bangert, C.; Quint, T.; Österreicher, Z.; Nussbaumer-Pröll, A.; Eberl, S.; Weber, M.; Karer, M.; Sommer, M.O.A.; Zeitlinger, M. Comparison of Non-Invasive Staphylococcus Aureus Sampling Methods on Lesional Skin in Patients with Atopic Dermatitis. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 245–252. [Google Scholar] [CrossRef]
- Kugelberg, E.; Norström, T.; Petersen, T.K.; Duvold, T.; Andersson, D.I.; Hughes, D. Establishment of a Superficial Skin Infection Model in Mice by Using Staphylococcus Aureus and Streptococcus Pyogenes. Antimicrob. Agents Chemother. 2005, 49, 3435–3441. [Google Scholar] [CrossRef] [Green Version]
- Onunkwo, C.C.; Hahn, L.B.; Sohnle, P.G. Clearance of Experimental Cutaneous Staphylococcus Aureus Infections in Mice. Natl. Inst. Health 2010, 302, 375–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, Y.; Oscherwitz, J.; Cease, K.B.; Chan, S.M.; Muñoz-Planillo, R.; Hasegawa, M.; Villaruz, A.E.; Cheung, G.Y.C.; McGavin, M.J.; Travers, J.B.; et al. Staphylococcus δ-Toxin Induces Allergic Skin Disease by Activating Mast Cells. Nature 2013, 503, 397–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, R.M.; Nakatsuji, T.; Gallo, L.R. Staphylococcus Aureus: Master Manipulator of the Skin. Health Hum. Serv. 2017, 22, 579–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurgoze, S.; Sahin, T.; Sevgili, M.; Ozkutlu, Z.; Ozan, S. The Effects of Ivermectin or Doramectin Treatment on Some Antioxidant Enzymes and the Level of Lipid Peroxidation in Sheep with Natural Sarcoptic Scap. Yuz. YIl Univ. Vet. Fak. Derg. 2003, 14, 30–34. [Google Scholar]
- DeJong, R.J.; Miller, L.M.; Molina-Cruz, A.; Gupta, L.; Kumar, S.; Barillas-Mury, C. Reactive Oxygen Species Detoxification by Catalase Is a Major Determinant of Fecundity in the Mosquito Anopheles Gambiae. Proc. Natl. Acad. Sci. USA. 2007, 104, 2121–2126. [Google Scholar] [CrossRef] [Green Version]
- Alghasham, A.; Salem, T.A.; Meki, A.R.M. Effect of Cadmium-Polluted Water on Plasma Levels of Tumor Necrosis Factor-α, Interleukin-6 and Oxidative Status Biomarkers in Rats: Protective Effect of Curcumin. Food Chem. Toxicol. 2013, 59, 160–164. [Google Scholar] [CrossRef]





| Factors | Levels | |
|---|---|---|
| Low (−1) | High (+1) | |
| X1: pH | 8 | 10 |
| X2: Reaction temperature (°C) | 30 | 70 |
| X3: Stirring rate (RPM) | 900 | 1500 |
| Responses | Units | Constraints |
| Y1: Particle size | Nm | Minimum |
| Y2: PDI | - | Minimum |
| Y3: Zeta potential | mV | Maximum |
| Formula Code | pH | Reaction Temperature (°C) | Stirring Rate (RPM) | Particle Size (Dnm ± SD) | Polydispersity Index (PDI) ± SD | Zeta Potential (mV ± SD) |
|---|---|---|---|---|---|---|
| F1 | 8 | 30 | 900 | 741.63 ± 36.04 | 0.265 ± 0.02 | −13.77 ± 0.35 |
| F2 | 8 | 30 | 1500 | 379.60 ± 39.80 | 0.33 ± 0.03 | −11.56 ± 1.53 |
| F3 | 8 | 70 | 900 | 496.27 ± 20.11 | 0.23 ± 0.04 | −16.20 ± 0.70 |
| F4 | 8 | 70 | 1500 | 445.27 ± 13.46 | 0.37 ± 0.04 | −11.57 ± 0.12 |
| F5 | 10 | 30 | 900 | 775.80 ± 24.70 | 0.50 ± 0.01 | −17.67 ± 0.76 |
| F6 | 10 | 30 | 1500 | 638.47 ± 41.95 | 0.40 ± 0.02 | −13.17 ± 0.40 |
| F7 | 10 | 70 | 900 | 397.40 ± 1.71 | 0.26 ± 0.02 | −9.94 ± 0.32 |
| F8 | 10 | 70 | 1500 | 622.27 ± 13.48 | 0.38 ± 0.02 | −18.87 ± 0.15 |
| Response | Predicted Value | Observed Value | % Error |
|---|---|---|---|
| Particle size (nm) | 489.871 | 496.27 | 1.31% |
| PDI (nm) | 0.233 | 0.233 | Zero% |
| Zeta potential (mV) | 16.2 | 16.2 | Zero% |
| Formulations | MIC | |||
|---|---|---|---|---|
| S. aureus | MRSA | K. pneumoniae | A. baumannii | |
| ZnO-NPs | 180 | 350 | 455 | 625 |
| Sterillium | 85 | *** NE | 68 | 59.5 |
| Groups | NegativeControl | Positive Control | ZnO-NPs | PVP (3% w/v) | Sterillium | |
|---|---|---|---|---|---|---|
| Epidermis | Hypertrophy | 0 | 0 | 0 | 0 | 0 |
| Hyperkeratosis | 0 | 0 | 0 | 0 | 0 | |
| Parakeratosis | 0 | 0 | 0 | 0 | 0 | |
| Erosion | 0 | 3 b,d | 0 a,c,d | 3 b,d | 1 a,b,c | |
| Inflammatory cells infiltration | 0 | 3 b,d | 0 a,c,d | 3 b,d | 1 a,b,c | |
| Extracellular edema | 0 | 0 | 0 | 0 | 0 | |
| Corium | Ulcer | 0 | 3 b,d | 0 a,c,d | 3 b,d | 0 a,b,c |
| Inflammatory cells infiltration | 0 | 3 b,d | 0 a,c,d | 3 b,d | 1 a,b,c | |
| Subcutis | Inflammatory cells infiltration | 0 | 3 b,d | 0 a,c,d | 2 b,d | 0 a,b,c |
| Animal Groups | PCV (%) ± SE | Hb(g/dL) ± SE | RBCs (×106/nL) ± SE | MCV (fL) ± SE | MCHC (%) ± SE |
|---|---|---|---|---|---|
| Negative Control | 39.53 ± 0.246 | 12.06 ± 0.635 | 7.84 ± 0.107 | 48.87 ± 0.483 | 37.58 ± 0.409 |
| Positive Control | 39.41 ± 0.247 | 12.34 ± 0.199 | 7.61 ± 0.140 | 48.72 ± 0.645 | 37.64 ± 0.354 |
| ZnO-NPs | 38.96 ± 0.325 | 12.60 ± 0.134 | 7.99 ± 0.112 | 47.92 ± 0.299 | 37.8 ± 0.224 |
| 3% PVP | 39.53 ± 0.246 | 13.07 ± 0.418 | 7.08 ± 0.270 | 48.51 ± 0.549 | 38.08 ± 0.445 |
| Sterillium | 39.96 ± 0.233 | 13.42 ± 0.306 | 8.34 ± 0.186 | 48.22 ± 0.481 | 38.06 ± 0.209 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismail, A.; Raya, N.R.; Orabi, A.; Ali, A.M.; Abo-zeid, Y. Investigating the Antibacterial Activity and Safety of Zinc Oxide Nanoparticles versus a Commercial Alcohol-Based Hand-Sanitizer: Can Zinc Oxide Nanoparticles Be Useful for Hand Sanitation? Antibiotics 2022, 11, 1606. https://doi.org/10.3390/antibiotics11111606
Ismail A, Raya NR, Orabi A, Ali AM, Abo-zeid Y. Investigating the Antibacterial Activity and Safety of Zinc Oxide Nanoparticles versus a Commercial Alcohol-Based Hand-Sanitizer: Can Zinc Oxide Nanoparticles Be Useful for Hand Sanitation? Antibiotics. 2022; 11(11):1606. https://doi.org/10.3390/antibiotics11111606
Chicago/Turabian StyleIsmail, Aliaa, Nermeen R. Raya, Ahmed Orabi, Alaa M. Ali, and Yasmin Abo-zeid. 2022. "Investigating the Antibacterial Activity and Safety of Zinc Oxide Nanoparticles versus a Commercial Alcohol-Based Hand-Sanitizer: Can Zinc Oxide Nanoparticles Be Useful for Hand Sanitation?" Antibiotics 11, no. 11: 1606. https://doi.org/10.3390/antibiotics11111606
APA StyleIsmail, A., Raya, N. R., Orabi, A., Ali, A. M., & Abo-zeid, Y. (2022). Investigating the Antibacterial Activity and Safety of Zinc Oxide Nanoparticles versus a Commercial Alcohol-Based Hand-Sanitizer: Can Zinc Oxide Nanoparticles Be Useful for Hand Sanitation? Antibiotics, 11(11), 1606. https://doi.org/10.3390/antibiotics11111606





