Andrographolide and 4-Phenylbutyric Acid Administration Increase the Expression of Antimicrobial Peptides Beta-Defensin-1 and Cathelicidin and Reduce Mortality in Murine Sepsis
Abstract
:1. Introduction
2. Results
2.1. Andrographolide, Levofloxacin, 4-Phenylbutyric Acid, Rosuvastatin and Valsartan Increase the Serum Level of Beta-Defensin-1
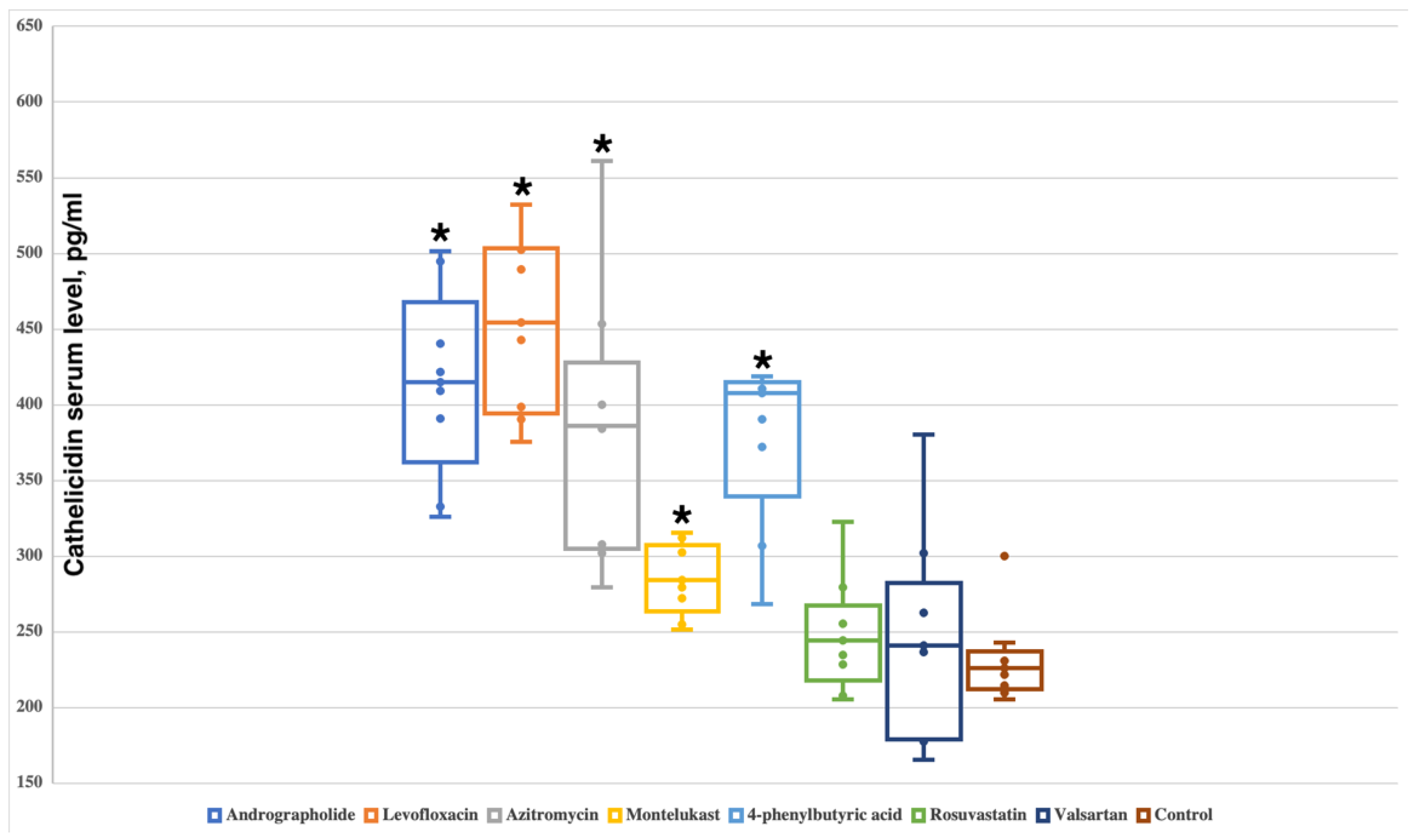
2.2. Andrographolide, Levofloxacin, Azithromycin, Montelukast and 4-Phenylbutyric Acid Increase the Serum Level of Cathelicidin
2.3. Andrographolide and 4-Phenylbutyric Acid Increase Survival in Murine Model of Sepsis
3. Discussion
4. Materials and Methods
4.1. Study of the Influence of Small Molecules on the Level of Endogenous AMPs
4.2. Effect of Andrographolide and 4-Phenylbutyric Acid on Survival in Experimental Sepsis
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- US CDC Antibiotic Resistance Threats in the United States; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2019.
- O’Neill, J. Review on Antimicrobial Resistance. Tackling a Global Health Crisis: Rapid Diagnostics: Stopping Unnecessary Use of Antibiotics. Indep. Rev. AMR 2015, 1–36. [Google Scholar]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Rodrigues, G.; Souza Santos, L.; Franco, O.L. Antimicrobial Peptides Controlling Resistant Bacteria in Animal Production. Front. Microbiol. 2022, 13, 874153. [Google Scholar] [CrossRef] [PubMed]
- Mookherjee, N.; Anderson, M.A.; Haagsman, H.P.; Davidson, D.J. Antimicrobial Host Defence Peptides: Functions and Clinical Potential. Nat. Rev. Drug Discov. 2020, 19, 311–332. [Google Scholar] [CrossRef]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 2559. [Google Scholar] [CrossRef]
- Barbosa, F.; Pinto, E.; Kijjoa, A.; Pinto, M.; Sousa, E. Targeting Antimicrobial Drug Resistance with Marine Natural Products. Int. J. Antimicrob. Agents 2020, 56, 106005. [Google Scholar] [CrossRef]
- Bolatchiev, A. Antimicrobial Peptides Epinecidin-1 and Beta-Defesin-3 Are Effective against a Broad Spectrum of Antibiotic-Resistant Bacterial Isolates and Increase Survival Rate in Experimental Sepsis. Antibiotics 2022, 11, 76. [Google Scholar] [CrossRef]
- Ivanenkov, Y.A.; Zhavoronkov, A.; Yamidanov, R.S.; Osterman, I.A.; Sergiev, P.V.; Aladinskiy, V.A.; Aladinskaya, A.V.; Terentiev, V.A.; Veselov, M.S.; Ayginin, A.A.; et al. Identification of Novel Antibacterials Using Machine-Learning Techniques. Front. Pharmacol. 2019, 10, 913. [Google Scholar] [CrossRef] [Green Version]
- Melo, M.C.R.; Maasch, J.R.M.A.; de la Fuente-Nunez, C. Accelerating Antibiotic Discovery through Artificial Intelligence. Commun. Biol. 2021, 4, 1050. [Google Scholar] [CrossRef]
- Klubthawee, N.; Adisakwattana, P.; Hanpithakpong, W.; Somsri, S.; Aunpad, R. A Novel, Rationally Designed, Hybrid Antimicrobial Peptide, Inspired by Cathelicidin and Aurein, Exhibits Membrane-Active Mechanisms against Pseudomonas Aeruginosa. Sci. Rep. 2020, 10, 9117. [Google Scholar] [CrossRef]
- Bolatchiev, A.; Baturin, V.; Shchetinin, E.; Bolatchieva, E. Novel Antimicrobial Peptides Designed Using a Recurrent Neural Network Reduce Mortality in Experimental Sepsis. Antibiotics 2022, 11, 411. [Google Scholar] [CrossRef] [PubMed]
- Meiyalaghan, S.; Latimer, J.M.; Kralicek, A.V.; Shaw, M.L.; Lewis, J.G.; Conner, A.J.; Barrell, P.J. Expression and Purification of the Antimicrobial Peptide GSL1 in Bacteria for Raising Antibodies. BMC Res. Notes 2014, 7, 777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.S.; Kim, S.W.; Song, J.M.; Kim, S.Y.; Kwon, K.C. A New Prokaryotic Expression Vector for the Expression of Antimicrobial Peptide Abaecin Using SUMO Fusion Tag. BMC Biotechnol. 2019, 19, 13. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.T.J.; Zhang, H.B.; Kim, D.; Liu, L.; Ganz, T. A Model for Antimicrobial Gene Therapy: Demonstration of Human β-Defensin 2 Antimicrobial Activities In Vivo. Hum. Gene Ther. 2002, 13, 2017. [Google Scholar] [CrossRef] [Green Version]
- Deslouches, B.; Peter Di, Y. Antimicrobial Peptides with Selective Antitumor Mechanisms: Prospect for Anticancer Applications. Oncotarget 2017, 8, 46635. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Zhai, Z.; Long, H.; Yang, G.; Deng, B.; Deng, J. Inducible Expression of Defensins and Cathelicidins by Nutrients and Associated Regulatory Mechanisms. Peptides 2020, 123, 170177. [Google Scholar] [CrossRef]
- Zhang, L.; Bao, M.; Liu, B.; Zhao, H.; Zhang, Y.; Ji, X.Y.; Zhao, N.; Zhang, C.; He, X.; Yi, J.; et al. Effect of Andrographolide and Its Analogs on Bacterial Infection: A Review. Pharmacology 2020, 105, 123–134. [Google Scholar] [CrossRef]
- Shao, Z.J.; Zheng, X.W.; Feng, T.; Huan, J.; Chen, J.; Wu, Y.Y.; Zhou, L.M.; Tu, W.W.; Li, H. Andrographolide Exerted Its Antimicrobial Effects by Upregulation of Human β-Defensin-2 Induced through P38 MAPK and NF-ΚB Pathway in Human Lung Epithelial Cells. Can. J. Physiol. Pharmacol. 2012, 90, 647–653. [Google Scholar] [CrossRef]
- Sechet, E.; Telford, E.; Bonamy, C.; Sansonetti, P.J.; Sperandio, B. Natural Molecules Induce and Synergize to Boost Expression of the Human Antimicrobial Peptide β-Defensin-3. Proc. Natl. Acad. Sci. USA 2018, 115, E9869–E9878. [Google Scholar] [CrossRef] [Green Version]
- Baturin, V.A.; Boshyan, R.O. Estimation of Blood Antimicrobial Peptide Levels in Women of Reproductive Age with Pelvic Inflammatory Disease before and after Antibiotic Therapy. Med. News North Cauc. 2021, 16, 159–161. [Google Scholar] [CrossRef]
- Steinmann, J.; Halldórsson, S.; Agerberth, B.; Gudmundsson, G.H. Phenylbutyrate Induces Antimicrobial Peptide Expression. Antimicrob. Agents Chemother. 2009, 53, 5127–5133. [Google Scholar] [CrossRef] [PubMed]
- Ottosson, H.; Nylén, F.; Sarker, P.; Miraglia, E.; Bergman, P.; Gudmundsson, G.H.; Raqib, R.; Agerberth, B.; Strömberg, R. Potent Inducers of Endogenous Antimicrobial Peptides for Host Directed Therapy of Infections. Sci. Rep. 2016, 6, 36692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE Guidelines 2.0: Updated Guidelines for Reporting Animal Research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 12.0. 2022. Available online: http://www.Eucast.org (accessed on 26 October 2022).



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolatchiev, A.; Baturin, V.; Bolatchieva, E. Andrographolide and 4-Phenylbutyric Acid Administration Increase the Expression of Antimicrobial Peptides Beta-Defensin-1 and Cathelicidin and Reduce Mortality in Murine Sepsis. Antibiotics 2022, 11, 1629. https://doi.org/10.3390/antibiotics11111629
Bolatchiev A, Baturin V, Bolatchieva E. Andrographolide and 4-Phenylbutyric Acid Administration Increase the Expression of Antimicrobial Peptides Beta-Defensin-1 and Cathelicidin and Reduce Mortality in Murine Sepsis. Antibiotics. 2022; 11(11):1629. https://doi.org/10.3390/antibiotics11111629
Chicago/Turabian StyleBolatchiev, Albert, Vladimir Baturin, and Elizaveta Bolatchieva. 2022. "Andrographolide and 4-Phenylbutyric Acid Administration Increase the Expression of Antimicrobial Peptides Beta-Defensin-1 and Cathelicidin and Reduce Mortality in Murine Sepsis" Antibiotics 11, no. 11: 1629. https://doi.org/10.3390/antibiotics11111629





